Magnetic Fe@FeOx, Fe@C and α-Fe2O3 Single-Crystal Nanoblends Synthesized by Femtosecond Laser Ablation of Fe in Acetone
Abstract
:1. Introduction
2. Materials and Methods
3. Results
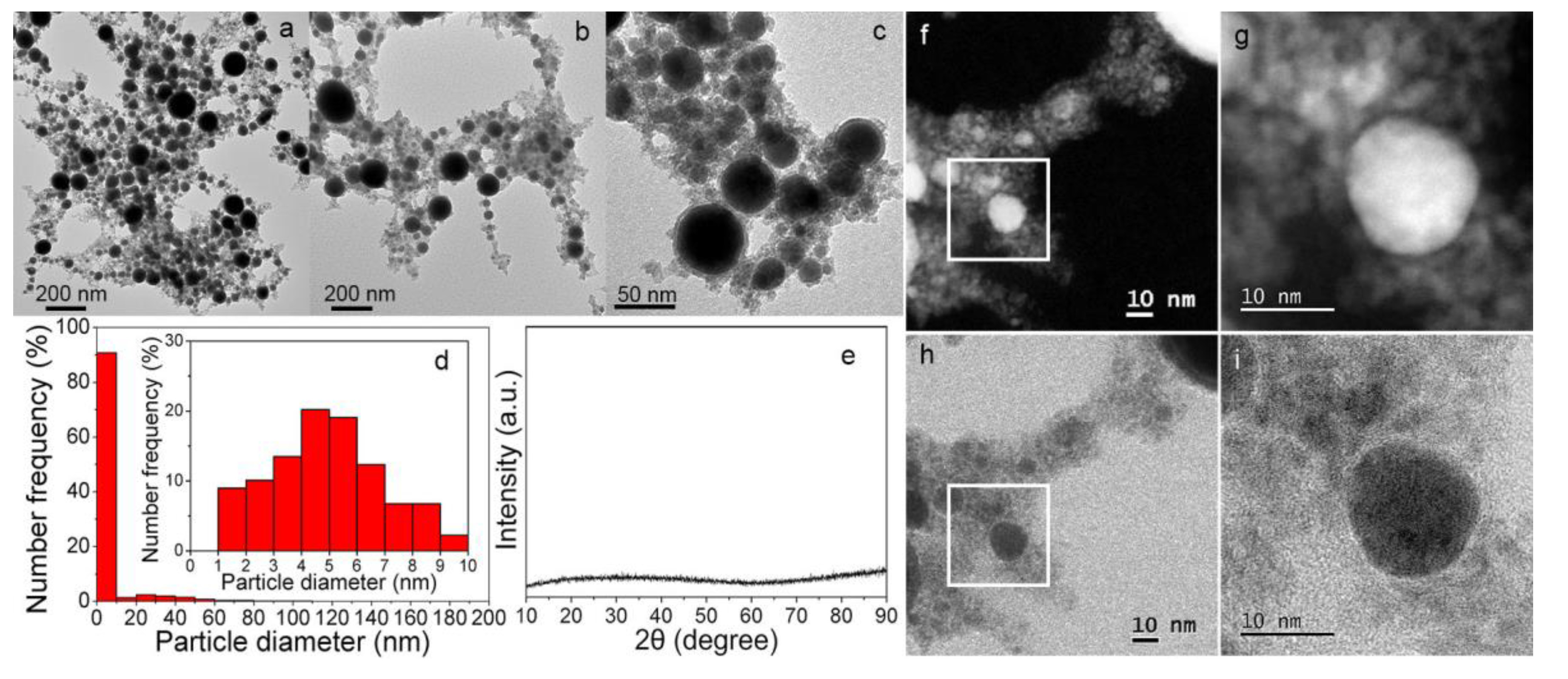
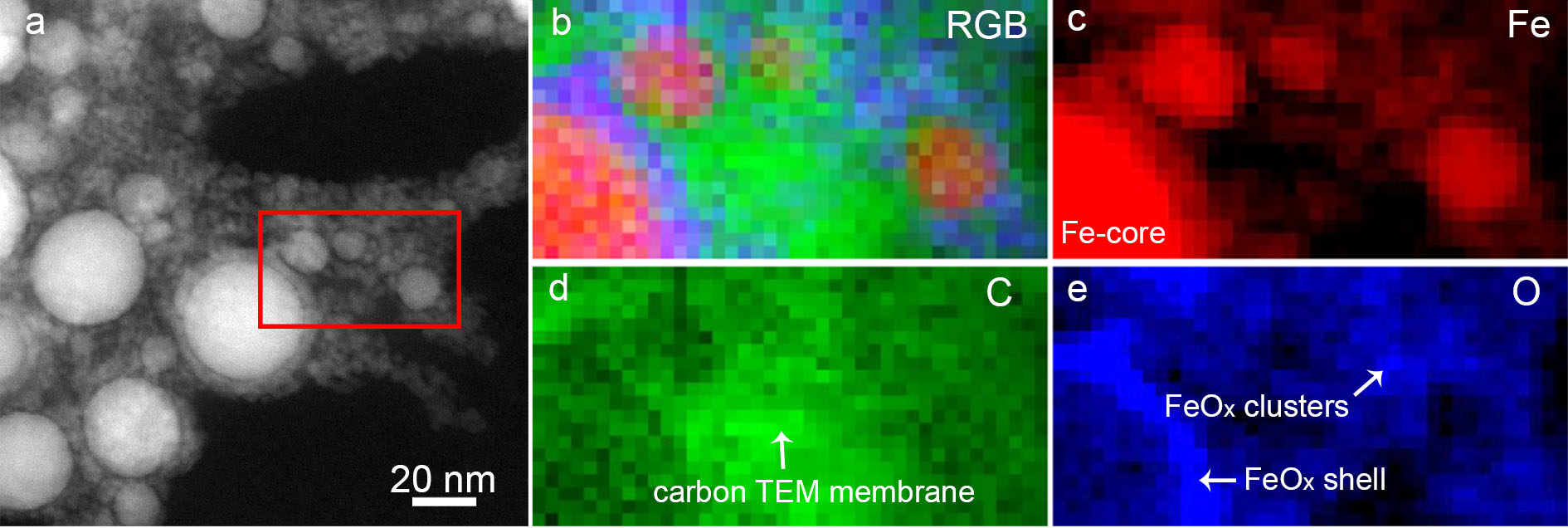
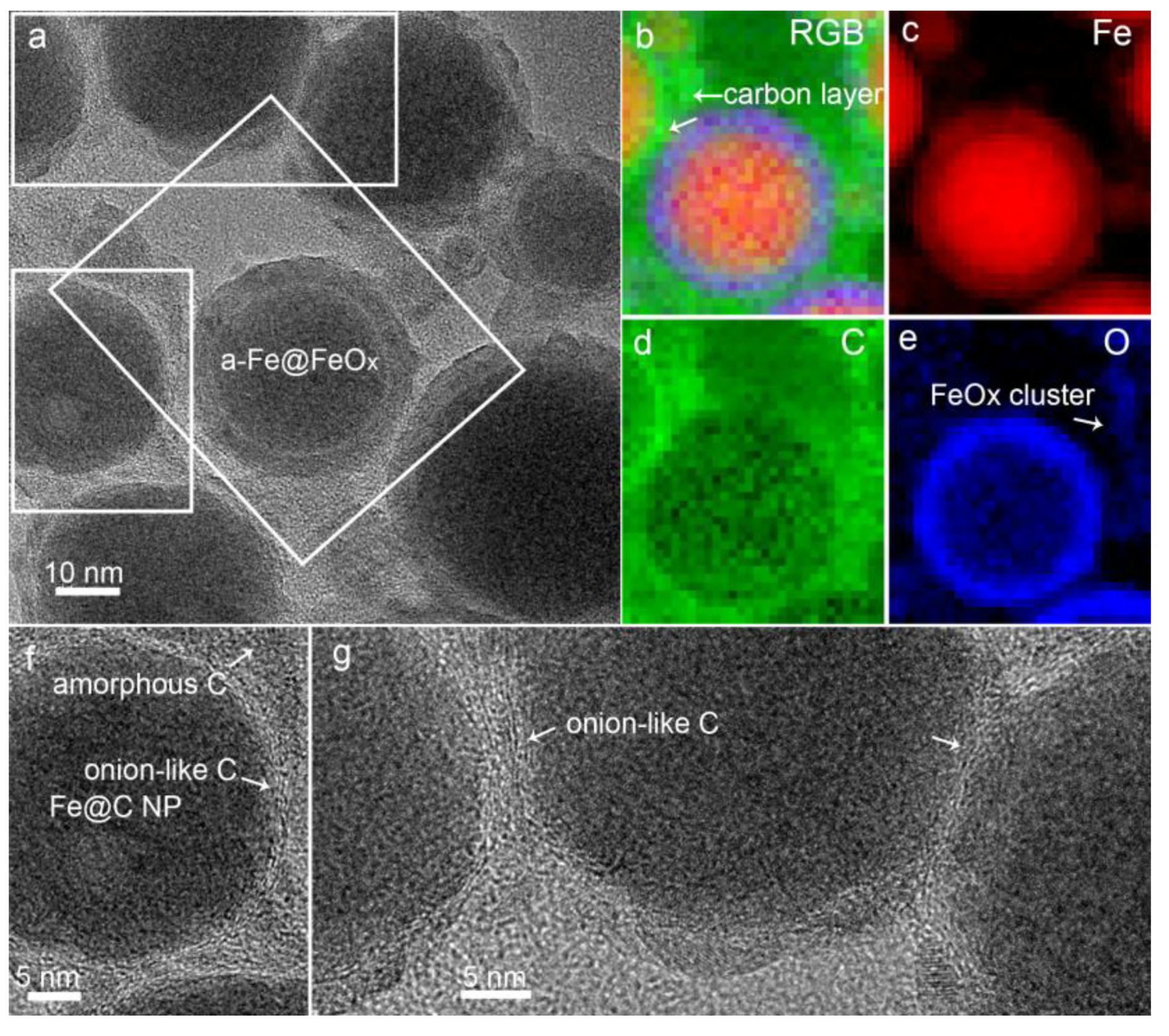
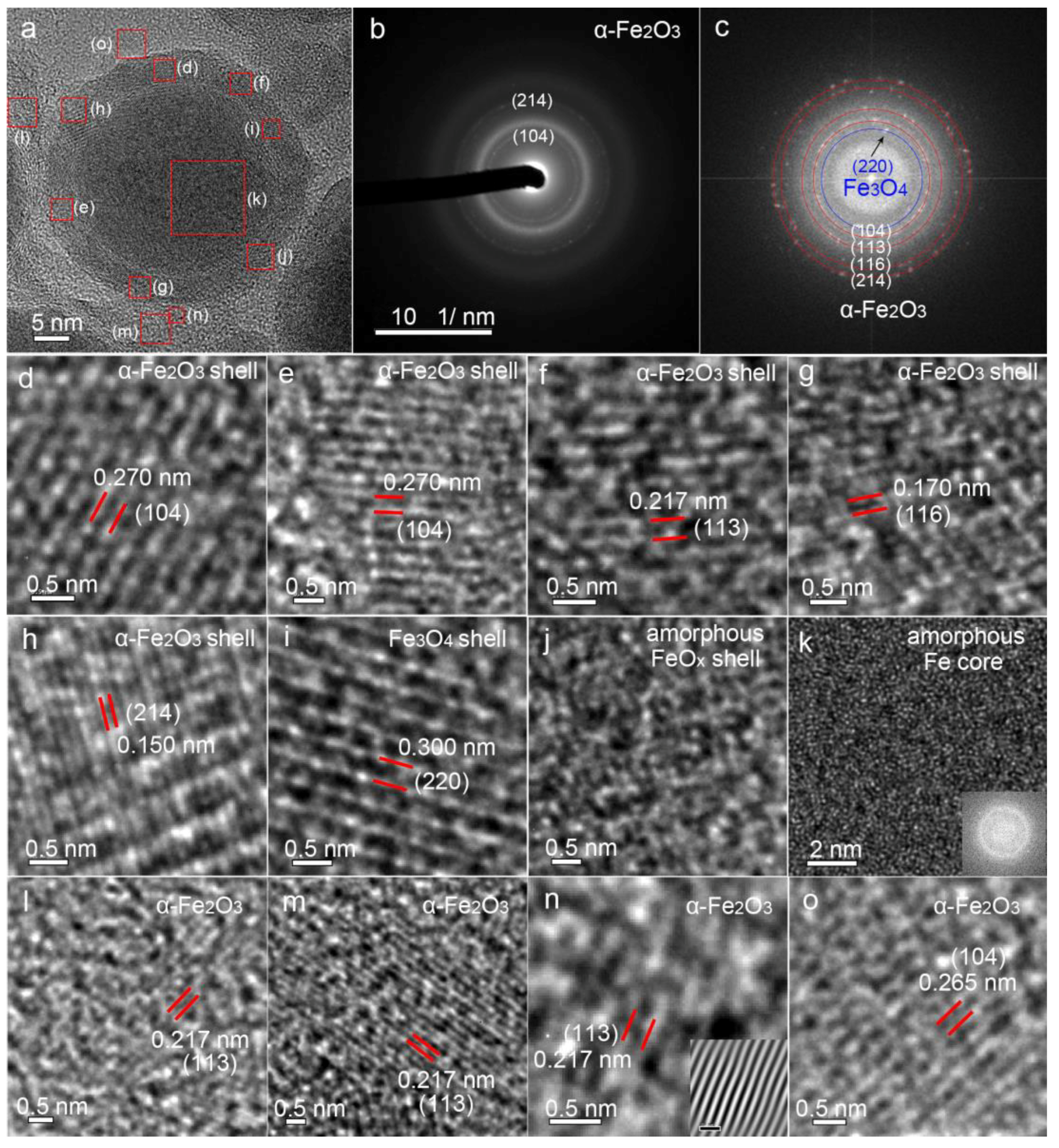
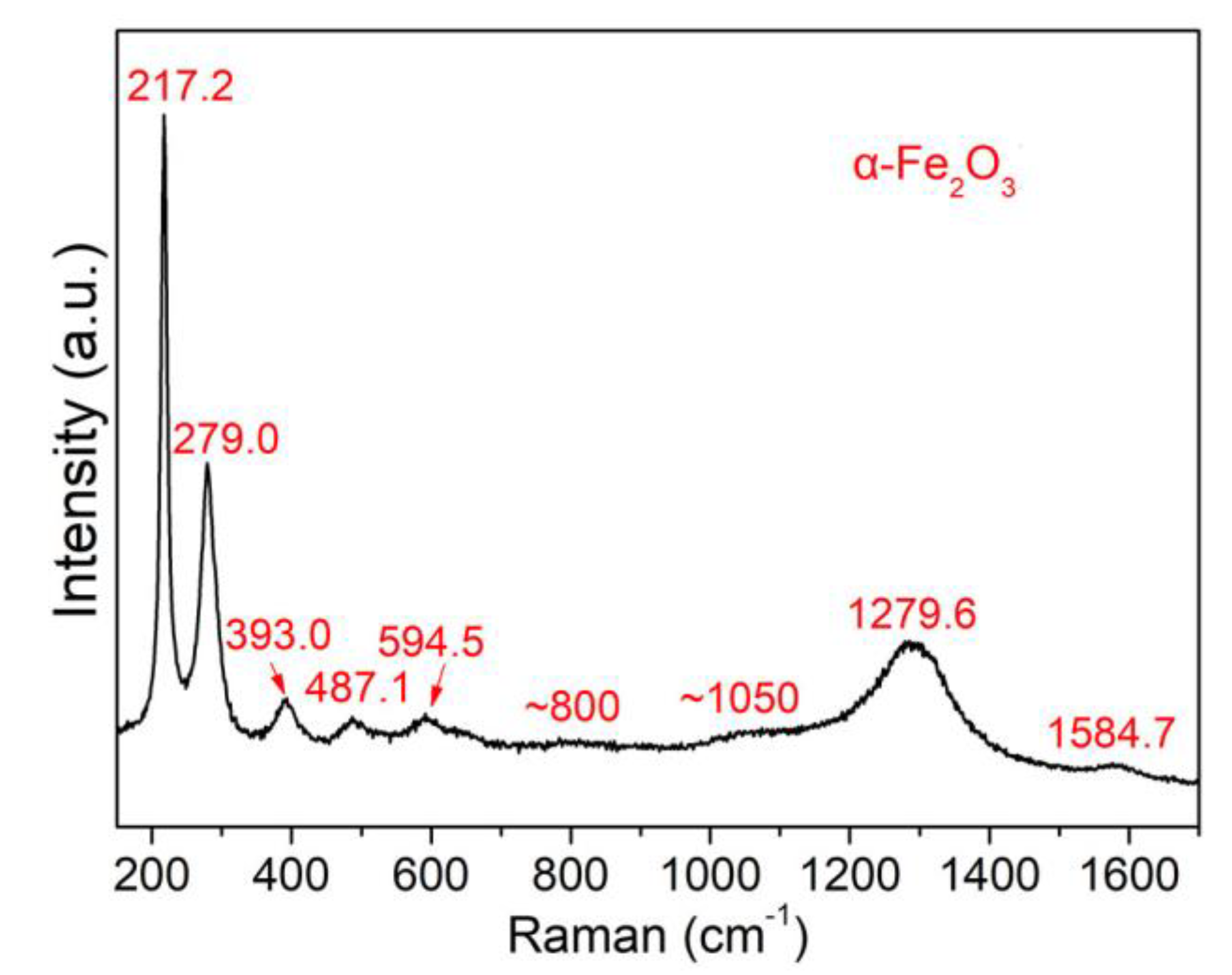
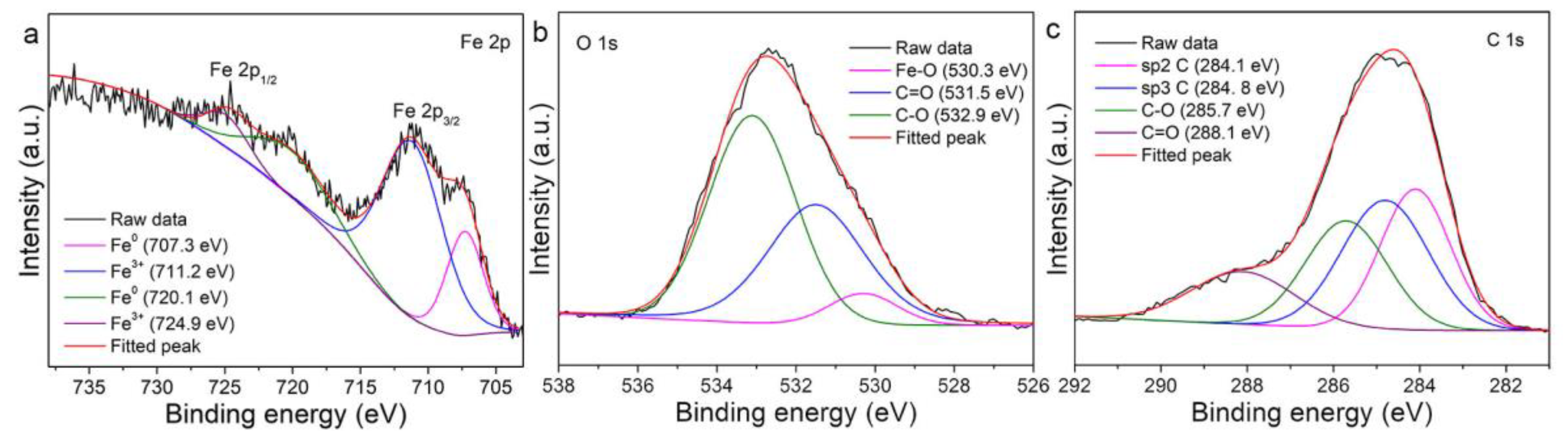
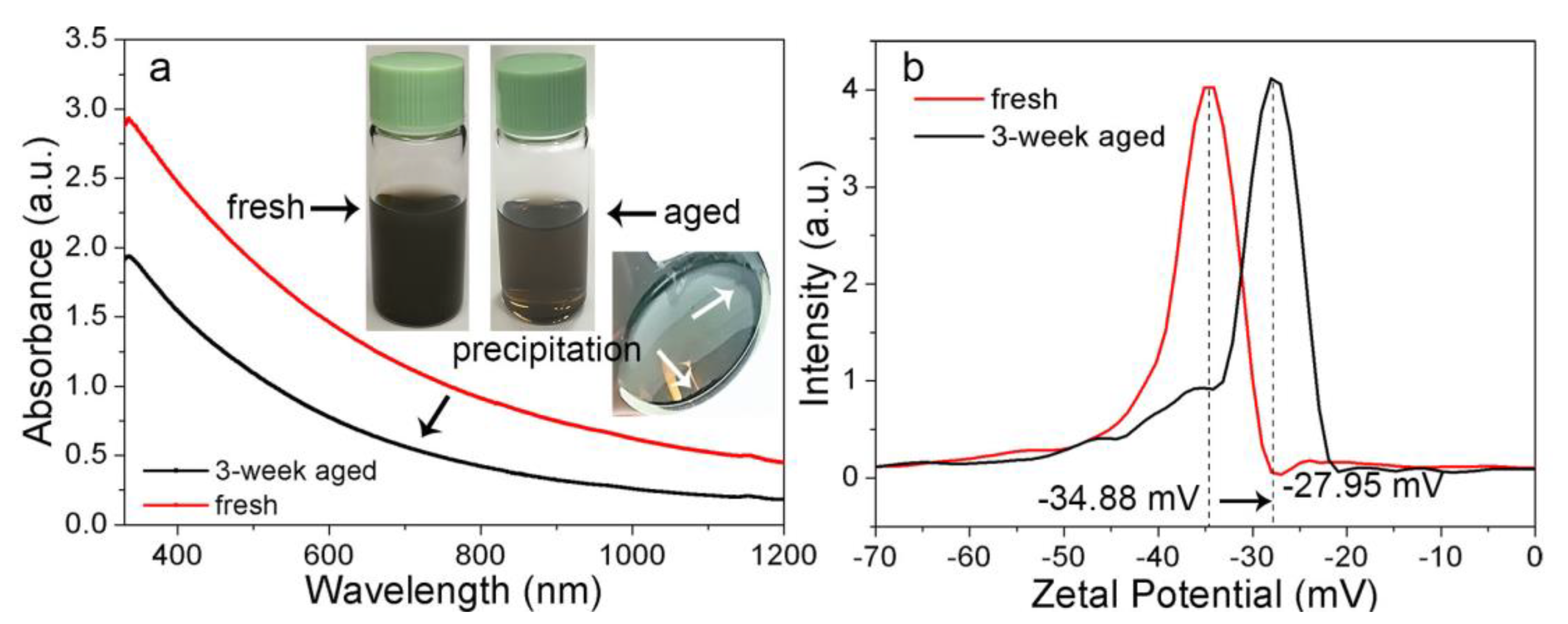
3.1. Material Property
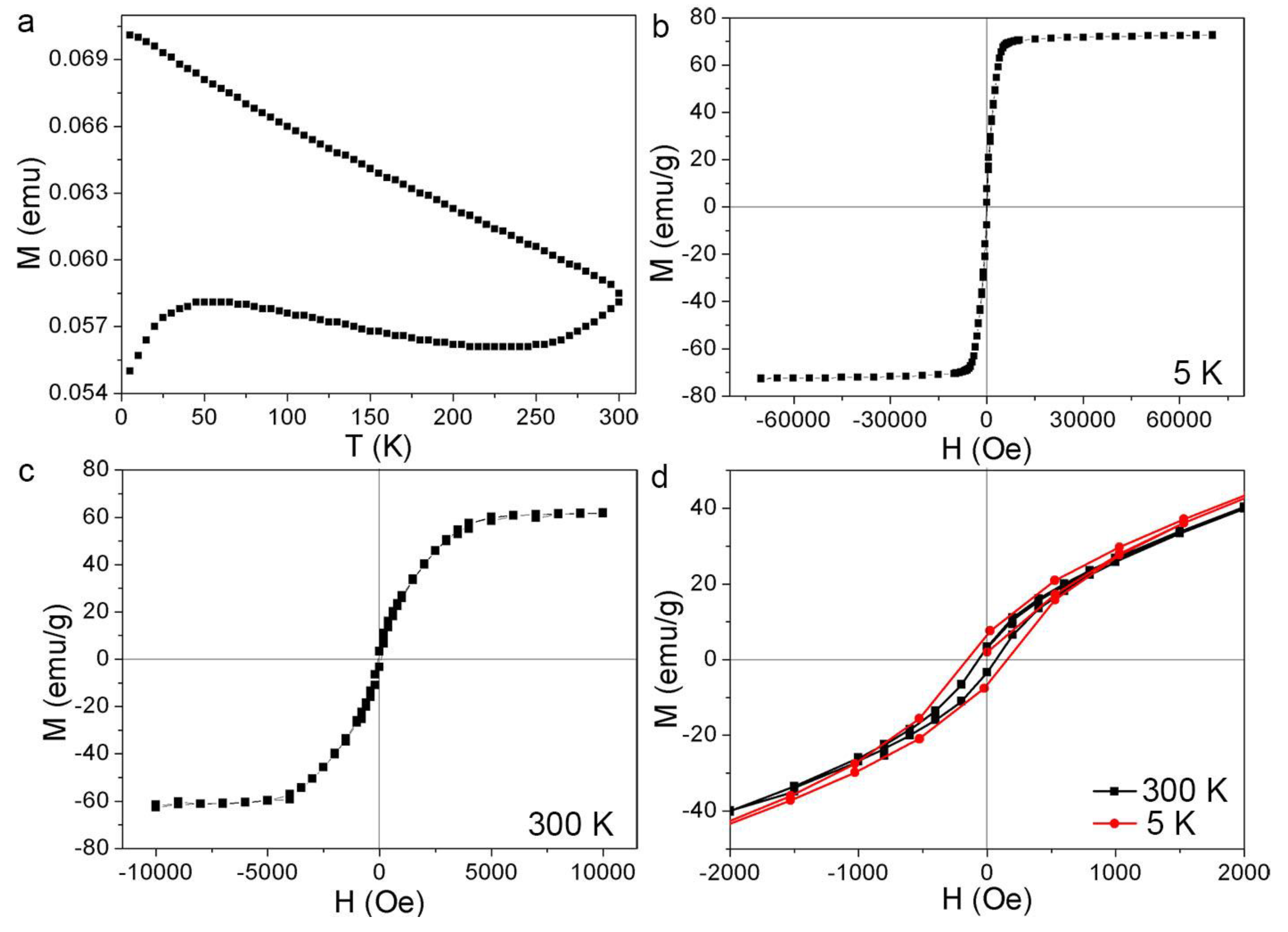
3.2. Magnetic Properties
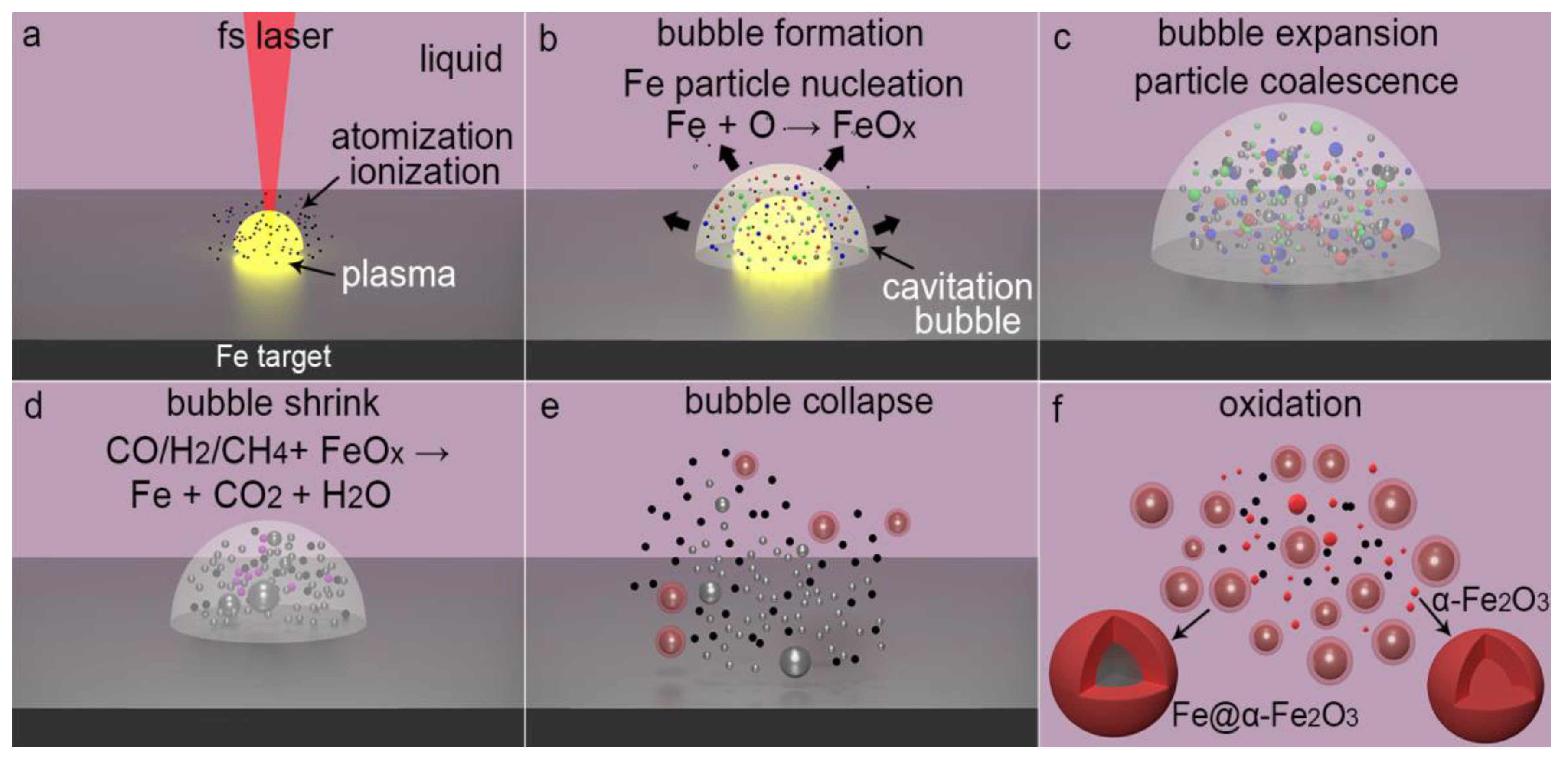
3.3. Formation Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Gökce, B. Perspective of laser-prototyping nanoparticle-polymer composites. Appl. Surf. Sci. 2017, 392, 991–1003. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Li, P.; Tian, Z.; Liang, C. Recent advances in surfactant-free, surface charged and defect-rich catalysts developed by laser ablation and processing in liquids. ChemNanoMat 2017, 3, 512–533. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Liang, C. Perspective on how laser-ablated particles grow in liquids. Sci. China Phys. Mech. Astron. 2017, 60, 074201. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B.; Sommer, S.; Streubel, R.; Barcikowski, S. Debris-free rear-side picosecond laser ablation of thin germanium wafers in water with ethanol. Appl. Surf. Sci. 2016, 367, 222–230. [Google Scholar] [CrossRef]
- Letzel, A.; Gökce, B.; Wagener, P.; Ibrahimkutty, S.; Menzel, A.; Plech, A.; Barcikowski, S. Size quenching during laser synthesis of colloids happens already in the vapor phase of the cavitation bubble. J. Phys. Chem. C 2017, 121, 5356–5365. [Google Scholar] [CrossRef]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size control of laser-fabricated surfactant-free gold nanoparticles with highly diluted electrolytes and their subsequent bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ma, Z.; Spasova, M.; Yelsukova, A.E.; Lu, S.; Farle, M.; Wiedwald, U.; Gökce, B. Formation mechanism of laser-synthesized iron-manganese alloy nanoparticles, manganese oxide nanosheets and nanofibers. Part. Part. Syst. Charact. 2017, 34, 1600225. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic nanoparticles of iron carbide, iron oxide, iron@iron oxide, and metal iron synthesized by laser ablation in organic solvents. J. Phys. Chem. C 2011, 115, 5140–5146. [Google Scholar] [CrossRef]
- Lasemi, N.; Bomati Miguel, O.; Lahoz, R.; Lennikov, V.; Pacher, U.; Rentenberger, C.; Kautek, W. Laser-assisted synthesis of colloidal fewxoy and fe/fexoy nanoparticles in water and ethanol. Chemphyschem 2018. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, E.; Torkamany, M.J. Iron oxide nanocrystals synthesis by laser ablation in water: Effect of laser wavelength. J. Clust. Sci. 2014, 25, 959–968. [Google Scholar] [CrossRef]
- Liu, P.; Cai, W.; Zeng, H. Fabrication and size-dependent optical properties of feo nanoparticles induced by laser ablation in a liquid medium. J. Phys. Chem. C 2008, 112, 3261–3266. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Polizzi, S.; Fiameni, S.; Innocenti, C.; Sangregorio, C.; Meneghetti, M. Magnetic iron oxide nanoparticles with tunable size and free surface obtained via a “green” approach based on laser irradiation in water. J. Mater. Chem. 2011, 21, 18665–18673. [Google Scholar] [CrossRef]
- Pandey, B.K.; Shahi, A.K.; Shah, J.; Kotnala, R.K.; Gopal, R. Optical and magnetic properties of fe2o3 nanoparticles synthesized by laser ablation/fragmentation technique in different liquid media. Appl. Surf. Sci. 2014, 289, 462–471. [Google Scholar] [CrossRef]
- Svetlichnyi, V.A.; Shabalina, A.V.; Lapin, I.N.; Goncharova, D.A.; Velikanov, D.A.; Sokolov, A.E. Characterization and magnetic properties study for magnetite nanoparticles obtained by pulsed laser ablation in water. Appl. Phys. A 2017, 123, 763. [Google Scholar] [CrossRef]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. C 2015, 53, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Kanitz, A.; Hoppius, J.S.; del Mar Sanz, M.; Maicas, M.; Ostendorf, A.; Gurevich, E.L. Synthesis of magnetic nanoparticles by ultrashort pulsed laser ablation of iron in different liquids. Chemphyschem 2017, 18, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Santillán, J.M.J.; Muñetón Arboleda, D.; Coral, D.F.; Fernández van Raap, M.B.; Muraca, D.; Schinca, D.C.; Scaffardi, L.B. Optical and magnetic properties of fe nanoparticles fabricated by femtosecond laser ablation in organic and inorganic solvents. Chemphyschem 2017, 18, 1192–1209. [Google Scholar]
- Jongnam, P.; Eunwoong, L.; Nong-Moon, H.; Misun, K.; Chul, K.S.; Yosun, H.; Je-Geun, P.; Han-Jin, N.; Jae-Young, K.; Jae-Hoon, P.; et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew. Chem. 2005, 117, 2932–2937. [Google Scholar]
- Franzel, L.; Bertino, M.F.; Huba, Z.J.; Carpenter, E.E. Synthesis of magnetic nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2012, 261, 332–336. [Google Scholar] [CrossRef]
- Khan, U.; Adeela, N.; Irfan, M.; Ali, H.; Han, X.F. Temperature mediated morphological and magnetic phase transitions of iron/iron oxide core/shell nanostructures. J. Alloys Compd. 2017, 696, 362–368. [Google Scholar] [CrossRef]
- Pardoe, H.; Chua-anusorn, W.; St. Pierre, T.G.; Dobson, J. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. [Google Scholar] [CrossRef]
- Qiang, Y.; Antony, J.; Sharma, A.; Nutting, J.; Sikes, D.; Meyer, D. Iron/iron oxide core-shell nanoclusters for biomedical applications. J. Nanopart. Res. 2006, 8, 489–496. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond laser three-dimensional micro- and nanofabrication. Appl. Phys. Rev. 2014, 1, 041303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Chen, F.; Fang, G.; Yang, Q.; Xie, D.; Qiao, G.; Li, W.; Si, J.; Hou, X. Wetting characteristics on hierarchical structures patterned by a femtosecond laser. J. Micromech. Microeng. 2010, 20, 075029. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, F.; Yang, Q.; Si, J.; Hou, X. Mutual wetting transition between isotropic and anisotropic on directional structures fabricated by femotosecond laser. Soft Matter 2011, 7, 8337–8342. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, F.; Yang, Q.; Yong, J.; Bian, H.; Ou, Y.; Si, J.; Meng, X.; Hou, X. A simple way to achieve pattern-dependent tunable adhesion in superhydrophobic surfaces by a femtosecond laser. ACS Appl. Mater. Interfaces 2012, 4, 4905–4912. [Google Scholar] [CrossRef] [PubMed]
- Moussa, S.; Atkinson, G.; El-Shall, M.S. Laser-assisted synthesis of magnetic fe/fe2o3 core: Carbon-shell nanoparticles in organic solvents. J. Nanopart. Res. 2013, 15, 1470. [Google Scholar] [CrossRef]
- Zufía-Rivas, J.; Morales, P.; Veintemillas-Verdaguer, S. Effect of the sodium polyacrylate on the magnetite nanoparticles produced by green chemistry routes: Applicability in forward osmosis. Nanomaterials 2018, 8, 470. [Google Scholar]
- Nguyen, V.; Gauthier, M.; Sandre, O. Templated synthesis of magnetic nanoparticles through the self-assembly of polymers and surfactants. Nanomaterials 2014, 4, 628. [Google Scholar] [CrossRef] [PubMed]
- Maneeratanasarn, P.; Khai, T.V.; Kim, S.Y.; Choi, B.G.; Shim, K.B. Synthesis of phase-controlled iron oxide nanoparticles by pulsed laser ablation in different liquid media. Phys. Status Solidi A 2013, 210, 563–569. [Google Scholar] [CrossRef]
- Sukhov, I.A.; Aleksandr, V.S.; Georgii, A.S.; Viau, G.; Garcia, C. Formation of nanoparticles during laser ablation of an iron target in a liquid. Quantum Electron. 2012, 42, 453. [Google Scholar] [CrossRef]
- De Bonis, A.; Lovaglio, T.; Galasso, A.; Santagata, A.; Teghil, R. Iron and iron oxide nanoparticles obtained by ultra-short laser ablation in liquid. Appl. Surf. Sci. 2015, 353, 433–438. [Google Scholar] [CrossRef]
- Marzun, G.; Bönnemann, H.; Lehmann, C.; Spliethoff, B.; Weidenthaler, C.; Barcikowski, S. Role of dissolved and molecular oxygen on cu and ptcu alloy particle structure during laser ablation synthesis in liquids. Chemphyschem 2017, 18, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Choi, W.; Jakobi, J.; Kalus, M.-R.; Barcikowksi, S.; Cho, S.-H.; Sugioka, K. Spontaneous shape alteration and size separation of surfactant-free silver particles synthesized by laser ablation in acetone during long-period storage. Nanomaterials 2018, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Shao, G. The formation of onion-like carbon-encapsulated cobalt carbide core/shell nanoparticles by the laser ablation of metallic cobalt in acetone. Carbon 2013, 55, 108–115. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Madrigal-Camacho, M.; Vilchis-Nestor, A.R.; Camacho-López, M.; Camacho-López, M.A. Synthesis of moc@graphite nps by short and ultra-short pulses laser ablation in toluene under n2 atmosphere. Diamond Relat. Mater. 2018, 82, 63–69. [Google Scholar] [CrossRef]
- De Bonis, A.; Santagata, A.; Galasso, A.; Laurita, A.; Teghil, R. Formation of titanium carbide (tic) and tic@c core-shell nanostructures by ultra-short laser ablation of titanium carbide and metallic titanium in liquid. J. Colloid Interfaces Sci. 2017, 489, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Tian, Z.; Ye, Y.; Cai, Y.; Liang, C.; Terabe, K. A general strategy toward transition metal carbide/carbon core/shell nanospheres and their application for supercapacitor electrode. Carbon 2016, 100, 590–599. [Google Scholar] [CrossRef]
- Giorgetti, E.; Muniz-Miranda, M.; Marsili, P.; Scarpellini, D.; Giammanco, F. Stable gold nanoparticles obtained in pure acetone by laser ablation with different wavelengths. J. Nanopart. Res. 2012, 14, 648. [Google Scholar] [CrossRef]
- Safronov, A.; Beketov, I.; Komogortsev, S.; Kurlyandskaya, G.; Medvedev, A.; Leiman, D.; Larrañaga, A.; Bhagat, S. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef] [Green Version]
- Osipov, V.V.; Platonov, V.V.; Uimin, M.A.; Podkin, A.V. Laser synthesis of magnetic iron oxide nanopowders. Tech. Phys. 2012, 57, 543–549. [Google Scholar] [CrossRef]
- Grinstaff, M.W.; Salamon, M.B.; Suslick, K.S. Magnetic properties of amorphous iron. Phys. Rev. B 1993, 48, 269. [Google Scholar] [CrossRef]
- Goya, G.; Berquo, T.; Fonseca, F.; Morales, M. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Hadjipanayis, G.; Sellmyer, D.J.; Brandt, B. Rare-earth-rich metallic glasses. I. Magnetic hysteresis. Phys. Rev. B 1981, 23, 3349. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Wiedwald, U.; Spasova, M.; Salabas, E.; Ulmeanu, M.; Farle, M.; Frait, Z.; Rodriguez, A.F.; Arvanitis, D.; Sobal, N.; Hilgendorff, M. Ratio of orbital-to-spin magnetic moment in co core-shell nanoparticles. Phys. Rev. B 2003, 68, 064424. [Google Scholar] [CrossRef]
- Han, L.; Wiedwald, U.; Biskupek, J.; Fauth, K.; Kaiser, U.; Ziemann, P. Nanoscaled alloy formation from self-assembled elemental co nanoparticles on top of pt films. Beilstein J. Nanotechnol. 2011, 2, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Y.; Rui, X.F.; Fu, Y.Y.; Zhang, H. Magnetic properties of α-fe2o3 nanowires. Chem. Phys. Lett. 2005, 410, 36–38. [Google Scholar] [CrossRef]
- Wu, C.; Yin, P.; Zhu, X.; OuYang, C.; Xie, Y. Synthesis of hematite (α-fe2o3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Bødker, F.; Hansen, M.F.; Koch, C.B.; Lefmann, K.; Mørup, S. Magnetic properties of hematite nanoparticles. Phys. Rev. B 2000, 61, 6826. [Google Scholar]
- Bødker, F.; Mørup, S. Size dependence of the properties of hematite nanoparticles. Europhys. Lett. 2000, 52, 217. [Google Scholar]
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.; Wu, C.; Schmidt, M.; Gokce, B.; Barcikowski, S.; Zhigilei, L. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: The origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimkutty, S.; Wagener, P.; Rolo, T.d.S.; Karpov, D.; Menzel, A.; Baumbach, T.; Barcikowski, S.; Plech, A. A hierarchical view on material formation during pulsed-laser synthesis of nanoparticles in liquid. Sci. Rep. 2015, 5, 16313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semaltianos, N.G.; Friedt, J.-M.; Chassagnon, R.; Moutarlier, V.; Blondeau-Patissier, V.; Combe, G.; Assoul, M.; Monteil, G. Oxide or carbide nanoparticles synthesized by laser ablation of a bulk hf target in liquids and their structural, optical, and dielectric properties. J. Appl. Phys. 2016, 119, 204903. [Google Scholar] [CrossRef]
- Kalus, M.-R.; Barsch, N.; Streubel, R.; Gokce, E.; Barcikowski, S.; Gokce, B. How persistent microbubbles shield nanoparticle productivity in laser synthesis of colloids—Quantification of their volume, dwell dynamics, and gas composition. Phys. Chem. Chem. Phys. 2017, 19, 7112–7123. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, W.; Vogel, A. Shock wave emission by laser generated bubbles. In Bubble Dynamics and Shock Waves; Springer: Berlin, Germany, 2013; pp. 67–103. [Google Scholar]
- Suslick, K.S.; Choe, S.-B.; Cichowlas, A.A.; Grinstaff, M.W. Sonochemical synthesis of amorphous iron. Nature 1991, 353, 414. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar]
- Lázár, K.; Varga, L.K.; Kovács Kis, V.; Fekete, T.; Klencsár, Z.; Stichleutner, S.; Szabó, L.; Harsányi, I. Electric explosion of steel wires for production of nanoparticles: Reactions with the liquid media. J. Alloys Compd. 2018, 763, 759–770. [Google Scholar]
- Beketov, I.V.; Safronov, A.P.; Bagazeev, A.V.; Larrañaga, A.; Kurlyandskaya, G.V.; Medvedev, A.I. In situ modification of fe and ni magnetic nanopowders produced by the electrical explosion of wire. J. Alloys Compd. 2014, 586, S483–S488. [Google Scholar] [CrossRef]
- Liu, P.; Cui, H.; Yang, G. Synthesis of body-centered cubic carbon nanocrystals. Cryst. Growth Des. 2008, 8, 581–586. [Google Scholar] [CrossRef]
- Seyedeh Zahra, M.; Parviz, P.; Ali, R.; Soghra, M.; Rasoul, S.-B. Generation of various carbon nanostructures in water using ir/uv laser ablation. J. Phys. D Appl. Phys. 2013, 46, 165303. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Choi, W.; Oshima, Y.; Wiedwald, U.; Cho, S.-H.; Lin, H.-P.; Li, Y.K.; Ito, Y.; Sugioka, K. Magnetic Fe@FeOx, Fe@C and α-Fe2O3 Single-Crystal Nanoblends Synthesized by Femtosecond Laser Ablation of Fe in Acetone. Nanomaterials 2018, 8, 631. https://doi.org/10.3390/nano8080631
Zhang D, Choi W, Oshima Y, Wiedwald U, Cho S-H, Lin H-P, Li YK, Ito Y, Sugioka K. Magnetic Fe@FeOx, Fe@C and α-Fe2O3 Single-Crystal Nanoblends Synthesized by Femtosecond Laser Ablation of Fe in Acetone. Nanomaterials. 2018; 8(8):631. https://doi.org/10.3390/nano8080631
Chicago/Turabian StyleZhang, Dongshi, Wonsuk Choi, Yugo Oshima, Ulf Wiedwald, Sung-Hak Cho, Hsiu-Pen Lin, Yaw Kuen Li, Yoshihiro Ito, and Koji Sugioka. 2018. "Magnetic Fe@FeOx, Fe@C and α-Fe2O3 Single-Crystal Nanoblends Synthesized by Femtosecond Laser Ablation of Fe in Acetone" Nanomaterials 8, no. 8: 631. https://doi.org/10.3390/nano8080631
APA StyleZhang, D., Choi, W., Oshima, Y., Wiedwald, U., Cho, S.-H., Lin, H.-P., Li, Y. K., Ito, Y., & Sugioka, K. (2018). Magnetic Fe@FeOx, Fe@C and α-Fe2O3 Single-Crystal Nanoblends Synthesized by Femtosecond Laser Ablation of Fe in Acetone. Nanomaterials, 8(8), 631. https://doi.org/10.3390/nano8080631







