Zinc Oxide Coated Tin Oxide Nanofibers for Improved Selective Acetone Sensing
Abstract
1. Introduction
2. Experiment and Characteristics
2.1. Materials
2.2. Electrospinning of SnO2 Nanofibers
2.3. Synthesis of ZnO Nanorods
2.4. Characterization of SnO2/ZnO 3D Hetero-Nanofibers
2.5. Fabrication and Testing of Gas Sensors
3. Results and Discussion
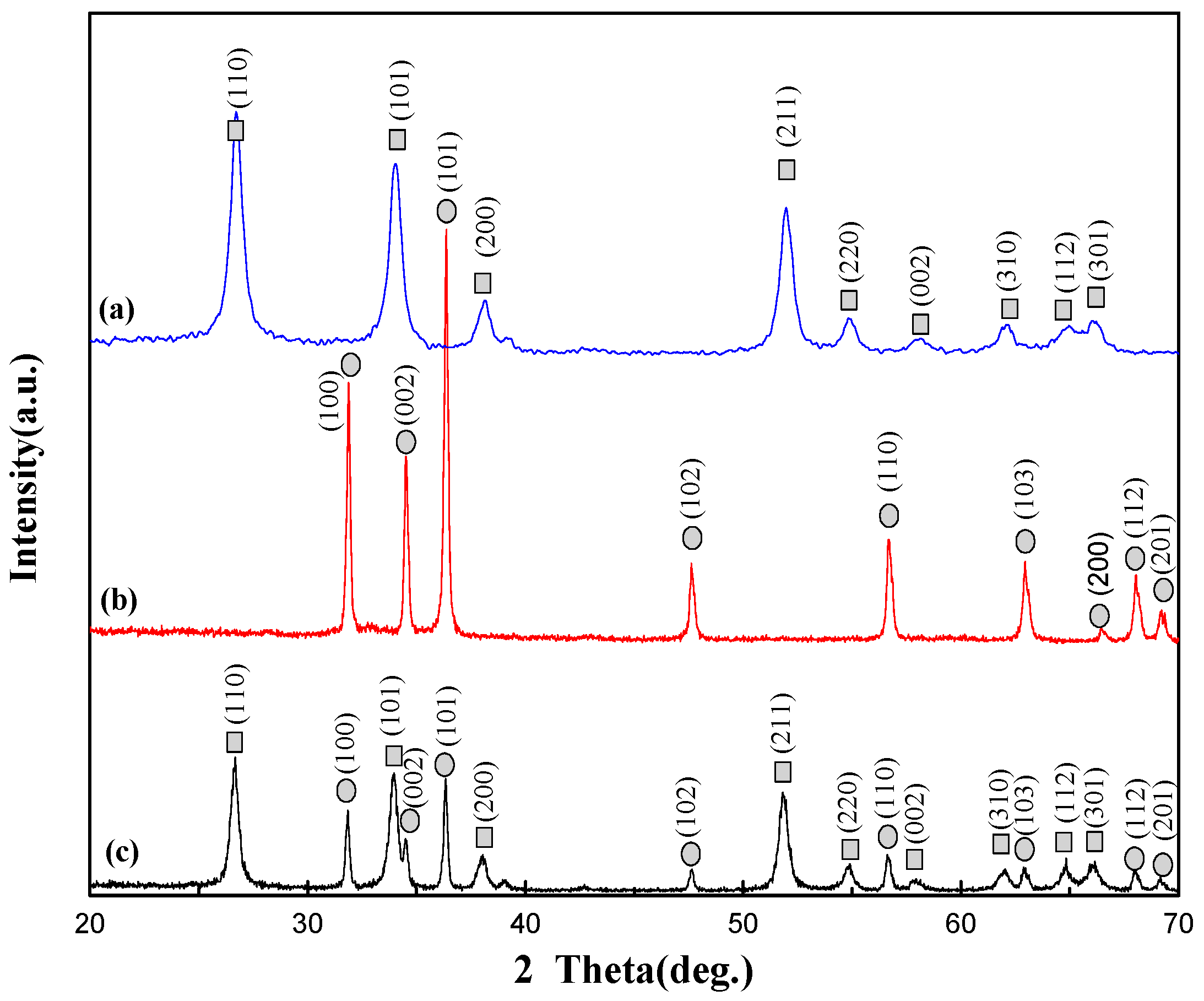
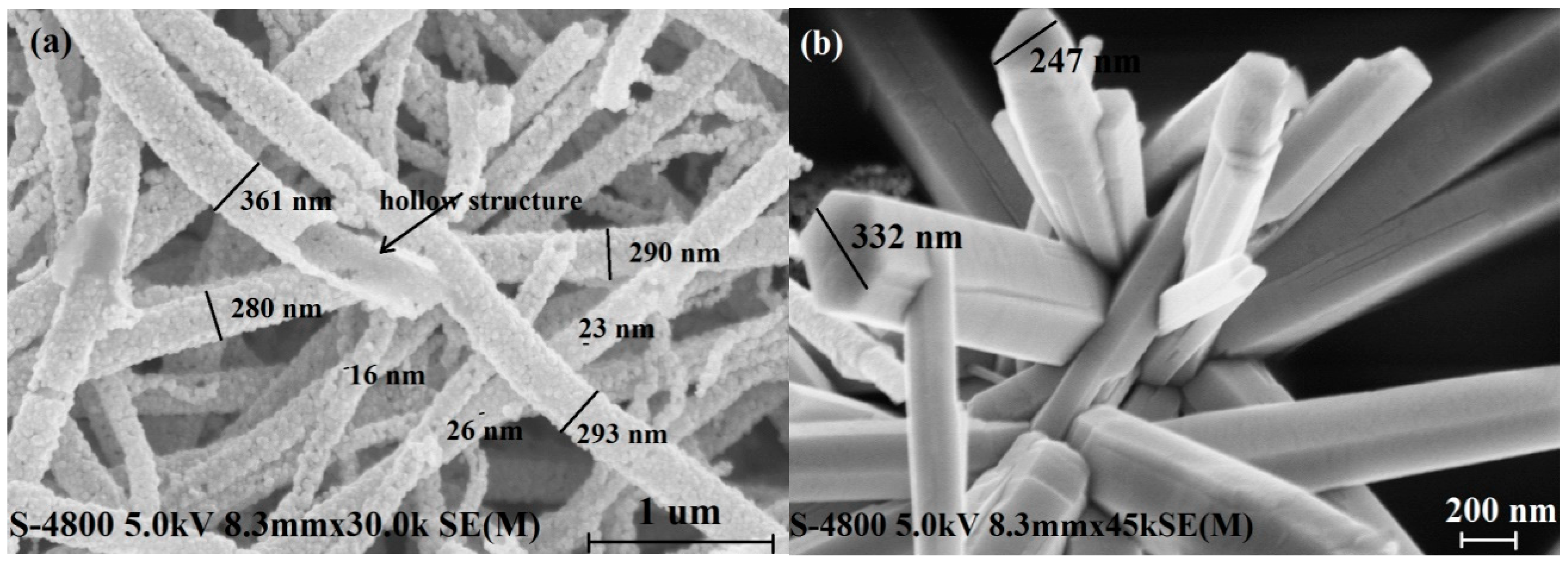
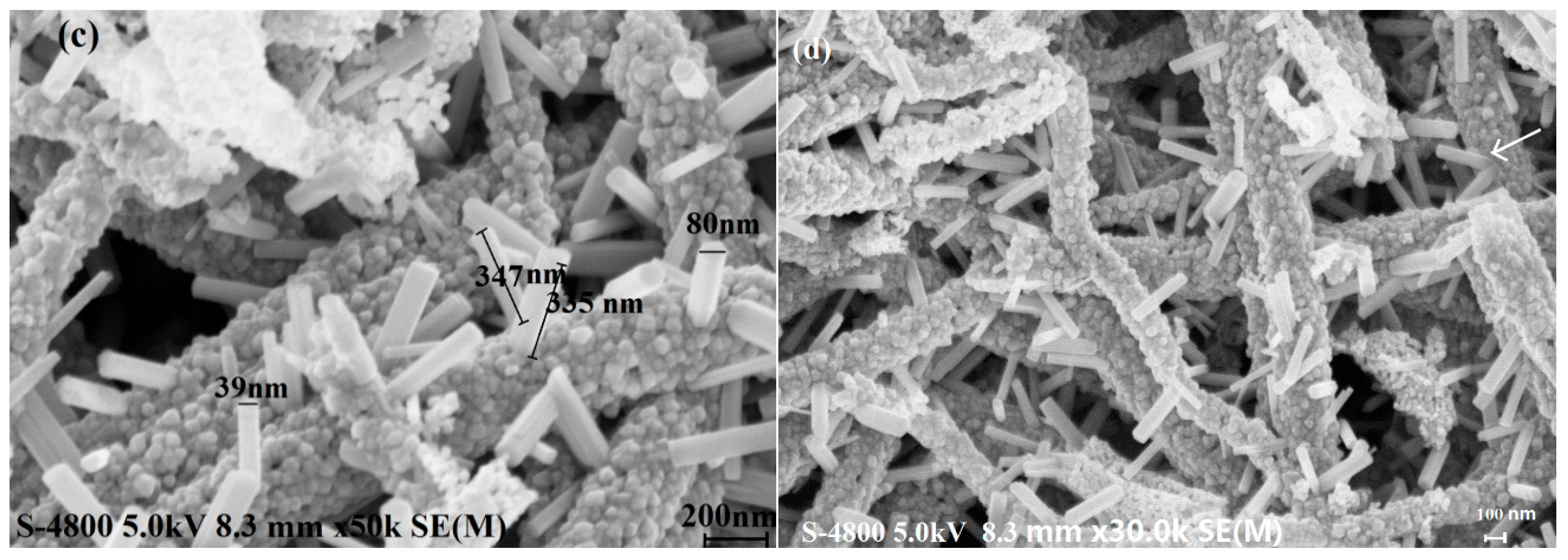
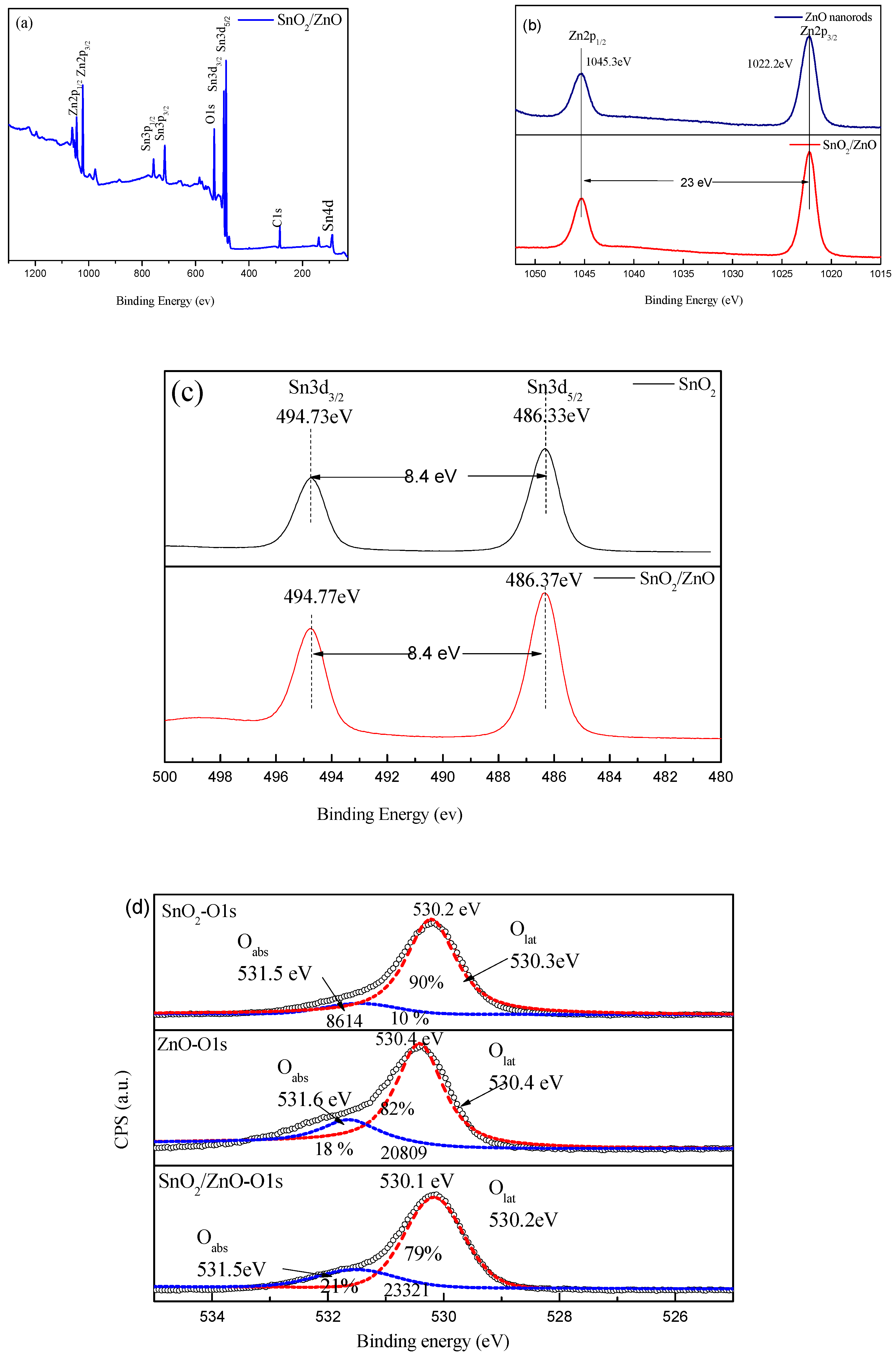
3.1. Structural Properties
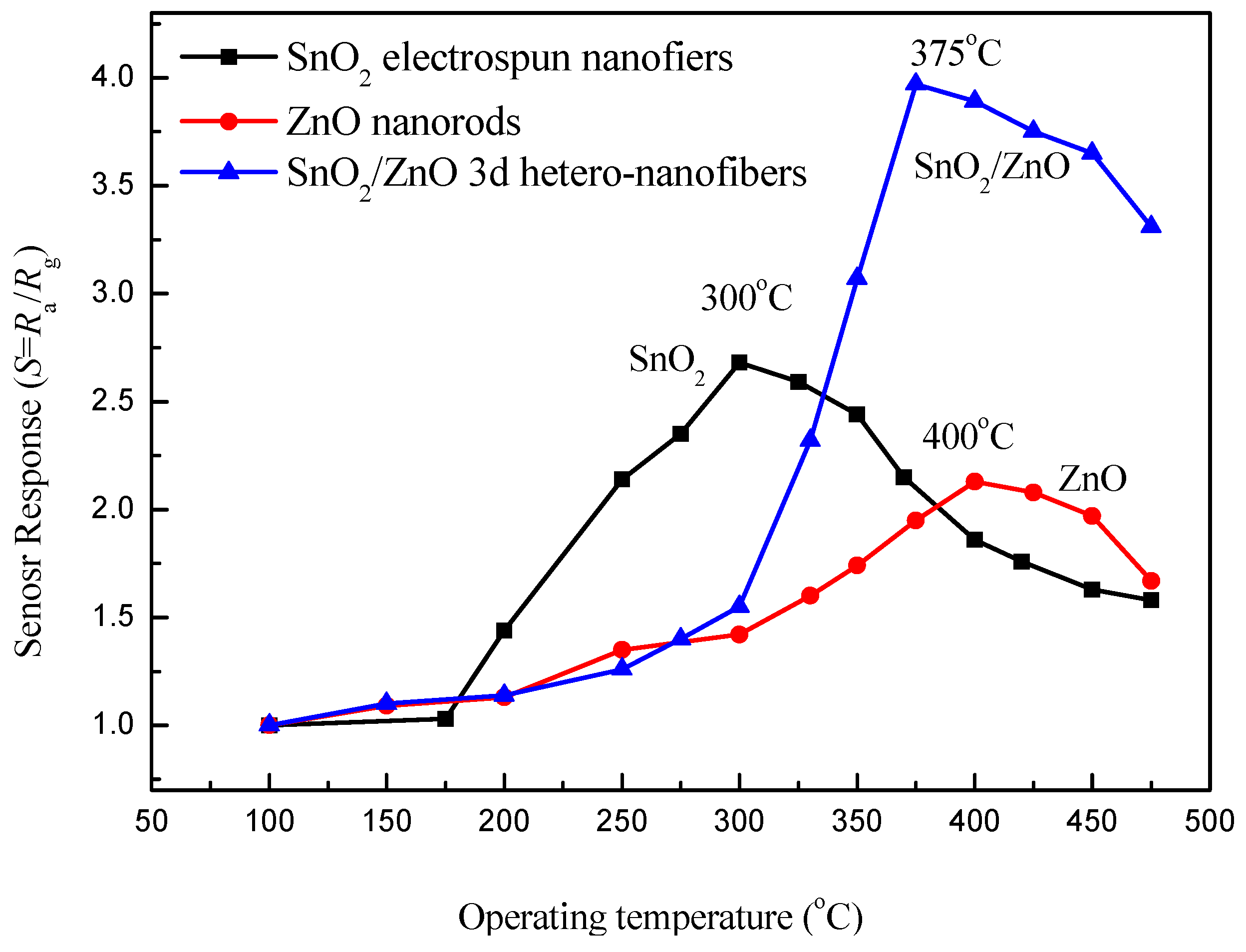
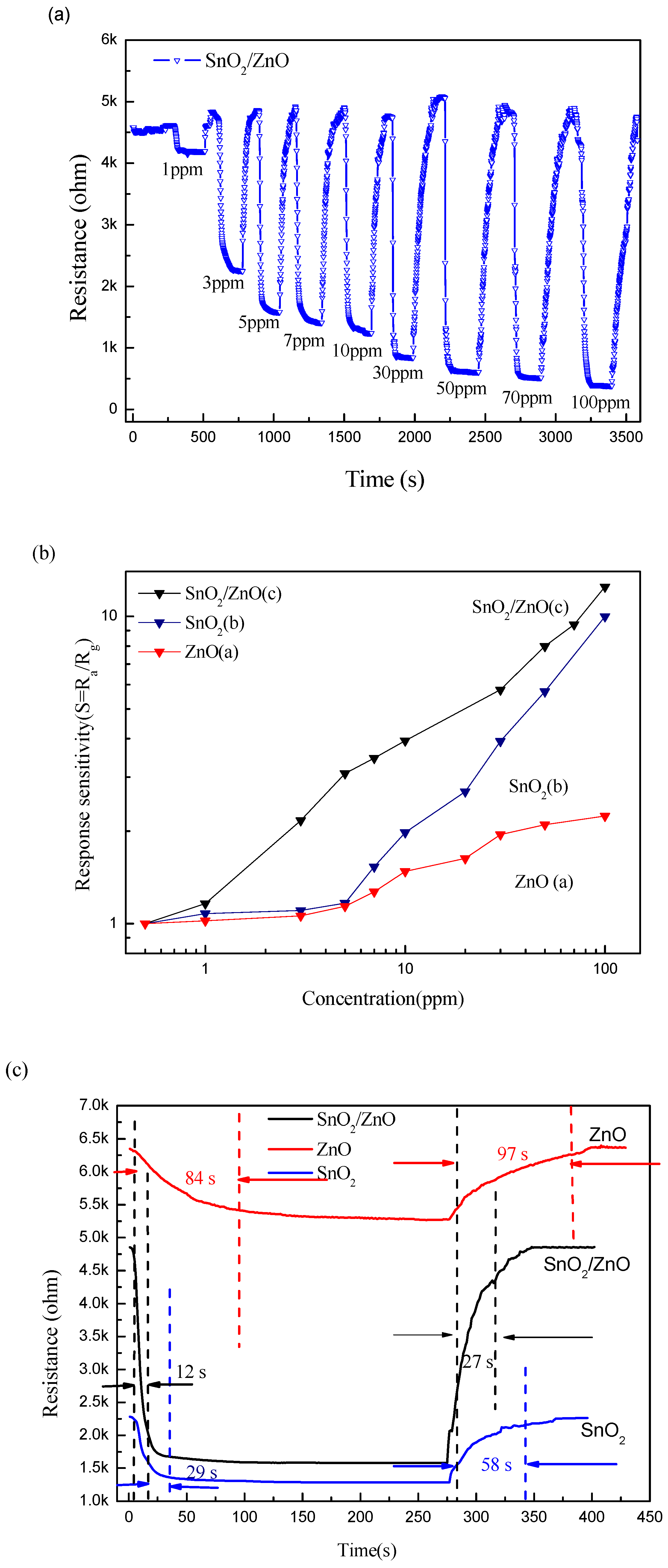
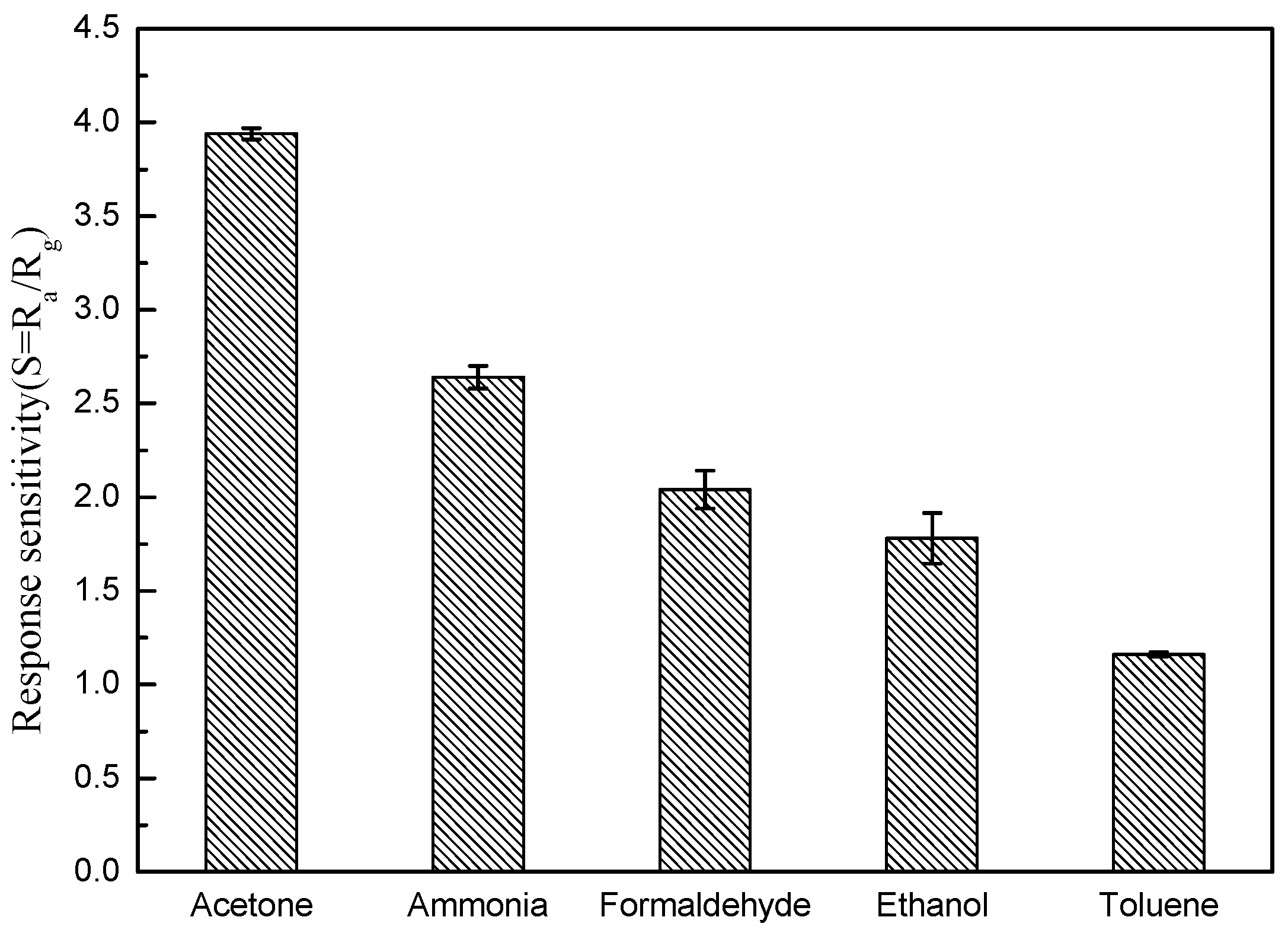
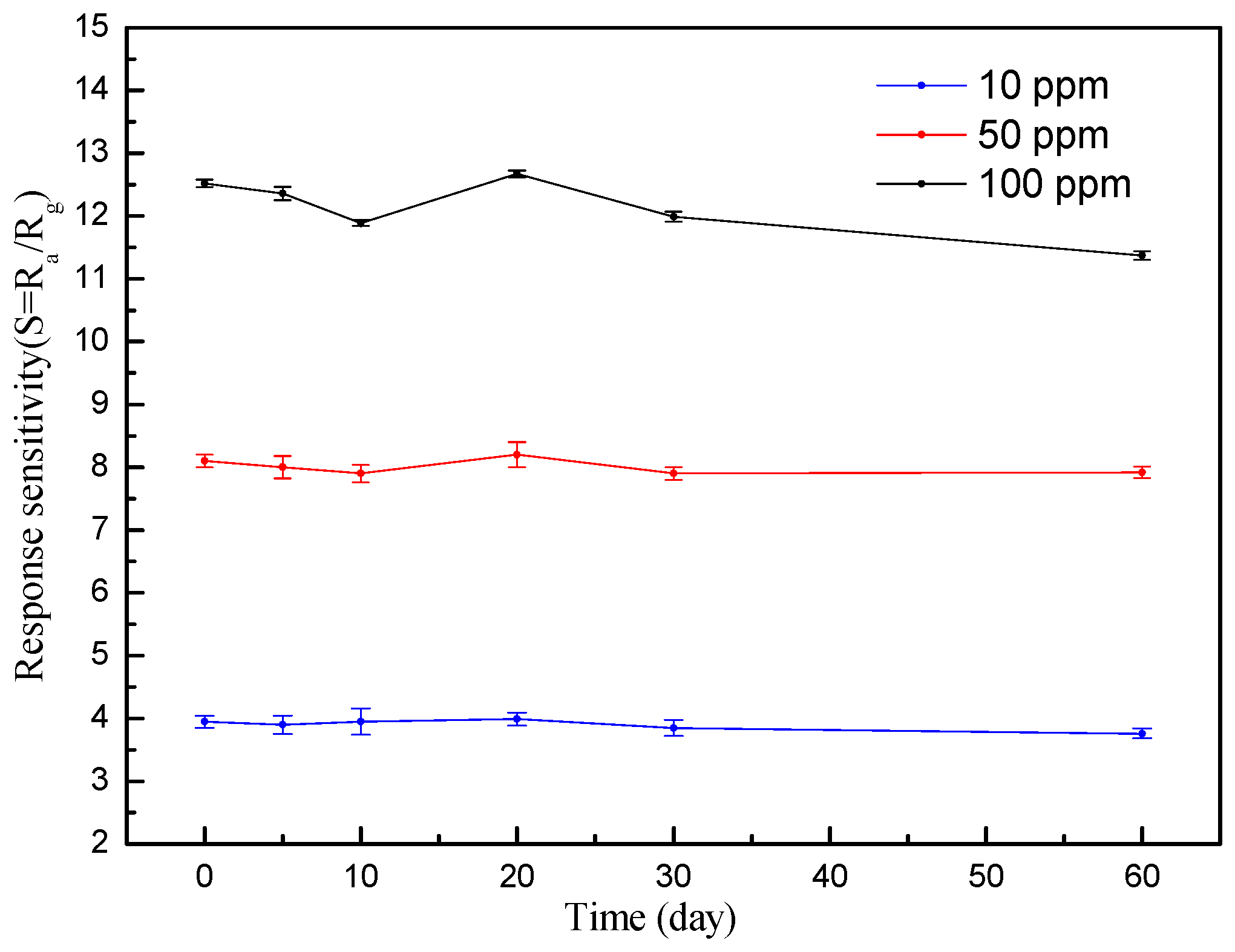
3.2. Gas-Sensing Properties
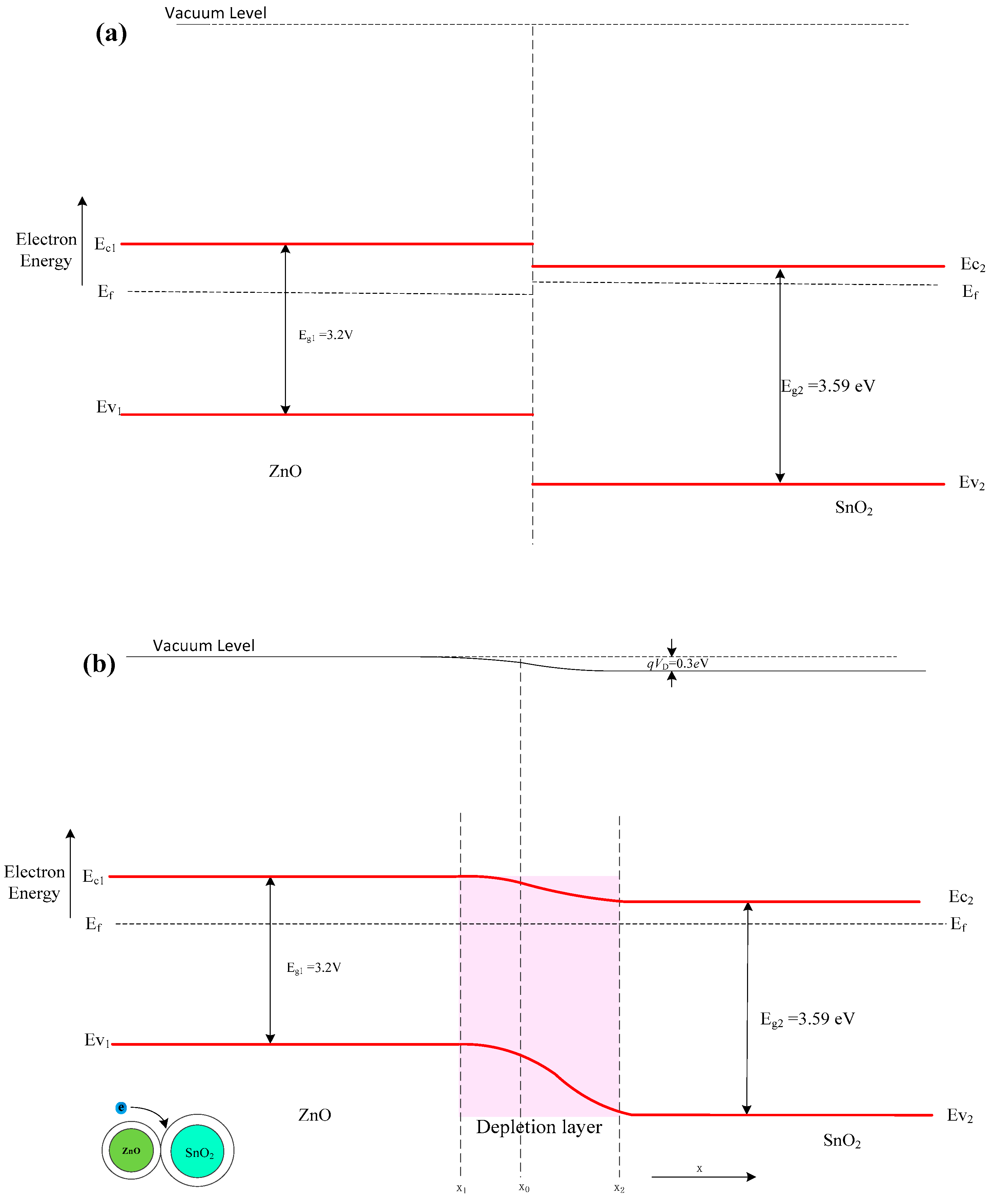
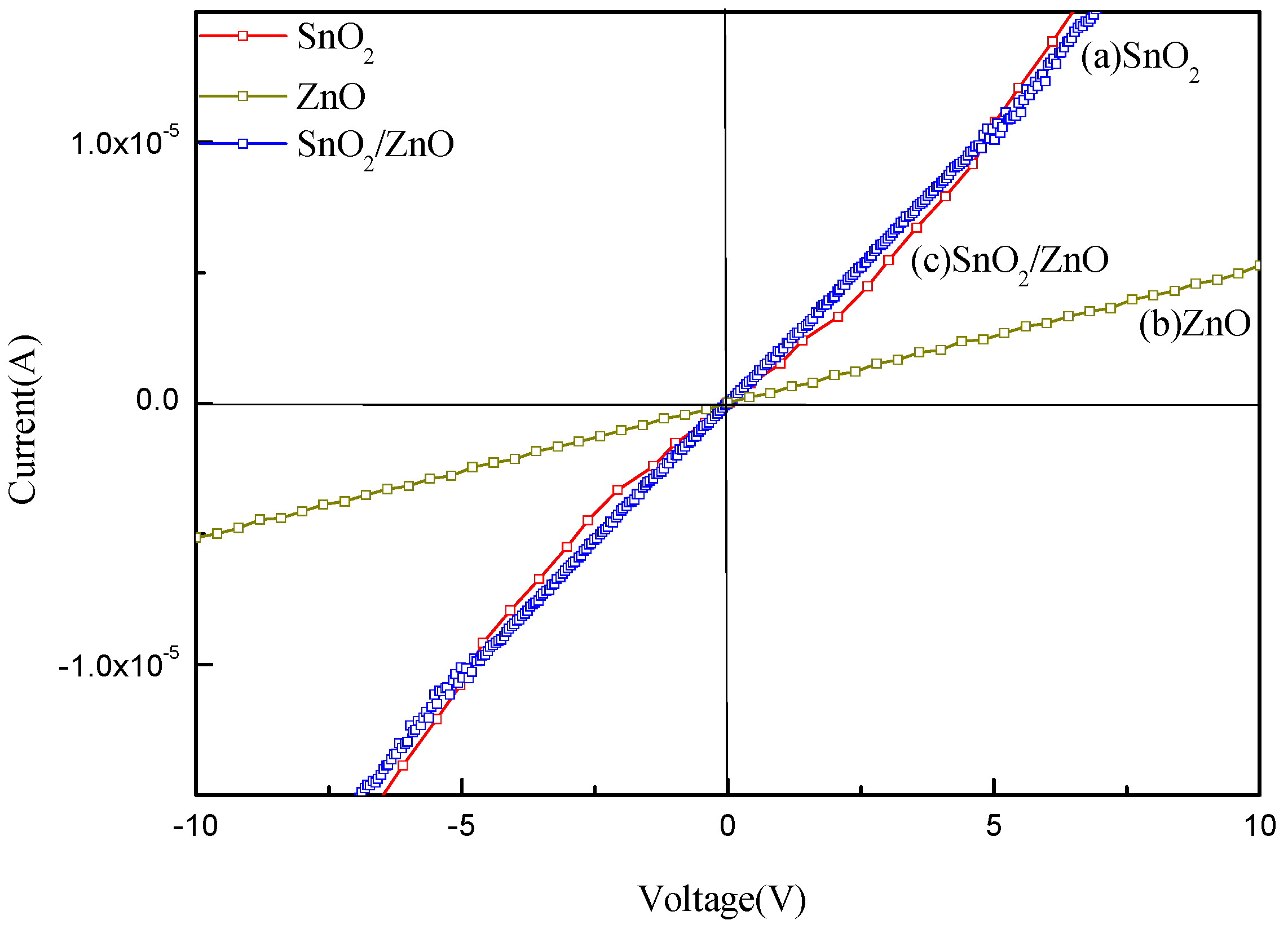
4. Gas-Sensing Mechanism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817. [Google Scholar] [CrossRef]
- Wu, X.-J.; Zhu, F.; Mu, C.; Liang, Y.; Xu, L.; Chen, Q.; Chen, R.; Xu, D. Electrochemical synthesis and applications of oriented and hierarchically quasi-1D semiconducting nanostructures. Coord. Chem. Rev. 2010, 254, 1135–1150. [Google Scholar] [CrossRef]
- Erickson, A.E.; Edmondson, D.; Chang, F.-C.; Wood, D.; Gong, A.; Levengood, S.L.; Zhang, M. High-throughput and high-yield fabrication of uniaxially-aligned chitosan-based nanofibers by centrifugal electrospinning. Carbohydr. Polym. 2015, 134, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Kong, B.; Wu, Z.; Sun, W.; Xu, Y.; Su, X. A novel electrospinning setup for the fabrication of thickness-controllable 3D scaffolds with an ordered nanofibrous structure. Mater. Lett. 2015, 160, 343–346. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.Y.; Wang, T.T.; Li, X.B.; Luo, J.; Li, W.Q.; Mao, Y.Z.; Gz, D.J. Synthesis and characterization of SnO2 hollow nanofibers by electrospinning for ethanol sensing properties. Mater. Lett. 2014, 131, 23–26. [Google Scholar] [CrossRef]
- Liang, X.; Jin, G.; Liu, F.; Zhang, X.; An, S.; Ma, J.; Lu, G. Synthesis of In2O3 hollow nanofibers and their application in highly sensitive detection of acetone. Ceram. Int. 2015, 41, 13780–13787. [Google Scholar] [CrossRef]
- Anjusree, G.S.; Deepak, T.G.; Nair, S.V.; Nair, A.S. Facile fabrication of TiO2 nanoparticle–TiO2 nanofiber composites by co-electrospinning–electrospraying for dye-sensitized solar cells. J. Energy Chem. 2015, 24, 762–769. [Google Scholar] [CrossRef]
- Giancaterini, L.; Emamjomeh, S.M.; De Marcellis, A.; Palange, E.; Resmini, A.; Anselmi-Tamburini, U.; Cantalini, C. The influence of thermal and visible light activation modes on the NO2 response of WO3 nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2016, 229, 387–395. [Google Scholar] [CrossRef]
- Di Mauro, A.; Zimbone, M.; Fragalà, M.E.; Impellizzeri, G. Synthesis of ZnO nanofibers by the electrospinning process. Mater. Sci. Semicond. Process. 2016, 42 Pt 1, 98–101. [Google Scholar] [CrossRef]
- Liu, S.; Jia, J.; Wang, J.; Liu, S.; Wang, X.; Song, H.; Hu, X. Synthesis of Fe-doped NiO nanofibers using electrospinning method and their ferromagnetic properties. J. Magn. Magn. Mater. 2012, 324, 2070–2074. [Google Scholar] [CrossRef]
- Wei, Y.; Song, Y.; Deng, X.; Han, B.; Zhang, X.; Shen, Y.; Lin, Y. Dielectric and Ferroelectric Properties of BaTiO3 Nanofibers Prepared via Electrospinning. J. Mater. Sci. Technol. 2014, 30, 743–747. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Zheng, W.; Wang, R.; Liu, X.; Xia, Y.; Zhao, J. Humidity sensing properties of BaTiO3 nanofiber prepared via electrospinning. Sens. Actuators B Chem. 2010, 146, 98–102. [Google Scholar] [CrossRef]
- Yan, S.H.; Ma, S.Y.; Li, W.Q.; Xu, X.L.; Cheng, L.; Song, H.S.; Liang, X.Y. Synthesis of SnO2–ZnO heterostructured nanofibers for enhanced ethanol gas-sensing performance. Sens. Actuators B Chem. 2015, 221, 88–95. [Google Scholar] [CrossRef]
- Firooz, A.A.; Hyodo, T.; Mahjoub, A.; Khodadadi, A.; Shimizu, Y. Synthesis of nano-and meso-porous MoO 3-doped SnO2 as a selective gas-sensing material to NO2. Sens. Actuators B 2010, 147, 554–560. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An efficient bicomponent TiO2/SnO2 nanofiber photocatalyst fabricated by electrospinning with a side-by-side dual spinneret method. Nano Lett. 2007, 7, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Yao, P. Formaldehyde sensing properties of electrospun NiO-doped SnO2 nanofibers. Sens. Actuators B Chem. 2011, 156, 723–730. [Google Scholar] [CrossRef]
- Xin, Y.; Lan, X.; Chang, P.; Huang, Y.; Wang, L.; Hu, X. Conformal spinel/layered heterostructures of Co3O4 shells grown on single-crystal Li-rich nanoplates for high-performance lithium-ion batteries. Appl. Surf. Sci. 2018, 447, 829–836. [Google Scholar] [CrossRef]
- Du, H.; Wang, J.; Su, M.; Yao, P.; Zheng, Y.; Yu, N. Formaldehyde gas sensor based on SnO2/In2O3 hetero-nanofibers by a modified double jets electrospinning process. Sens. Actuators B Chem. 2012, 166–167, 746–752. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Liu, Y.; Wang, C.; Li, L. A highly sensitive ethanol sensor based on mesoporous ZnO–SnO2 nanofibers. Nanotechnology 2009, 20, 075501. [Google Scholar] [CrossRef] [PubMed]
- Du, H.-Y.; Wang, J.; Yu, P.; Yu, N.-S.; Sun, Y.-H.; Tian, J.-L. Investigation of gas sensing materials tin oxide nanofibers treated by oxygen plasma. J. Nanopart. Res. 2014, 16, 2216. [Google Scholar] [CrossRef]
- Du, H.-Y.; Wang, J.; Qiao, Q.; Sun, Y.-H.; Shao, Q.; Li, X.-G. Synthesis and Characterization of ZnO/PPy Hetero-Nanocomposites and Their Gas Sensing Properties. Acta Physico-Chim. Sin. 2015, 31, 800–806. [Google Scholar] [CrossRef]
- Hanson, D.R.; Koppes, M.; Stoffers, A.; Harsdorf, R.; Edelen, K. Proton transfer mass spectrometry at 11 hPa with a circular glow discharge: Sensitivities and applications. Int. J. Mass Spectrom. 2009, 282, 28–37. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.Y.; Kim, S.S. Synthesis of SnO2–ZnO core–shell nanofibers via a novel two-step process and their gas sensing properties. Nanotechnology 2009, 20, 465603. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Cho, P.-S.; Kim, S.-J.; Lee, J.-H.; Kang, C.-Y.; Kim, J.-S.; Yoon, S.-J. The selective detection of C2H5OH using SnO2–ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens. Actuators B Chem. 2007, 123, 318–324. [Google Scholar] [CrossRef]
- Yu, J.H.; Choi, G.M. Electrical and CO gas sensing properties of ZnO–SnO2 composites. Sens. Actuators B Chem. 1998, 52, 251–256. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gong, H.; Chow, C.L.; Guo, J.; White, T.J.; Tse, M.S.; Tan, O.K. Low-temperature growth of SnO2 nanorod arrays and tunable n–p–n sensing response of a ZnO/SnO2 heterojunction for exclusive hydrogen sensors. Adv. Funct. Mater. 2011, 21, 2680–2686. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Yao, P.; Li, X. Hollow hierarchical SnO2-ZnO composite nanofibers with heterostructure based on electrospinning method for detecting methanol. Sens. Actuators B Chem. 2014, 192, 543–549. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Network structured SnO2/ZnO heterojunction nanocatalyst with high photocatalytic activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, J.; Zhang, Y.; Sun, Y.A.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B Chem. 2008, 132, 334–339. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Sze, S. Semiconductor Sensors; John Wiley & Sons Inc.: New York, NY, USA, 1994. [Google Scholar]
- Lantto, V.; Rantala, T. Computer simulation of the surface energy barrier of oxidic semiconductors with mobile donors. Sens. Actuators B Chem. 1994, 19, 711–715. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kanazawa, E.; Takao, Y.; Egashira, M. Modification of H 2-sensitive breakdown voltages of SnO2 varistors with noble metals. Sens.Actuators B Chem. 1998, 52, 38–44. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, M.; Shan, G.; Li, Y.; Huang, B.; Yang, G. Biocompatible ZnO/Au nanocomposites for ultrasensitive DNA detection using resonance Raman scattering. J. Phys. Chem. B 2008, 112, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun nanofibers of ZnO−SnO2 heterojunction with high photocatalytic activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Xiong, C.; Balkus, K.J. Mesoporous molecular sieve derived TiO2 nanofibers doped with SnO2. J. Phys. Chem. C 2007, 111, 10359–10367. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z. Sensitivity improvement of TiO2-doped SnO2 to volatile organic compounds. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 43, 633–638. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Weis, T.; Lipperheide, R.; Wille, U.; Brehme, S. Barrier-controlled carrier transport in microcrystalline semiconducting materials: Description within a unified model. J. Appl. Phys. 2002, 92, 1411–1418. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, K.; Lu, H.; Wang, X.; Liu, G.; Jin, K.; He, M.; Wang, C.; Yang, G. Visible-blind, ultraviolet-sensitive photodetector based on SrTiO3 single crystal. Opt. Lett. 2007, 32, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Das, S.N.; Kar, J.P.; Myoung, J.M. Junction Properties and Applications of ZnO Single Nanowire Based Schottky Diode, Nanowires—Fundamental Research; InTech: Rijeka, Croatia, 2011; p. 140318. [Google Scholar]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mandalapu, L.; Yang, Z.; Xiu, F.; Zhao, D.; Liu, J. Homojunction photodiodes based on Sb-doped p-type ZnO for ultraviolet detection. Appl. Phys. Lett. 2006, 88, 092103. [Google Scholar] [CrossRef]
- Hoffman, R.; Wager, J.; Jayaraj, M.; Tate, J. Electrical characterization of transparent p–i–n heterojunction diodes. J. Appl. Phys. 2001, 90, 5763–5767. [Google Scholar] [CrossRef]
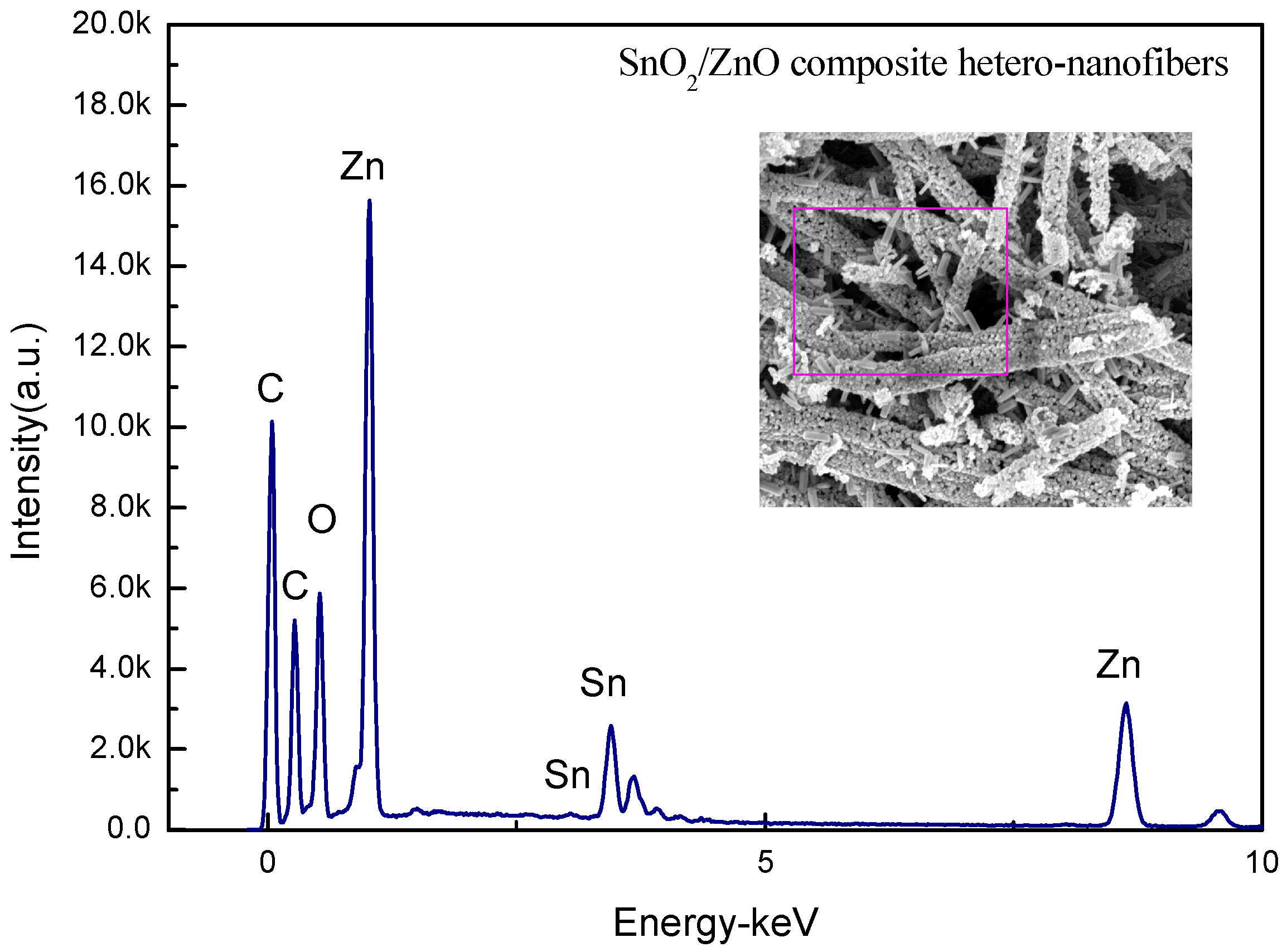

| Elements | Weight (%) | Atomic (%) |
|---|---|---|
| O K | 16.9 | 56.5 |
| Zn K | 28.9 | 23.4 |
| Sn L | 54.2 | 20.1 |
| Types | Preparation Method | Detect Gas | Structure | Operating Temperature (°C) | Response Value (Concentration) | Response Time /Recovery Time (s) |
|---|---|---|---|---|---|---|
| SnO2/ZnO reported by other journals | Two steps electrospinning and atomic layer deposition [25] | O2 | SnO2–ZnO core-shell nanofiber | 300 | S = 1.02 (70 ppm) | 250 s/500 s |
| NO2 | S = 3.08 (5 ppm) | 40 s/120 s | ||||
| A combinatorial solution deposition technique [26] | C2H5OH | SnO2/ZnO films | 300 | S = 4.69 (200 ppm) | Excellent selective | |
| A combination of surfactant-directed assembly and an electrospinning [21] | C2H5OH | A mesoporous structure | 300 | S = 4 (5 ppm) | 3 s/8 s | |
| The pellet by sintering [27] | CO | More porous microstructure | 360 | S = 12 (200 ppm) | ─ | |
| The thermal evaporation of Sn powders followed by the ALD of ZnO [28]. | NO2 | SnO2-Core/ZnO-Shell | Room temperature | S = 1.04 (5 ppm) | 110 s/230 s | |
| (SnO2) PECVD and ZnO deposited by spin coating [29]. | H2 | ZnO Surface Modifi cation of the SnO2 Nanorod Arrays | 350 | S = 2.6 (100ppm) | 7 s/30 s | |
| Mix-electrospun [30] | CH3OH | Hollow hierarchical, and heterostructure | 350 | S = 8.5 (10 ppm) | 20 s/40 s | |
| Two-step solvothermal method [31] | Photocatalytic Activity | Network Structured | High Photocatalytic Activity | ─ | ─ | |
| SnO2/ZnO 3D hetero-nanofibers | Electrospinning followed by a low-temperature water bath treatment | Acetone | ZnO nanorod grew on the SnO2 nanofibers | 350 | S = 3.08 (5 ppm) | 12 s/27 s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Li, X.; Yao, P.; Wang, J.; Sun, Y.; Dong, L. Zinc Oxide Coated Tin Oxide Nanofibers for Improved Selective Acetone Sensing. Nanomaterials 2018, 8, 509. https://doi.org/10.3390/nano8070509
Du H, Li X, Yao P, Wang J, Sun Y, Dong L. Zinc Oxide Coated Tin Oxide Nanofibers for Improved Selective Acetone Sensing. Nanomaterials. 2018; 8(7):509. https://doi.org/10.3390/nano8070509
Chicago/Turabian StyleDu, Haiying, Xiaogan Li, Pengjun Yao, Jing Wang, Yanhui Sun, and Liang Dong. 2018. "Zinc Oxide Coated Tin Oxide Nanofibers for Improved Selective Acetone Sensing" Nanomaterials 8, no. 7: 509. https://doi.org/10.3390/nano8070509
APA StyleDu, H., Li, X., Yao, P., Wang, J., Sun, Y., & Dong, L. (2018). Zinc Oxide Coated Tin Oxide Nanofibers for Improved Selective Acetone Sensing. Nanomaterials, 8(7), 509. https://doi.org/10.3390/nano8070509







