Synthesis and Characterization of Hollow Mesoporous Silica Nanoparticles for Smart Corrosion Protection
Abstract
1. Introduction
2. Materials and Methods
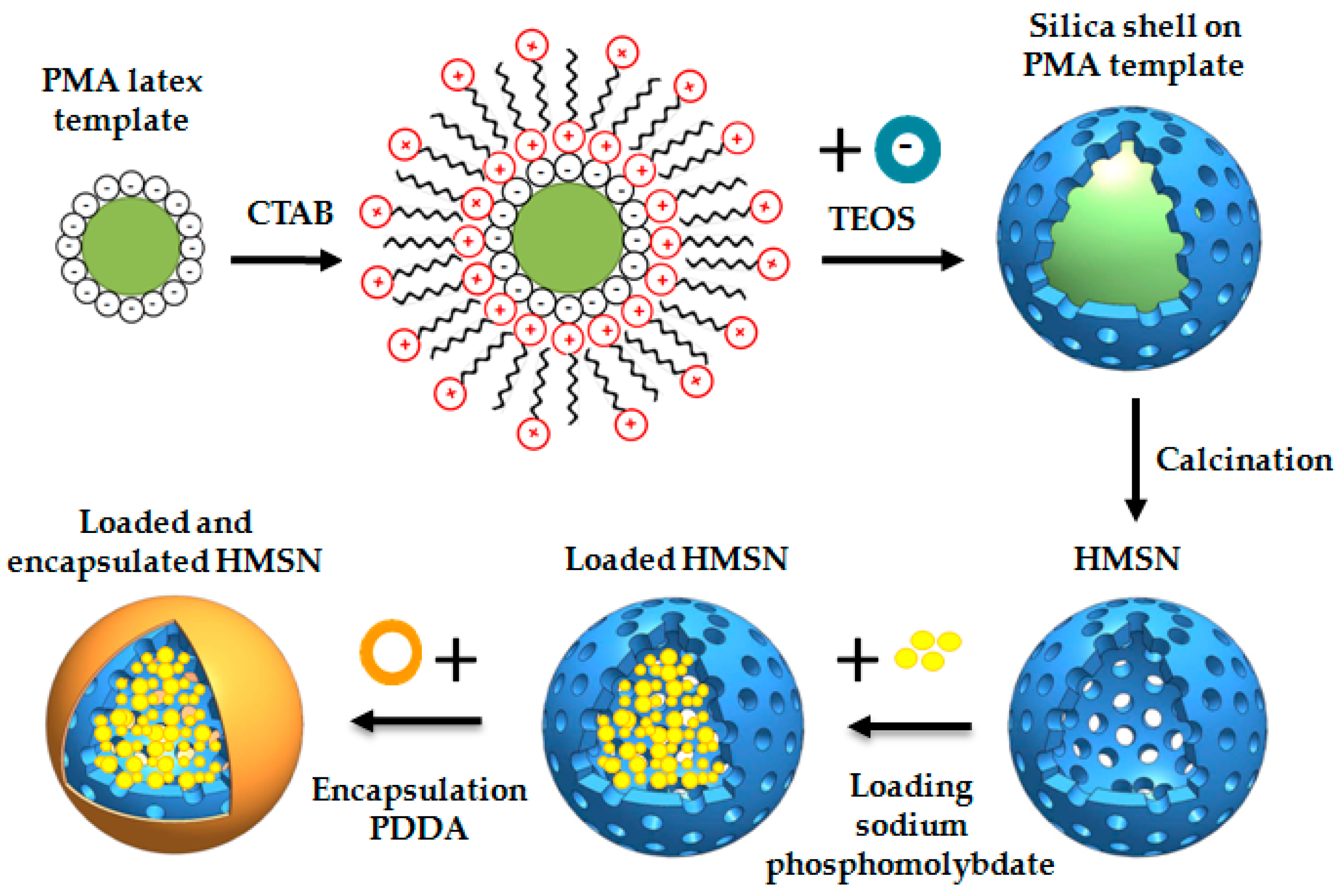
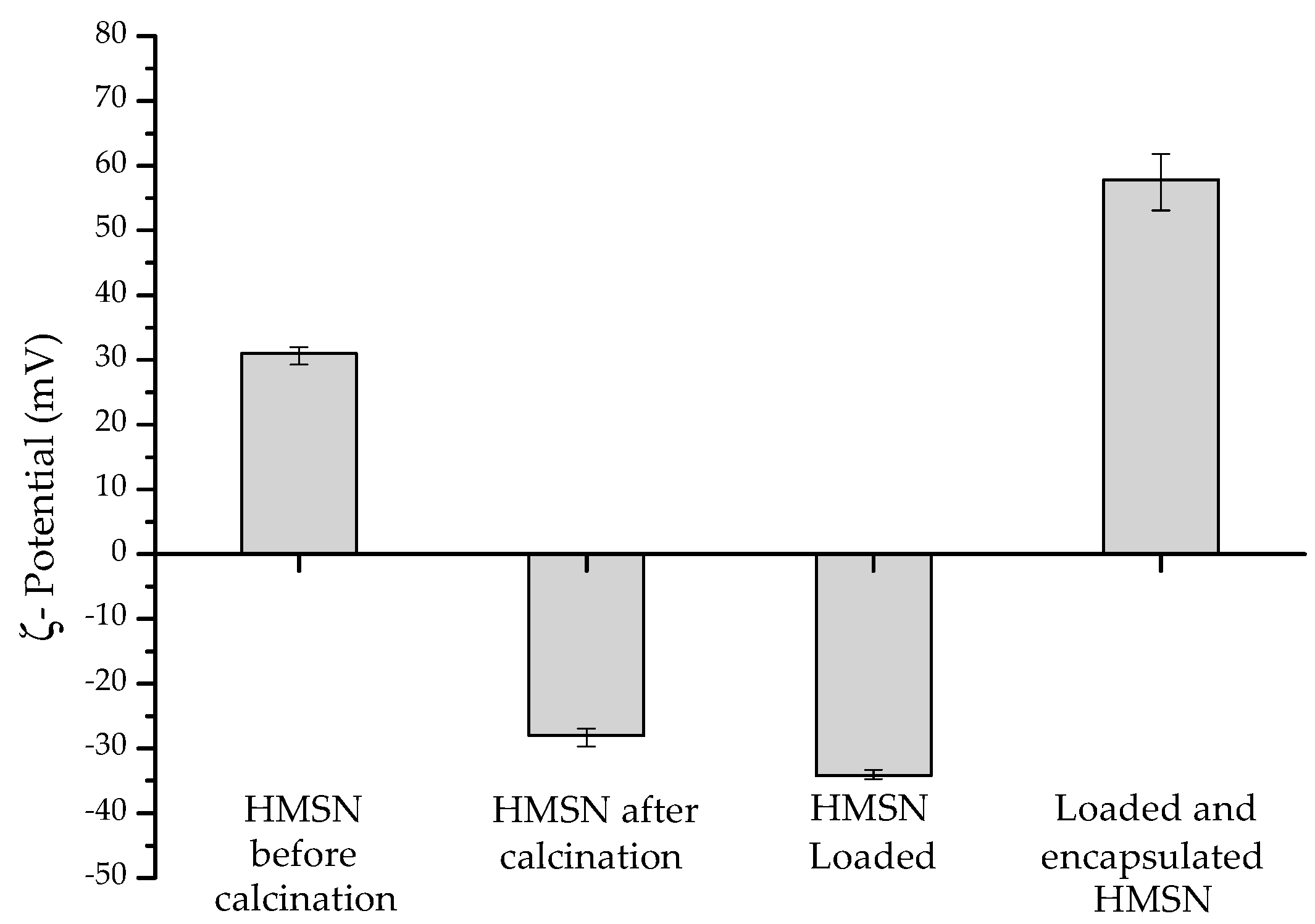
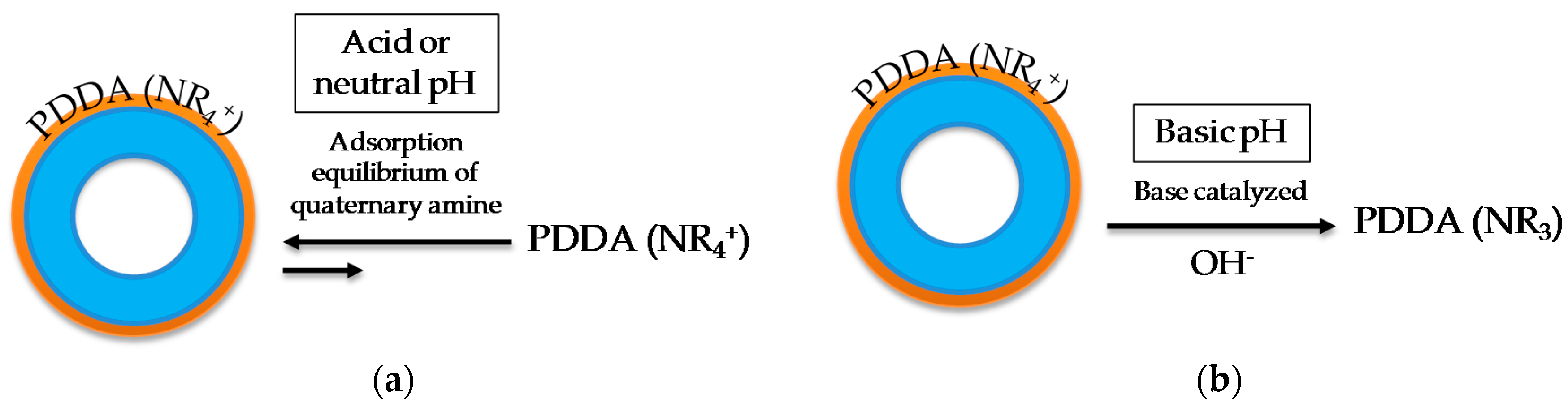
2.1. Synthesis, Loading with Inhibitor, and Encapsulation of HMSN
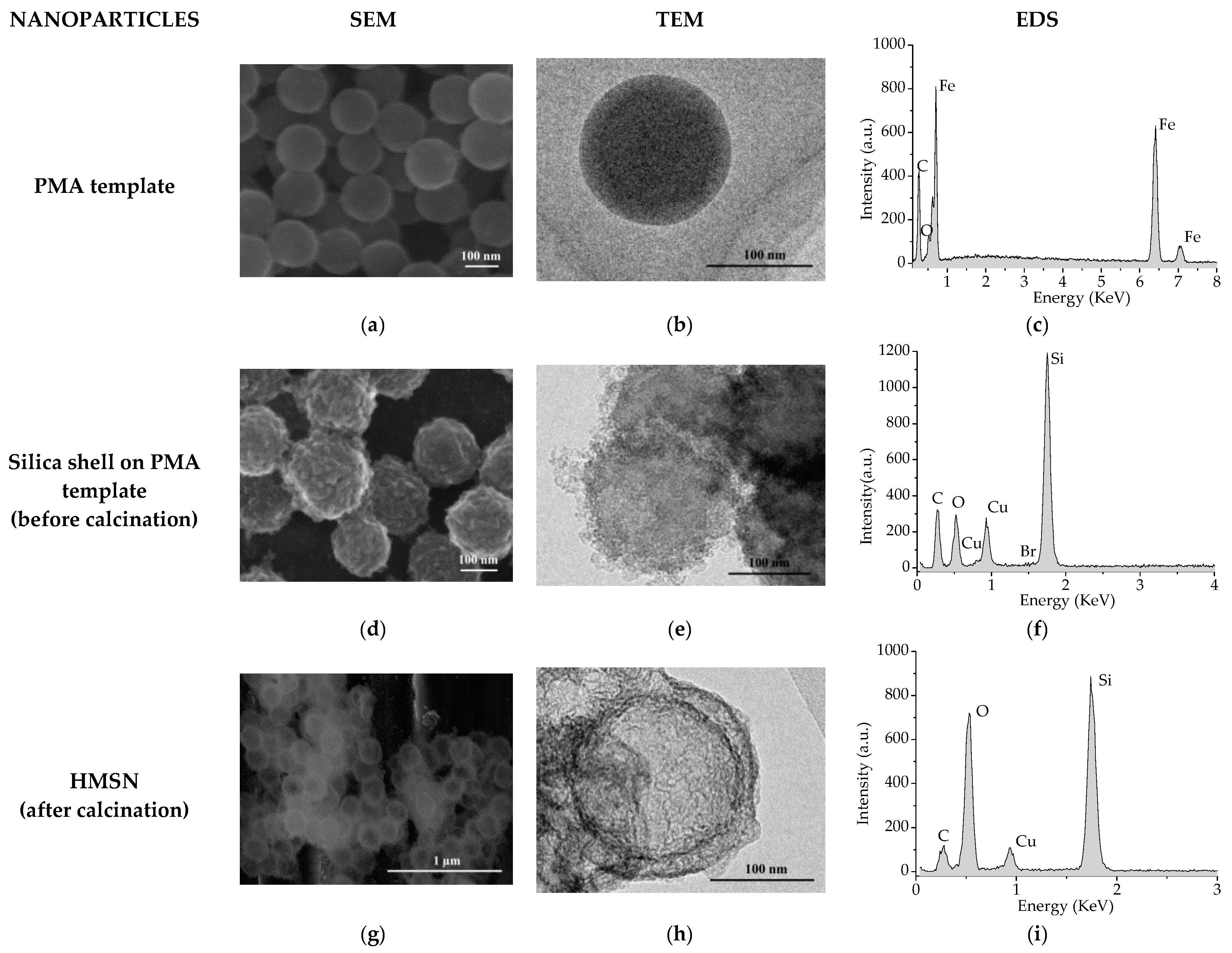
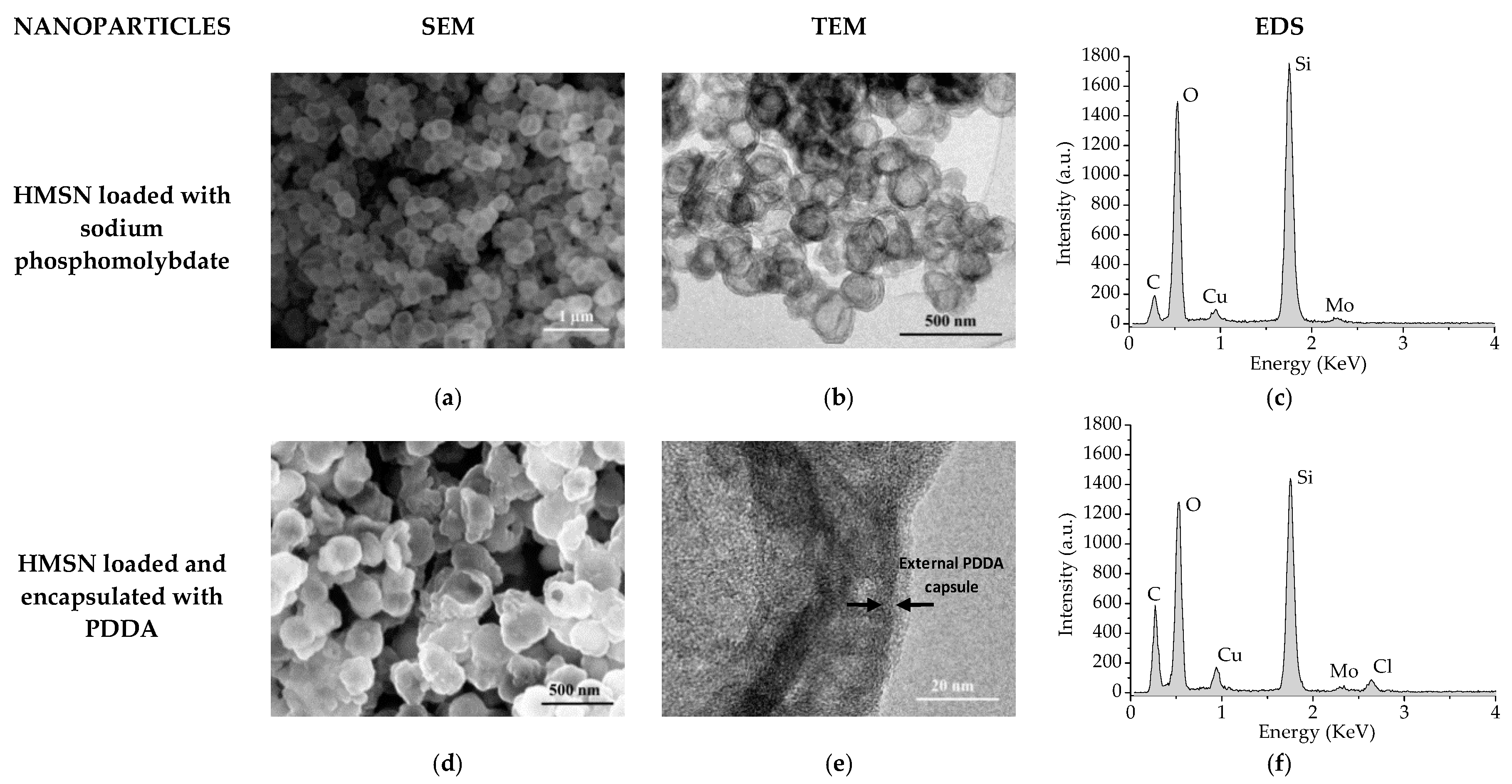
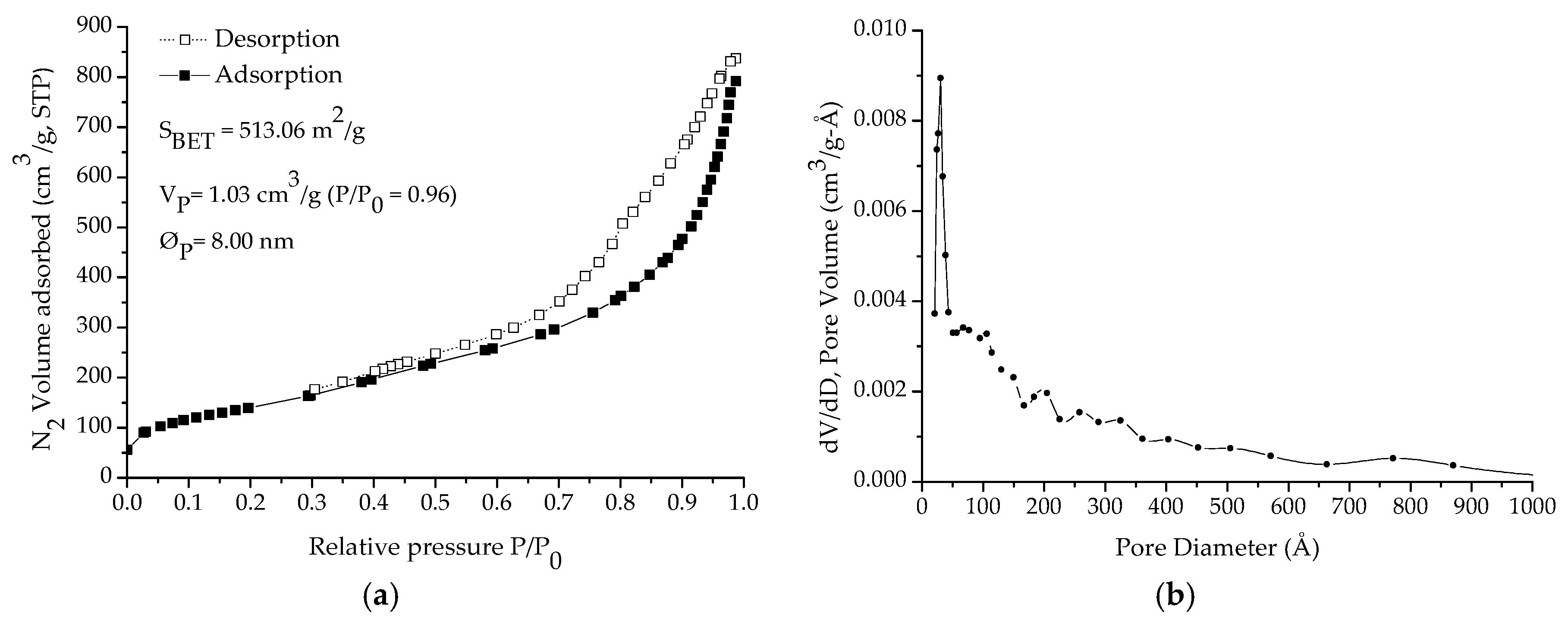
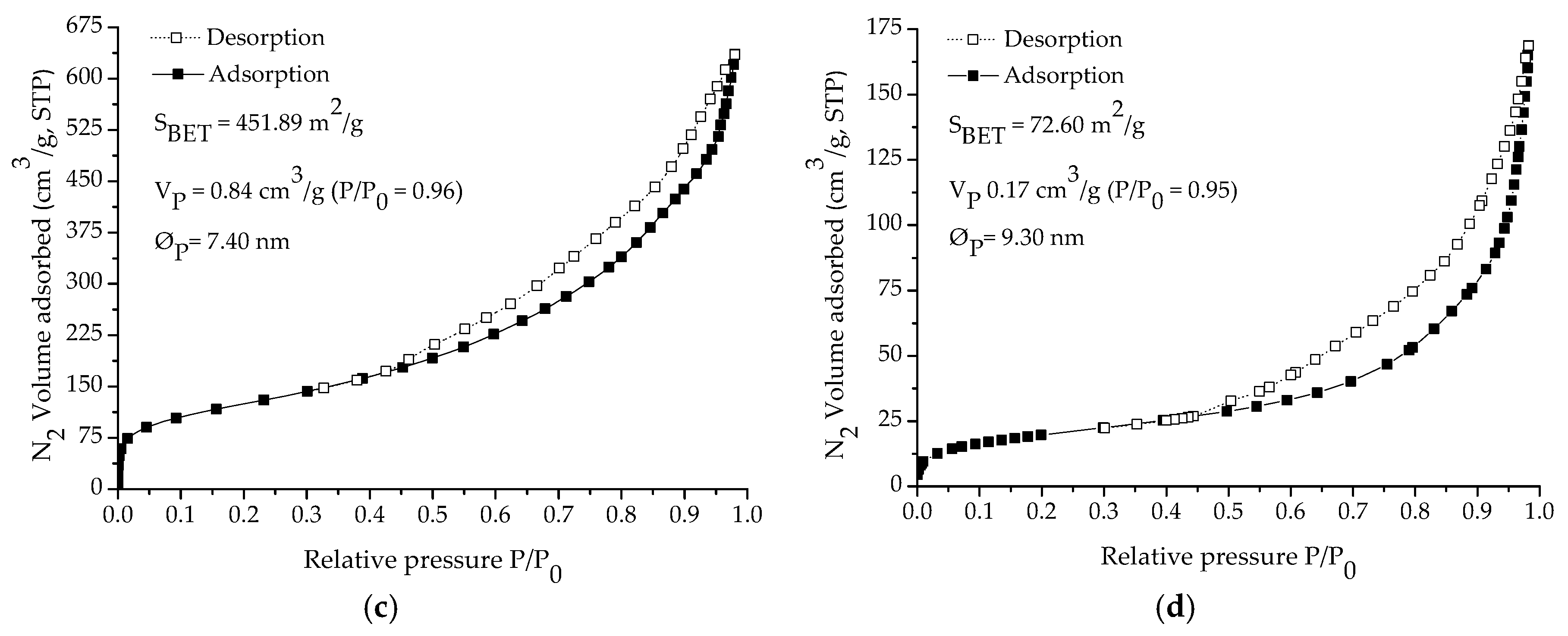
2.2. Characterization Study
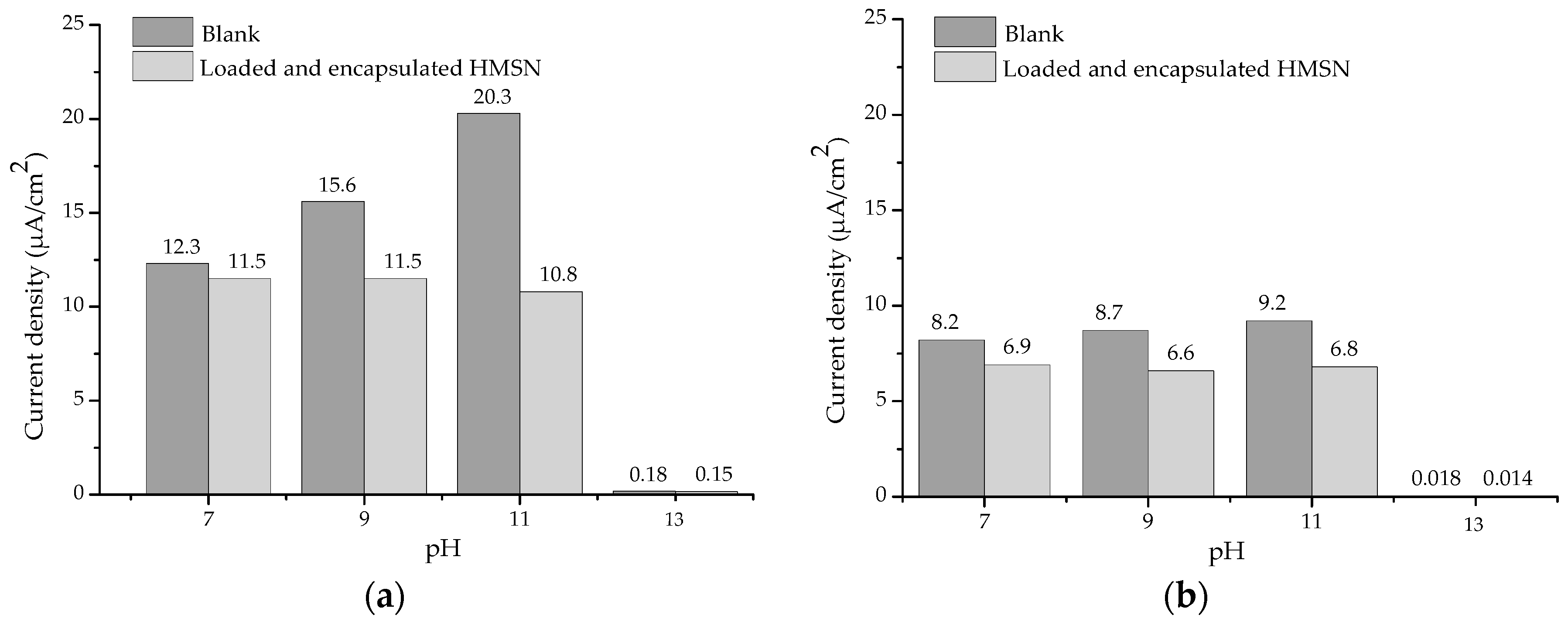
2.3. Evaluation of Inhibitor Release as a Function of pH
2.4. Evaluation of Smart Anticorrosive Behaviour
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almeida, E.; Santos, D.; Fragata, F.; de la Fuente, D.; Morcillo, M. Anticorrosive painting for a wide spectrum of marine atmospheres: Environmental-friendly versus traditional paint systems. Prog. Org. Coat. 2006, 57, 11–22. [Google Scholar] [CrossRef]
- Langard, S.; Norseth, T. A cohort study of bronchial carcinomas in workers producing chromate pigments. Br. J. Ind. Med. 1975, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Hamdy, A.S. Stimuli-responsive Polyelectrolyte Multilayers for fabrication of self-healing coatings—A review. Surf. Coat. Technol. 2016, 303, 406–424. [Google Scholar] [CrossRef]
- Li, G.; Zheng, Z.; Möhwald, H.; Shchukin, D. Silica/Polymer Double-Walled Hybrid Nanotubes: Synthesis and Application as Stimuli-Responsive Nanocontainers in Self-Healing Coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Pirhady-Tavandashti, N.; Ghorbani, M.; Shojaei, A.; Gonzalez-Garcia, Y.; Terryn, H.; Mol, J.M.C. pH responsive Ce(III) loaded polyaniline nanofibers for self-healing corrosion protection of AA2024-T3. Prog. Org. Coat. 2016, 99, 197–209. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Salak, A.N.; Lisenkov, A.D.; Ferreira, M. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464–15470. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Surface-Engineered Nanocontainers for Entrapment of Corrosion Inhibitors. Adv. Funct. Mater. 2007, 17, 1451–1458. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Inhibition of Filiform Corrosion on Polymer Coated AA2024-T3 by Hydrotalcite-Like Pigments Incorporating Organic Anions. Electrochem. Solid-State Lett. 2004, 7, B13–B15. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.; Ferreira, M. Novel Inorganic Host Layered Double Hydroxides Intercalated with Guest Organic Inhibitors for Anticorrosion Applications. ACS Appl. Mater. Interfaces 2009, 1, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Kamburova, K.; Radeva, T. Polyelectrolyte-modified kaolinite nanocontainers for entrapment of corrosion inhibitor benzotriazole. Colloid Polym. Sci. 2018, 296, 1157–1164. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.; Möhwald, H. Layer-by-Layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Kopeć, M.; Szczepanowicz, K.; Mordarski, G.; Podgórna, K.; Socha, R.P.; Nowak, P.; Warszyński, P.; Hack, T. Self-healing epoxy coatings loaded with inhibitor-containing polyelectrolyte nanocapsules. Prog. Org. Coat. 2015, 84, 97–106. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Fu, J. An intelligent anticorrosion coating based on pH-responsive smart nanocontainers fabricated via a facile method for protection of carbon steel. J. Mater. Chem. A 2015, 3, 6423–6431. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.A.; Kordas, G.C. Improvement of anti-corrosive properties of epoxy-coated AA 2024-T3 with TiO2 nanocontainers loaded with 8-hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Noiville, R.; Jaubert, O.; Gressier, M.; Bonino, J.P.; Taberna, P.L.; Fori, B.; Menu, M.J. Ce(III) corrosion inhibitor release from silica and boehmite nanocontainers. Mater. Sci. Eng. B 2018, 229, 144–154. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Lamaka, S.V.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S.; Möhwald, H. Active Anticorrosion Coatings with Halloysite Nanocontainers. J. Phys. Chem. C 2008, 112, 958–964. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.; Gabor, A.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M. Release Studies on Mesoporous Microcapsules for New Corrosion Protection Systems. Ph.D. Thesis, Rühr-University, Bochum, Germany, 2007. [Google Scholar]
- Falcón, J.M.; Otubo, L.M.; Aoki, I.V. Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf. Coat. Technol. 2016, 303, 319–329. [Google Scholar] [CrossRef]
- Yeganeh, M.; Saremi, M.; Rezaeyan, H. Corrosion inhibition of steel using mesoporous silica nanocontainers incorporated in the polypyrrole. Prog. Org. Coat. 2014, 77, 1428–1435. [Google Scholar] [CrossRef]
- Skorb, E.V.; Fix, D.; Andreeva, D.V.; Möhwald, H.; Shchukin, D.G. Surface-modified mesoporous SiO2 containers for corrosion protection. Adv. Funct. Mater. 2009, 19, 2373–2379. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-García, R.; Alcántara, J.; Simancas, J.; Morcillo, M.; de la Fuente, D. pH-dependent release of environmentally friendly corrosion inhibitor from mesoporous silica nanoreservoirs. Microporous Mesoporous Mater. 2018, 255, 166–173. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-García, R.; Chico, B.; Díaz, I.; Morcillo, M.; De La Fuente, D. Smart Mesoporous Silica Nanocapsules as Environmentally Friendly Anticorrosive Pigments. Int. J. Corros. 2015, 2015, 426397. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Hollamby, M.J.; Fix, D.; Dönch, I.; Borisova, D.; Möhwald, H.; Shchukin, D. Hybrid polyester coating incorporating functionalized mesoporous carriers for the holistic protection of steel surfaces. Adv. Mater. 2011, 23, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Fang, L.; Zhao, Z.; Ge, Y.; Piletsky, S.; Turner, A. Hierarchical Structures: Hierachically Structured Hollow Silica Spheres for High Efficiency Immobilization of Enzymes. Adv. Funct. Mater. 2013, 23, 2162–2167. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Zhang, L.; Li, X.; Cai, X.; Du, Y.; Zhang, L.; Shi, J. A salt-assisted acid etching strategy for hollow mesoporous silica/organosilica for pH-responsive drug and gene co-delivery. J. Mater. Chem. B 2015, 3, 766–775. [Google Scholar] [CrossRef]
- Wang, J.; Ding, H.; Tao, X.; Chen, J. Storage and sustained release of volatile substances from a hollow silica matrix. Nanotechnology 2007, 18, 245705. [Google Scholar] [CrossRef]
- Song, G.; Li, Z.; Li, K.; Zhang, L.; Meng, A. SiO2/ZnO Composite Hollow Sub-Micron Fibers: Fabrication from Facile Single Capillary Electrospinning and Their Photoluminescence Properties. Nanomaterials 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, D.; Hu, Z. A smart anticorrosion coating based on hollow silica nanocapsules with inorganic salt in shells. J. Coat. Technol. Res. 2017, 14, 85–94. [Google Scholar] [CrossRef]
- Chen, T.; Fu, J. An intelligent anticorrosion coating based on pH-responsive supramolecular nanocontainers. Nanotechnology 2012, 23, 505705. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chen, T.; Wang, M.; Yang, N.; Li, S.; Wang, Y.; Liu, X. Acid and Alkaline Dual Stimuli-Responsive Mechanized Hollow Mesoporous Silica Nanoparticles as Smart Nanocontainers for Intelligent Anticorrosion Coatings. ACS Nano 2013, 7, 11397–11408. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.; Hashemi, T. Adsorbed corrosion inhibitors studied by electron spectroscopy: Benzotriazole on copper and copper alloys. Corros. Sci. 1978, 18, 39–51. [Google Scholar] [CrossRef]
- Brusic, V.; Frisch, M.A.; Eldridge, B.N.; Novak, F.P.; Kaufman, F.B.; Rush, B.M.; Frankel, G.S. Copper Corrosion with and without Inhibitors. J. Electrochem. Soc. 1991, 138, 2253–2259. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Pinilla, P.; Cano, E.; Polo, J.L.; Miguel, S. Copper corrosion inhibition by triphenylmethane derivatives in sulphuric acid media. Corros. Sci. 2003, 45, 427–449. [Google Scholar] [CrossRef]
- Paliwoda-Porebska, G.; Stratmann, M.; Rohwerder, M.; Potje-Kamloth, K.; Lu, Y.; Pich, A.Z.; Adler, H.J. On the development of polypyrrole coatings with self-healing properties for iron corrosion protection. Corros. Sci. 2005, 47, 3216–3233. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, D.; Wang, A.; Yin, H.; Ren, M.; Liu, Y.; Jiang, T.; Yu, L. Synthesis of porous hollow silica spheres using polystyrene–methyl acrylic acid latex template at different temperatures. J. Phys. Chem. Solids 2009, 70, 1432–1437. [Google Scholar] [CrossRef]
- Agrawal, M.; Pich, A.; Gupta, S.; Zafeiropoulos, N.E.; Simon, P.; Stamm, M. Synthesis of Novel Tantalum Oxide Sub-micrometer Hollow Spheres with Tailored Shell Thickness. Langmuir 2008, 24, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-Controlled Macromolecule Encapsulation in and Release from Polyelectrolyte Multilayer Nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Smith, J.T.; el Rassi, Z. Capillary zone electrophoresis of biological substances with fused silica capillaries having zero or constant electroosmotic flow. Electrophoresis 1993, 14, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, F.; Hartwick, R.A. Poly(diallyldimethylammonium chloride) as a Cationic Coating for Capillary Electrophoresis. J. Chromatogr. Sci. 1997, 35, 126–130. [Google Scholar] [CrossRef]
- Nehmé, R.; Perrin, C.; Cottet, H.; Blanchin, M.D.; Fabre, H. Influence of polyelectrolyte coating conditions on capillary coating stability and separation efficiency in capillary electrophoresis. Electrophoresis 2008, 29, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dubin, P.L. Capillary Modification by Noncovalent Polycation Adsorption: Effects of Polymer Molecular Weight and Adsorption Ionic Strength. Anal. Chem. 1999, 71, 3463–3468. [Google Scholar] [CrossRef]
- Nehmé, R.; Perrin, C.; Cottet, H.; Blanchin, M.D.; Fabre, H. Stability of capillaries coated with highly charged polyelectrolyte monolayers and multilayers under various analytical conditions-application to protein analysis. J. Chromatogr. A 2011, 1218, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.C.; Trumbull, E.R. Olefins from Amines: The Hofmann Elimination Reaction and Amine Oxide Pyrolysis. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zea, C.; Alcántara, J.; Barranco-García, R.; Morcillo, M.; De la Fuente, D. Synthesis and Characterization of Hollow Mesoporous Silica Nanoparticles for Smart Corrosion Protection. Nanomaterials 2018, 8, 478. https://doi.org/10.3390/nano8070478
Zea C, Alcántara J, Barranco-García R, Morcillo M, De la Fuente D. Synthesis and Characterization of Hollow Mesoporous Silica Nanoparticles for Smart Corrosion Protection. Nanomaterials. 2018; 8(7):478. https://doi.org/10.3390/nano8070478
Chicago/Turabian StyleZea, Cristina, Jenifer Alcántara, Rosa Barranco-García, Manuel Morcillo, and Daniel De la Fuente. 2018. "Synthesis and Characterization of Hollow Mesoporous Silica Nanoparticles for Smart Corrosion Protection" Nanomaterials 8, no. 7: 478. https://doi.org/10.3390/nano8070478
APA StyleZea, C., Alcántara, J., Barranco-García, R., Morcillo, M., & De la Fuente, D. (2018). Synthesis and Characterization of Hollow Mesoporous Silica Nanoparticles for Smart Corrosion Protection. Nanomaterials, 8(7), 478. https://doi.org/10.3390/nano8070478






