Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Fe3O4@CEF Nanoparticles
2.2.2. Synthesis of PLGA/Fe3O4@CEF Coatings
2.3. Physicochemical Characterisation
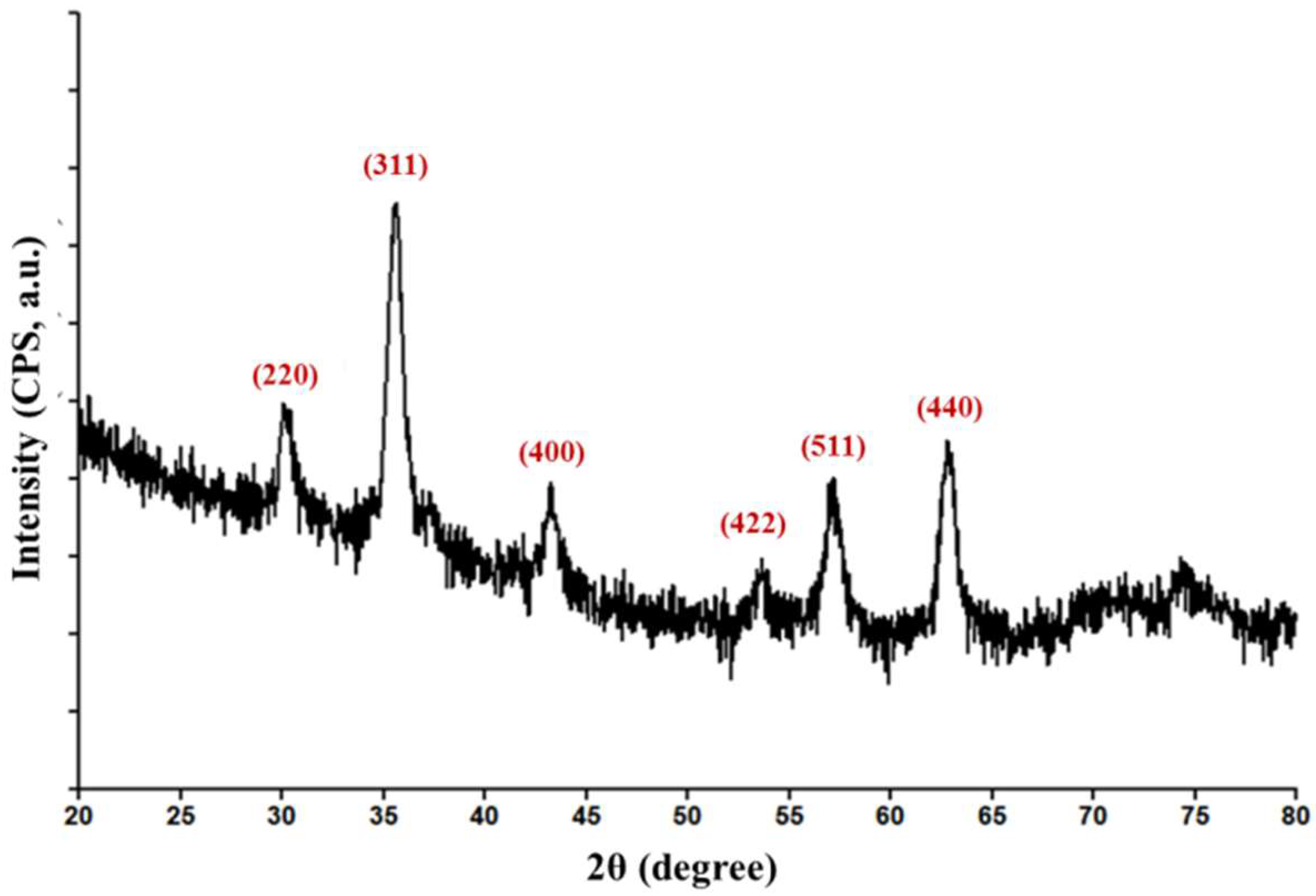
2.3.1. Grazing Incidence X-ray Diffraction (GIXRD)
2.3.2. Thermogravimetric Analysis (TGA)
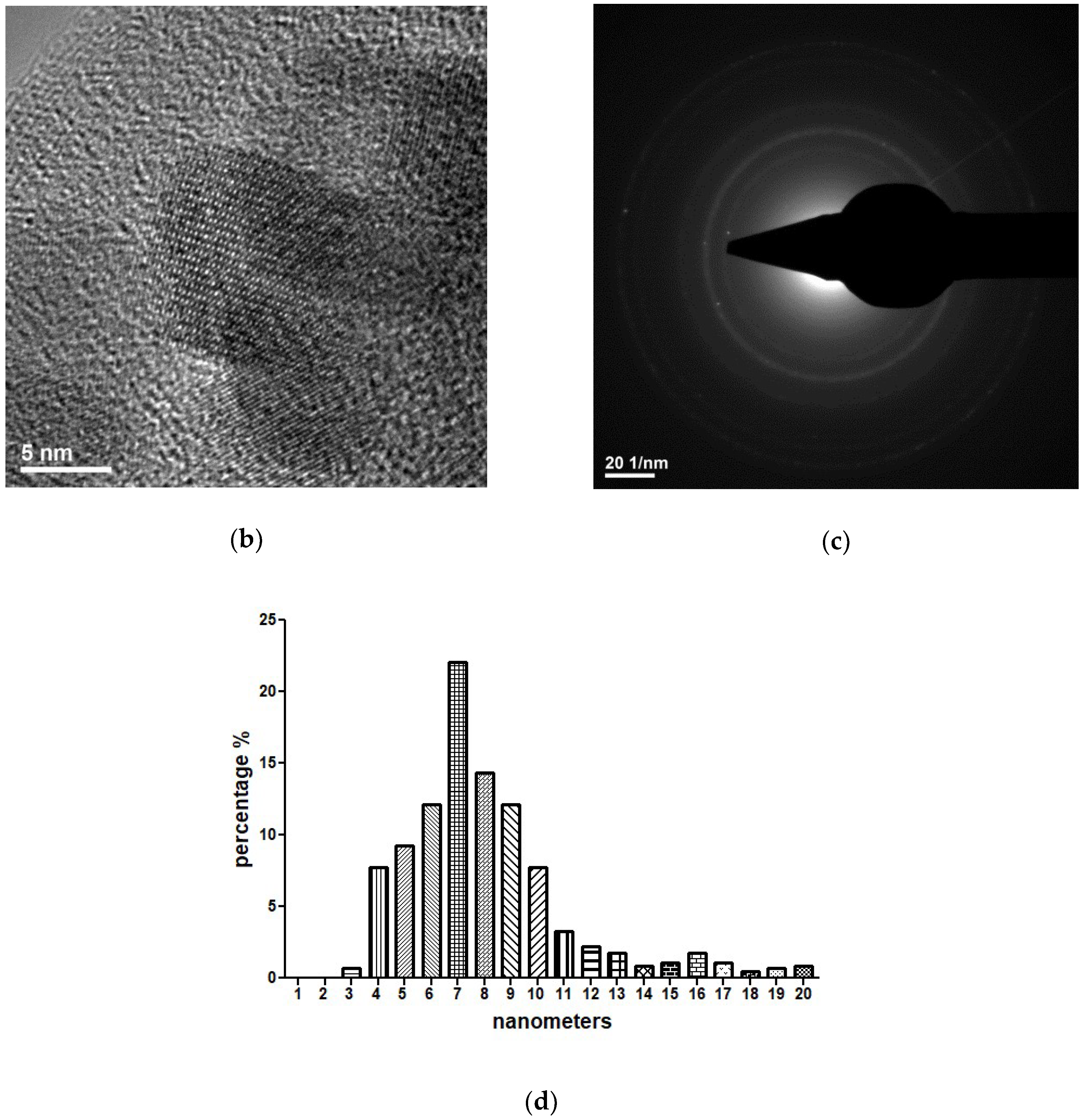
2.3.3. Transmission Electron Microscopy (TEM)
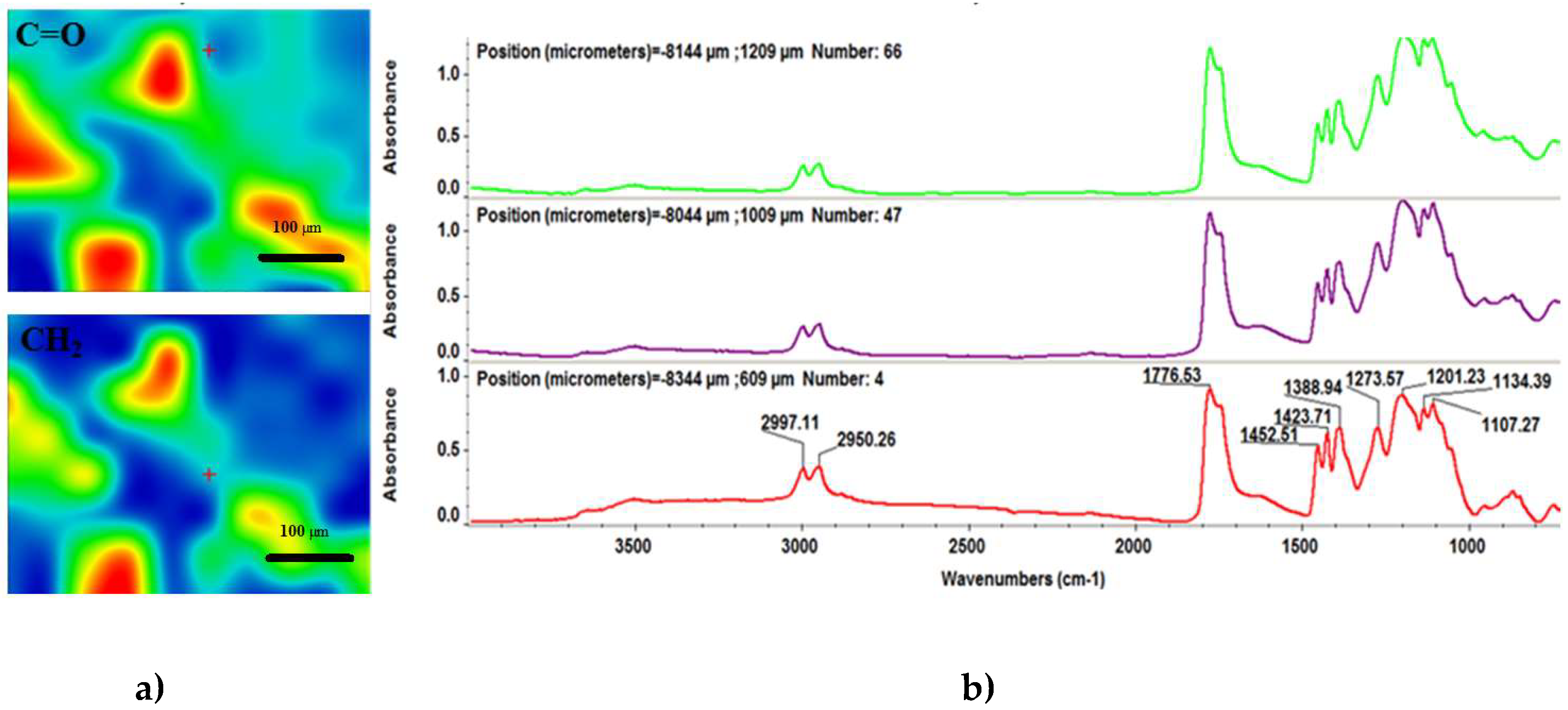
2.3.4. Infrared Microscopy (IRM)
2.4. Biological Evaluation
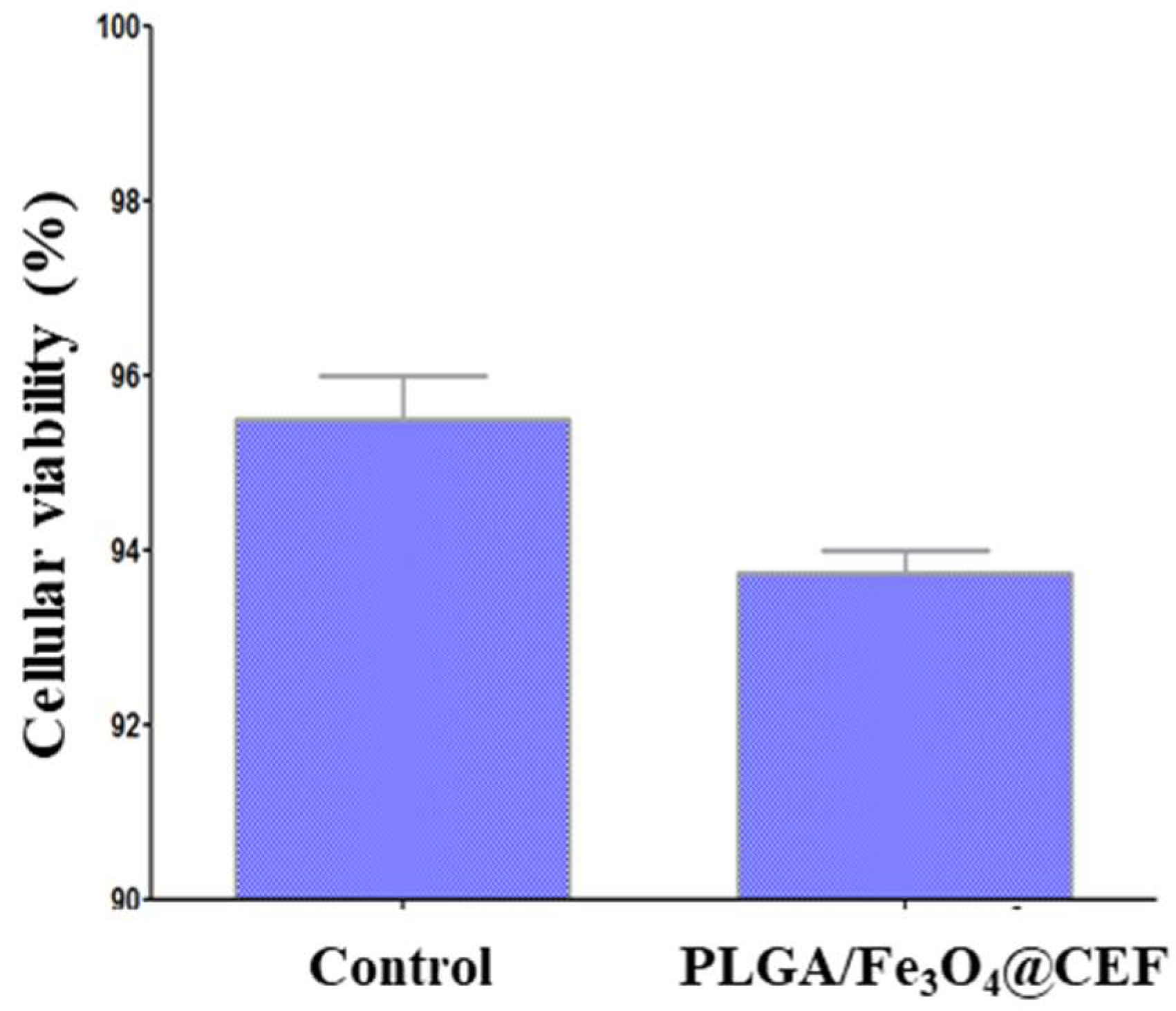
2.4.1. Biocompatibility Evaluation
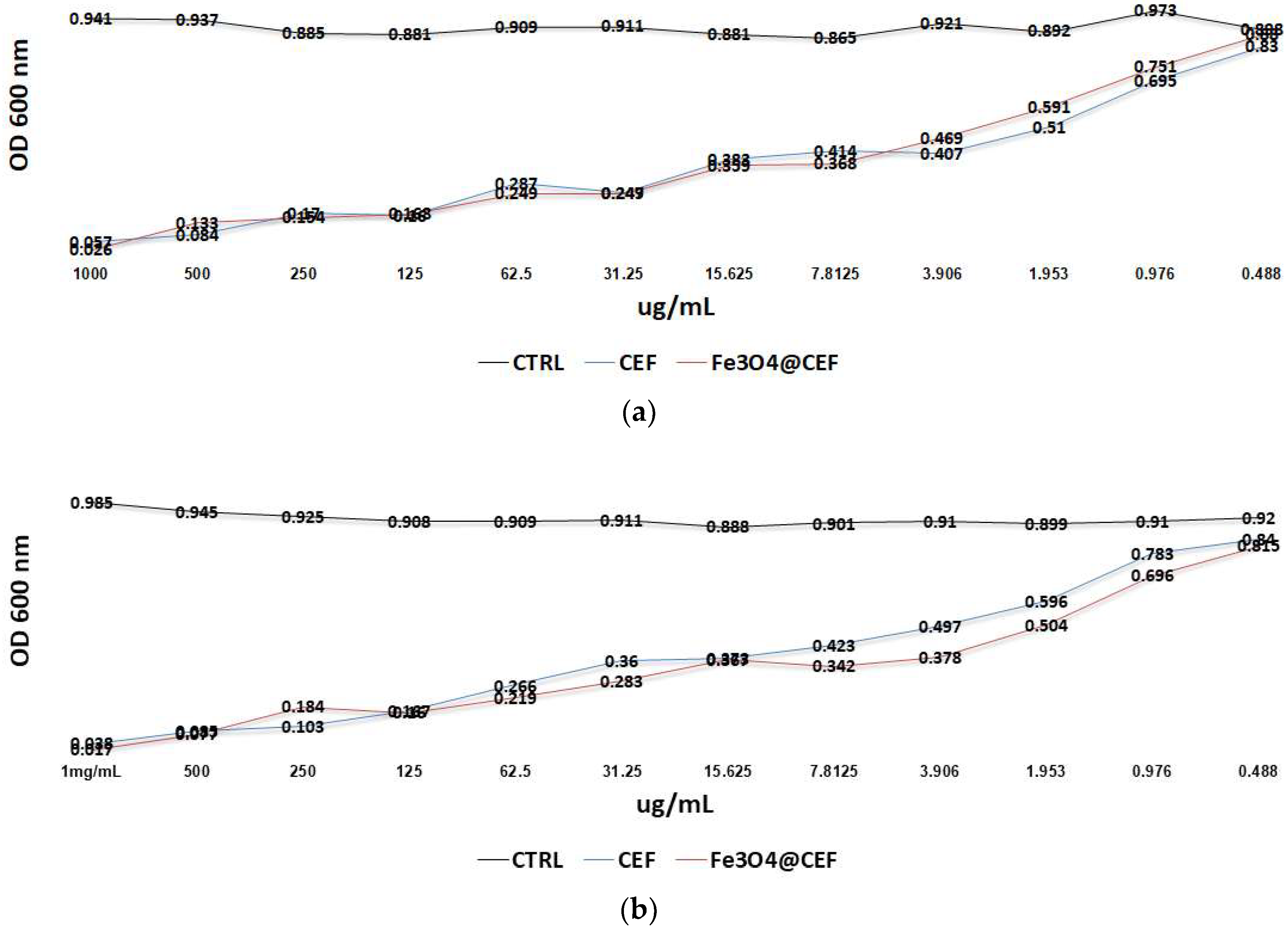
2.4.2. Antimicrobial Evaluation
Antimicrobial Evaluation of the Core-Shell Nanoparticles
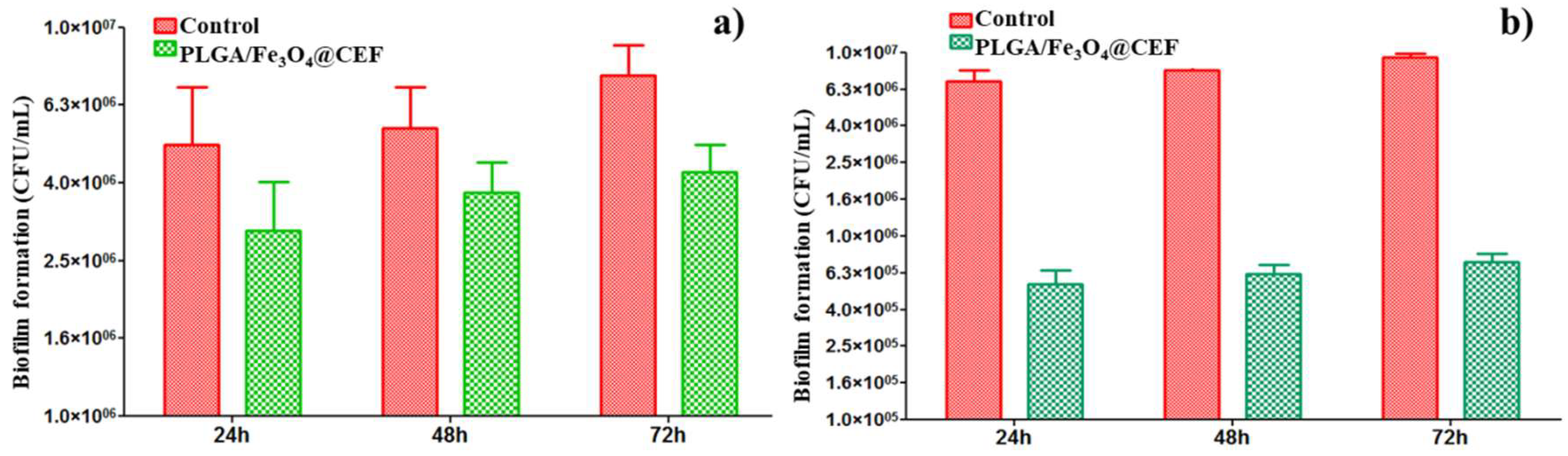
Anti-Biofilm Effect of the Coatings
2.4.3. In Vivo Biocompatibility and Biodistribution
3. Results
3.1. Physicochemical Characterisation of Fe3O4@CEF Nanoparticles
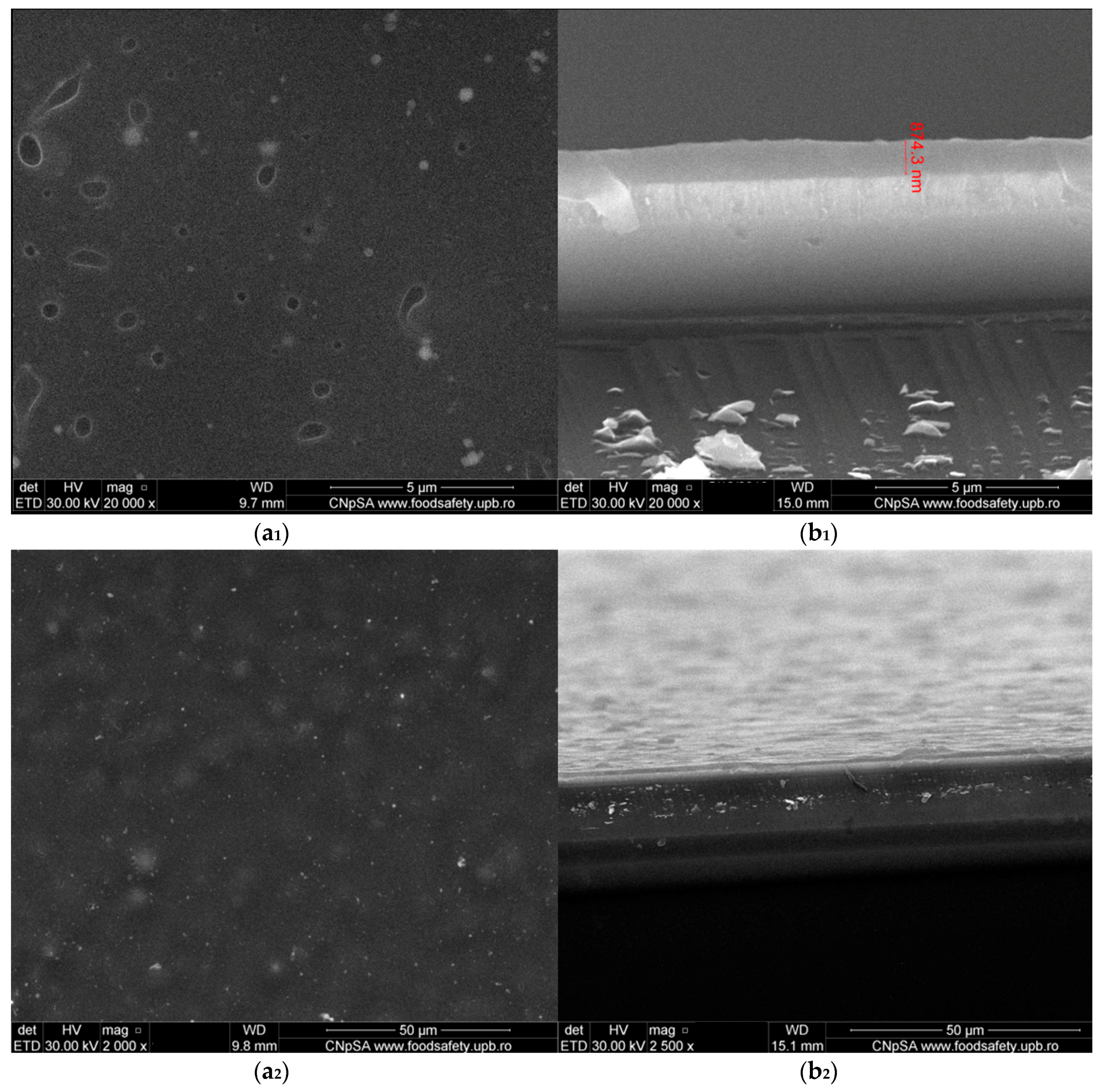
3.2. Physicochemical Characterisation of PLGA/Fe3O4@CEF Coatings
3.3. Biological Evaluation of the PLGA/ Fe3O4@CEF Coatings
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Cavling Arendrup, M. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Zhang, H.J.; Liu, J.; Dong, Q.; Duan, S.; Ge, J.Q.; Wang, Z.H.; Zhang, Z. Antimicrobial resistance of 3 types of gram-negative bacteria isolated from hospital surfaces and the hands of health care workers. Am. J. Infect. Control 2017, 45, e143–e147. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Darby, R.R.; Raibagkar, P.; Gonzalez Castro, L.N.; Berkowitz, A.L. Antibiotic-associated encephalopathy. Neurology 2016, 86, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.K.W.; Chow, K.M.; Choy, A.S.M.; Kwan, B.C.H.; Szeto, C.C.; Li, P.K.T. Clinical manifestation of macrolide antibiotic toxicity in CKD and dialysis patients. Clin. Kidney J. 2014, 7, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Wijdeven, R.H.; Pang, B.; Assaraf, Y.G.; Neefjes, J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist. Updates 2016, 28, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Busl, K.M. Nosocomial Infections in the Neurointensive Care Unit. Neurol. Clin. 2018, 29, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Merezeanu, N.; Gheorghe, I.; Popa, M.; Chifiriuc, M.C.; Lazăr, V.; Pântea, O.; Banu, O.; Bolocan, A.; Grigore, R.; Vifor Bereșteanu, Ș. Virulence and resistance features of Pseudomonas aeruginosa strains isolated from patients with cardiovascular diseases. Biointerface Res. Appl. Chem. 2016, 6, 1117–1121. [Google Scholar]
- Tatu, A.L.; Merezeanu, N.; Pântea, O.; Gheorghe, I.; Popa, M.; Banu, O.; Cristea, V.C.; Chifiriuc, M.C.; Lazăr, V.; Marutescu, L. Resistance features of Pseudomonas aeruginosa strains isolated from patients with infectious complications of cardiovascular surgery. Biointerface Res. Appl. Chem. 2017, 7, 2004–2008. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2016, 44, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization in health care facilities: An overview and current issues. Infect. Dis. Clin. N. Am. 2016, 30, 609–637. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Nayak, S. Disinfection, sterilization and disposables. Anaesth. Intensive Care Med. 2013, 14, 423–427. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Alam, N.; Koul, M.; Mintoo, M.J.; Khare, V.; Gupta, R.; Rawat, N.; Sharma, P.R.; Singh, S.K.; Mondhe, D.M.; Gupta, P.N. Development and characterization of hyaluronic acid modified PLGA based nanoparticles for improved efficacy of cisplatin in solid tumor. Biomed. Pharmacother. 2017, 95, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.W.; Seol, D.; Ding, L.; Heo, D.N.; Lee, S.J.; Martin, J.A.; Kwon, I.K. Ultrasound-triggered PLGA microparticle destruction and degradation for controlled delivery of local cytotoxicity and drug release. Int. J. Biol. Macromol. 2018, 106, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.A.; Yaghoubi, S.; Abdollahi, E.; Tekie, F.S.M.; Kamali, H.; Khodaverdi, E.; Hadizadeh, F. In-vivo study of naltrexone hydrochloride release from an in-situ forming PLGA-PEG-PLGA system in the rabbit. J. Drug Deliv. Sci. Technol. 2017, 36, 156–160. [Google Scholar] [CrossRef]
- Jalali, N.; Rezvani, Z.; Singh Chauhan, N.P.; Mozafari, M. Synthesis and characterization of surface-modified poly (lactide-co-glycolide) nanoparticles by chitosan molecules for on-demand drug delivery applications. Biointerface Res. Appl. Chem. 2016, 6, 1200–1202. [Google Scholar]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Markoutsa, E.; Zhan, Y.; Zhang, J.; Xu, P. Mussel-inspired PLGA/polydopamine core-shell nanoparticle for light induced cancer thermochemotherapy. Acta Biomater. 2017, 59, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Pei, J.; Hyun, H.; Castanares, M.A.; Collins, D.S.; Yeo, Y. Small molecule delivery to solid tumors with chitosan-coated PLGA particles: A lesson learned from comparative imaging. J. Control. Release 2017, 268, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, D.; Guo, Z.F.; Zhang, Y.M.; Liu, Y.; Askin, S.; Craig, D.Q.M.; Zhao, M. The application of novel nano-thermal and imaging techniques for monitoring drug microstructure and distribution within PLGA microspheres. Int. J. Pharm. 2017, 522, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Keshvari, H.; Fasehee, H.; Dinarvand, R.; Faghihi, S. Combining NT3-overexpressing MSCs and PLGA microcarriers for brain tissue engineering: A potential tool for treatment of Parkinson’s disease. Mater. Sci. Eng. C 2017, 76, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Santoveña, A.; Monzón, C.; Alvarez-Lorenzo, C.; del Rosario, C.; Delgado, A.; Evora, C.; Concheiro, A.; Llabrés, M.; Fariña, J.B. Structure-Performance relationships of temperature-responsive PLGA-PEG-PLGA gels for sustained release of bone morphogenetic Protein-2. J. Pharm. Sci. 2017, 106, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Schardosim, M.; Soulié, J.; Poquillon, D.; Cazalbou, S.; Duployer, B.; Tenailleau, C.; Rey, C.; Hübler, R.; Combes, C. Freeze-casting for PLGA/carbonated apatite composite scaffolds: Structure and properties. Mater. Sci. Eng. C 2017, 77, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Bisht, R.; Jaiswal, J.K.; Oliver, V.F.; Eurtivong, C.; Reynisson, J.; Rupenthal, I.D. Preparation and evaluation of PLGA nanoparticle-loaded biodegradable light-responsive injectable implants as a promising platform for intravitreal drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 142–156. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Li, B.; Yang, X.; Zeng, R.; Liu, Y.; Li, T.; Ho, R.J.Y.; Shao, J. PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment. J. Colloid Interface Sci. 2017, 490, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Gil, S.; Simón-Yarza, T.; Garbayo, E.; Prósper, F.; Blanco-Prieto, M.J. Cytokine-loaded PLGA and PEG-PLGA microparticles showed similar heart regeneration in a rat myocardial infarction model. Int. J. Pharm. 2017, 523, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Deng, C.; Meng, F.; Zhang, J.; Sun, H.; Zhong, Z. Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J. Control. Release 2017, 259, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Abdel Gawad, S.A.; El-Ads, E.H.; El-Gohary, A.R.M.; Galal, A. A new strategy for NADH sensing using ionic liquid crystals-carbon nanotubes/nano-magnetite composite platform. Sens. Actuators B Chem. 2017, 251, 65–73. [Google Scholar] [CrossRef]
- Jaime, J.; Rangel, G.; Muñoz-Bonilla, A.; Mayoral, A.; Herrasti, P. Magnetite as a platform material in the detection of glucose, ethanol and cholesterol. Sens. Actuators B Chem. 2017, 238, 693–701. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, L.; Zhu, C.; Li, B.; Xiao, H.; Jiang, H.; Zhou, X. Magnetically controlled electrochemical sensing membrane based on multifunctional molecularly imprinted polymers for detection of insulin. Electrochim. Acta 2016, 218, 91–100. [Google Scholar] [CrossRef]
- Atashi, Z.; Divband, B.; Keshtkar, A.; Khatamian, M.; Farahmand-Zahed, F.; Nazarlo, A.K.; Gharehaghaji, N. Synthesis of cytocompatible Fe3O4@ZSM-5 nanocomposite as magnetic resonance imaging contrast agent. J. Magn. Magn. Mater. 2017, 438, 46–51. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Wu, H.; Zang, F.; Ma, M.; Hua, Z.; Gu, N.; Zhang, Y. Improving sensitivity of magnetic resonance imaging by using a dual-targeted magnetic iron oxide nanoprobe. Colloid Surf. B Biointerfaces 2018, 161, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.; Fedorova, M.; Naumenko, V.; Shchetinin, I.; Abakumov, M.; Erofeev, A.; Gorelkin, P.; Meshkov, G.; Beloglazkina, E.; Ivanenkov, Y.; et al. Synthesis, characterization and MRI application of magnetite water-soluble cubic nanoparticles. J. Magn. Magn. Mater. 2017, 441, 6–13. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; Nalin de Silva, K.M.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Je, J.Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Sodium alginate-polyvinyl alcohol-bovin serum albumin coated Fe3O4 nanoparticles as anticancer drug delivery vehicle: Doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater. Sci. Eng. C 2017, 79, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Yurenya, A.Y.; Polikarpov, M.A.; Chukalova, A.A.; Moskaleva, E.Y.; Taldenkov, A.N.; Panchenko, V.Y. The magnetic introduction of magnetite nanoparticles into live cells for radiosensibility enhancement. J. Magn. Magn. Mater. 2017, 427, 111–113. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzogou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cotar, A.I.; Grumezescu, A.M.; Hunag, K.S.; Voicu, G.; Chifiriuc, M.C.; Radulescu, R. Magnetite nanoparticles influence the efficacy of antibiotics against biofilm embedded Staphylococcus aureus cells. Biointerface Res. Appl. Chem. 2013, 3, 559–565. [Google Scholar]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1766–1775. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, M.C. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Mogoantă, L.; Mogoşanu, G.D.; Grumezescu, A.M.; Stănculescu, A.; Socol, G.; Iordache, F.; Maniu, H.; et al. MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Appl. Surf. Sci. 2015, 336, 188–195. [Google Scholar] [CrossRef]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoșanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ș.; Trușcă, R.; Chifiriuc, M.C.; Maniu, H. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)-polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Grumezescu, V.; Andronescu, E.; Holban, A.M.; Socol, G.; Grumezescu, A.M.; Ficai, A.; Lazăr, V.; Chifiriuc, M.C.; Trușcă, R. Fabrication and characterization of functionalized surfaces with 3-amino propyltrimethoxysilane films for anti-infective therapy applications. Appl. Surf. Sci. 2015, 336, 401–406. [Google Scholar] [CrossRef]
- Iordache, F.; Grumezescu, V.; Grumezescu, A.M.; Curuțiu, C.; Dițu, L.M.; Socol, G.; Ficai, A.; Trușcă, R.; Holban, A.M. Gamma-cyclodextrin/usnic acid thin film fabricated by MAPLE for improving the resistance of medical surfaces to Staphylococcus aureus colonization. Appl. Surf. Sci. 2015, 336, 407–412. [Google Scholar] [CrossRef]
- Guan, D.; Gao, Z.; Yang, W.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.; Liu, L. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Balaure, P.C.; Andronescu, E.; Grumezescu, A.M.; Ficai, A.; Huang, K.S.; Yang, C.H.; Chifiriuc, C.M.; Lin, Y.S. Fabrication, characterization and in vitro profile based interaction with eukaryotic and prokaryotic cells of alginate-chitosan-silica biocomposite. Int. J. Pharm. 2013, 441, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, Y.Y.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Shi, S.Q.; Che, W.; Liang, K.; Xia, C.; Zhang, D. Phase transitions of carbon-encapsulated iron oxide nanoparticles during the carbonization of cellulose at various pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2015, 115, 1–6. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Machado, D.; Laia, C.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Thermal and magnetic properties of chitosan-iron oxide nanoparticles. Carbohydr. Polym. 2016, 149, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L.; Kim, D.; Kim, S. Development of hematin conjugated PLGA nanoparticle for selective cancer targeting. Eur. J. Pharm. Sci. 2016, 91, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Ali, A.E.; Ghareeb, D.A.; Nasr, N.M. Synthesis, molecular spectroscopy and thermal analysis of some cefepime complexes. J. Mol. Struct. 2016, 1107, 189–201. [Google Scholar] [CrossRef]
- D’Avila Carvalho Erbetta, C.; Alves, R.J.; Magalhães Resende, J.; de Souza Freitas, R.F.; de Sousa, R.G. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Rescignano, N.; Tarpani, L.; Romani, A.; Bicchi, I.; Mattioli, S.; Emiliani, C.; Torre, L.; Kenny, J.M.; Martino, S.; Latterini, L.; et al. In-vitro degradation of PLGA nanoparticles in aqueous medium and in stem cell cultures by monitoring the cargo fluorescence spectrum. Polym. Degrad. Stab. 2016, 134, 296–304. [Google Scholar] [CrossRef]
- Sima, F.; Axente, E.; Iordache, I.; Luculescu, C.; Gallet, O.; Anselme, K.; Mihăilescu, I.N. Combinatorial Matrix Assisted Pulsed Laser Evaporation of a biodegradable polymer and fibronectin for protein immobilization and controlled release. Appl. Surf. Sci. 2014, 306, 75–79. [Google Scholar] [CrossRef]
- Socol, G.; Mihăilescu, I.N.; Albu, A.M.; Antohe, Ș.; Stănculescu, F.; Stănculescu, A.; Mihuț, L.; Preda, N.; Socol, M.; Rașoga, O. MAPLE prepared polymeric thin films for non-linear optic applications. Appl. Surf. Sci. 2009, 255, 5611–5614. [Google Scholar] [CrossRef]
- Vișan, A.; Stan, G.E.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Beșleagă, C.; Luculescu, C.; Chifiriuc, M.C.; Hussien, M.D.; Marsan, O.; et al. Combinatorial MAPLE deposition of antimicrobial orthopedic maps fabricated from chitosan and biomimetic apatite powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Visan, A.; Socol, G.; Surdu, A.V.; Oprea, A.E.; Grumezescu, A.M.; Chifiriuc, M.C.; Boehm, R.D.; Yamaleyeva, D.; Taylord, M.; et al. Antimicrobial activity of biopolymeric thin films containing flavonoid natural compounds and silver nanoparticles fabricated by MAPLE: A comparative study. Appl. Surf. Sci. 2016, 374, 290–296. [Google Scholar] [CrossRef]
- Visan, A.; Cristescu, R.; Stefan, N.; Miroiu, M.; Nita, C.; Socol, M.; Florica, C.; Rasoga, O.; Zgura, I.; Sima, L.E.; et al. Antimicrobial polycaprolactone/polyethylene glycol embedded lysozyme coatings of Ti implants for osteoblast functional properties in tissue engineering. Appl. Surf. Sci. 2017, 417, 234–243. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Tan, S.; Bandara, H.M.H.N.; Fu, Y.; Liu, S.; Smyth, H.D.C. Externally Controlled Triggered-Release of Drug from PLGA Micro and Nanoparticles. PLoS ONE. 2014, 9, e114271. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Gestal, M.C.; Holban, A.M.; Grumezescu, V.; Vasile, B.Ș.; Mogoantă, L.; Iordache, F.; Bleotu, C.; Mogoșanu, G.D. Biocompatible Fe3O4 Increases the Efficacy of Amoxicillin Delivery against Gram-Positive and Gram-Negative Bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Kariminia, S.; Shamsipur, A.; Shamsipur, M. Analytical characteristics and application of novel chitosan coated magnetic nanoparticles as an efficient drug delivery system for ciprofloxacin. Enhanced drug release kinetics by low-frequency ultrasounds. J. Pharm. Biomed. Anal. 2016, 129, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.M.; Sathiya, S.M.; Manivannan, G.; Rajan, M.A.J. A comparative study on the biopolymer functionalized iron oxide nanocomposite for antimicrobial activity. Mater. Today Proc. 2016, 3, 3866–3871. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.; Lazar, V. Inhibitory Activity of Fe3O4/Oleic Acid/Usnic Acid—Core/Shell/Extra-Shell Nanofluid on S. aureus biofilm development. IEEE Trans. Nanobiosci. 2011, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoșanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficai, D.; Grumezescu, V.; Fufă, O.M.; Popescu, R.C.; Holban, A.M.; Ficai, A.; Grumezescu, A.M.; Mogoanta, L.; Mogosanu, G.D.; Andronescu, E. Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite. Nanomaterials 2018, 8, 633. https://doi.org/10.3390/nano8090633
Ficai D, Grumezescu V, Fufă OM, Popescu RC, Holban AM, Ficai A, Grumezescu AM, Mogoanta L, Mogosanu GD, Andronescu E. Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite. Nanomaterials. 2018; 8(9):633. https://doi.org/10.3390/nano8090633
Chicago/Turabian StyleFicai, Denisa, Valentina Grumezescu, Oana Mariana Fufă, Roxana Cristina Popescu, Alina Maria Holban, Anton Ficai, Alexandru Mihai Grumezescu, Laurentiu Mogoanta, George Dan Mogosanu, and Ecaterina Andronescu. 2018. "Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite" Nanomaterials 8, no. 9: 633. https://doi.org/10.3390/nano8090633
APA StyleFicai, D., Grumezescu, V., Fufă, O. M., Popescu, R. C., Holban, A. M., Ficai, A., Grumezescu, A. M., Mogoanta, L., Mogosanu, G. D., & Andronescu, E. (2018). Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite. Nanomaterials, 8(9), 633. https://doi.org/10.3390/nano8090633








