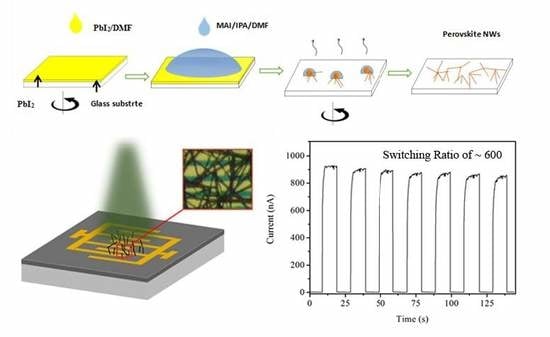
High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of CH3NH3I (MAI)
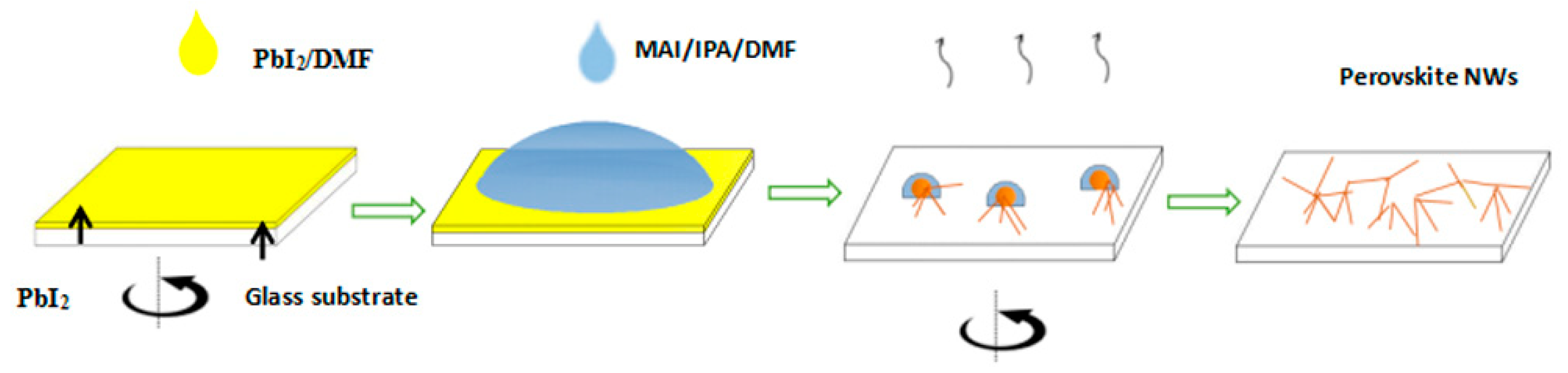
2.3. Preparation of CH3NH3PbI3 (MAPbI3) Nanowires
2.4. Device Fabrication
2.5. Structural Characterization
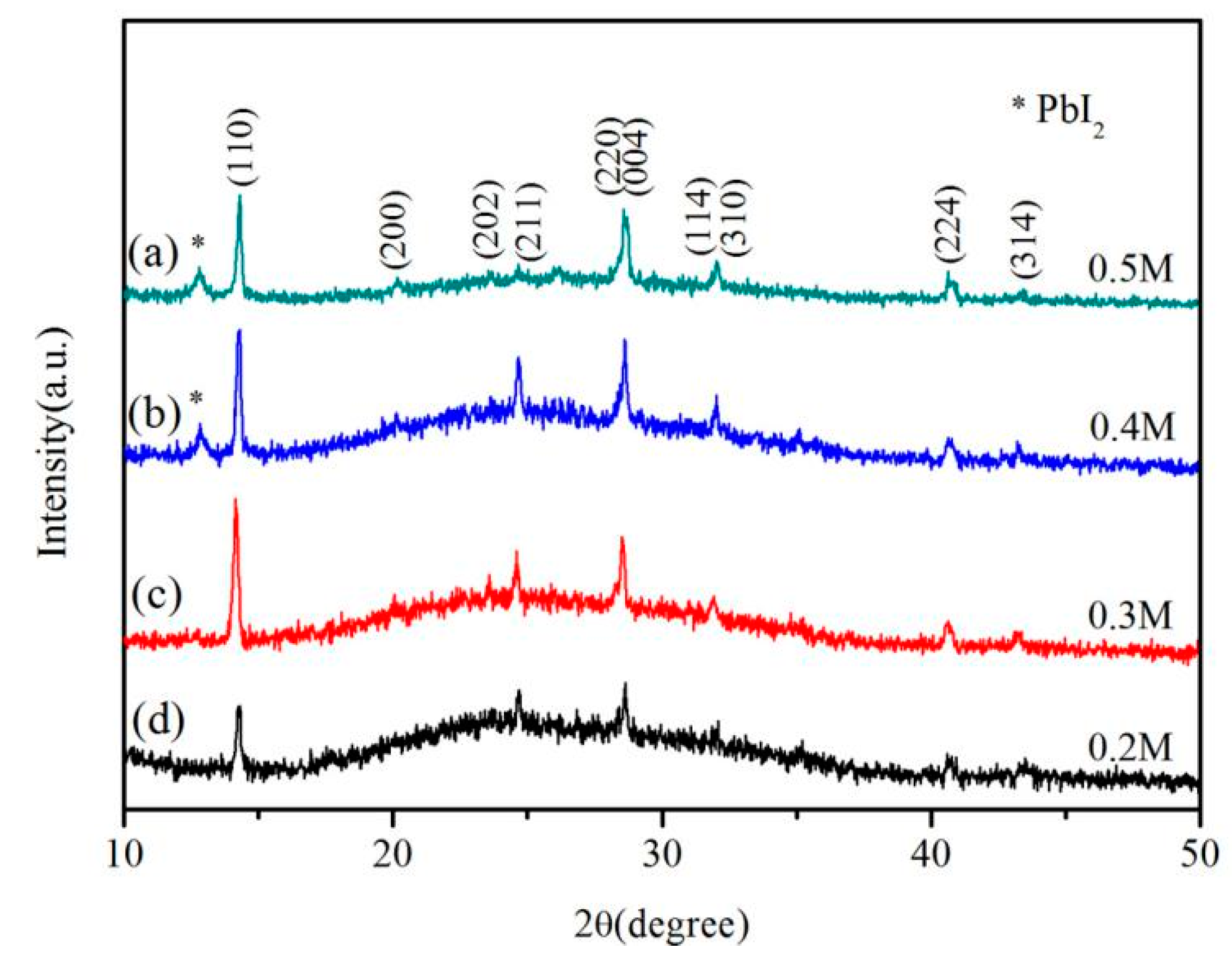
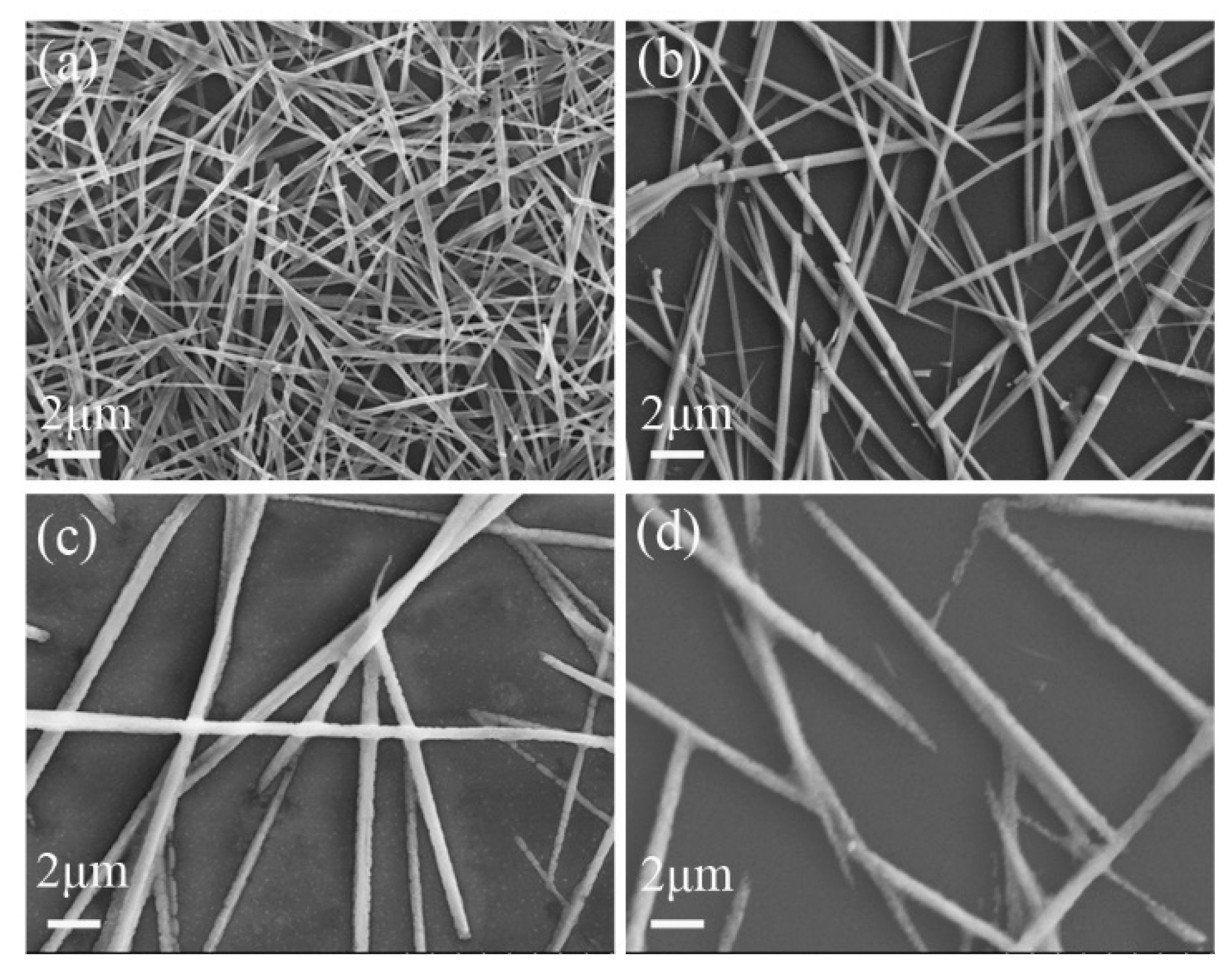
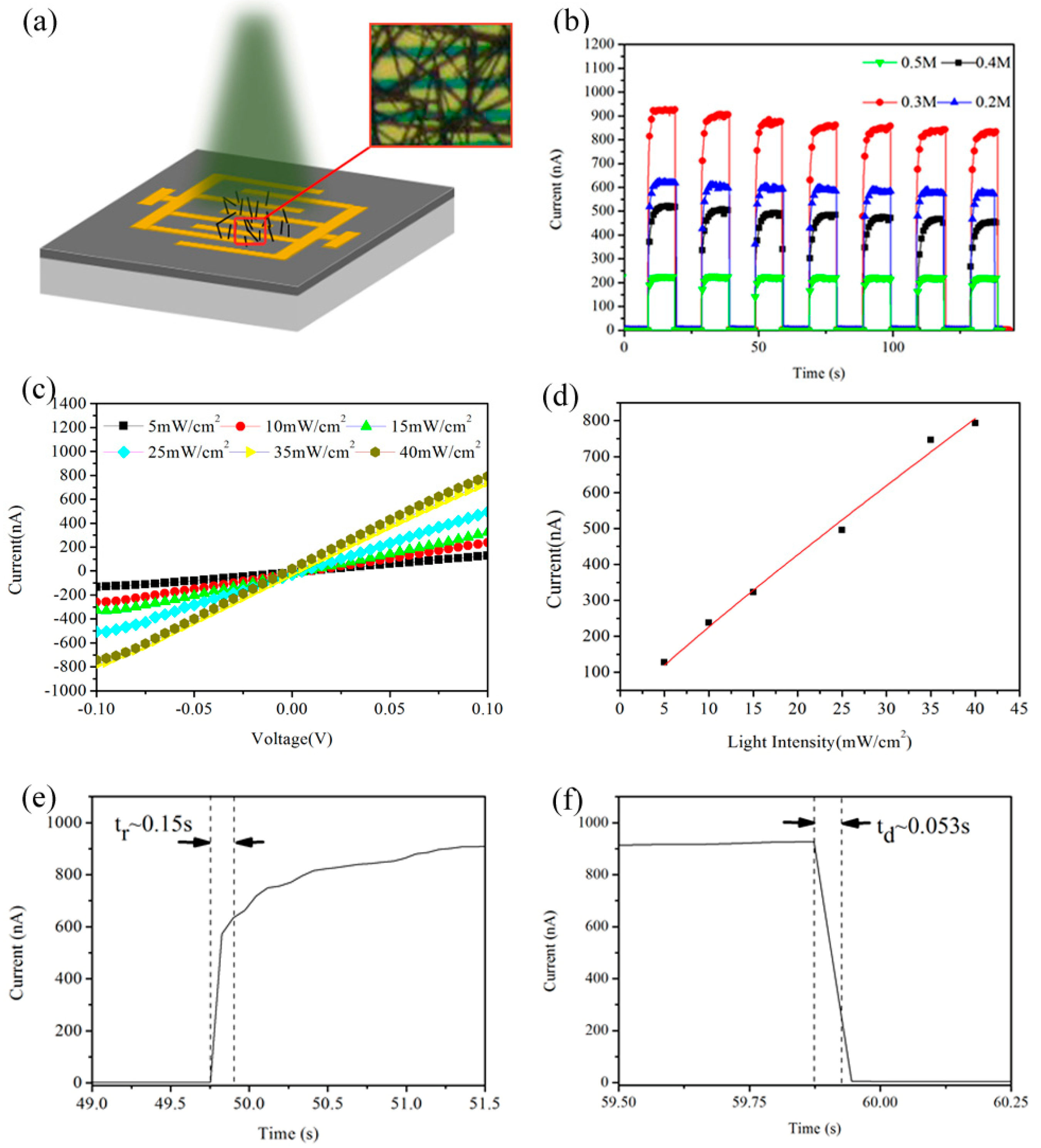
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fang, X.; Bando, Y.; Liao, M.U.; Gautam, K.; Zhi, C.; Dierre, B.; Liu, B.; Zhai, T.; Sekiguchi, T.; Koide, Y.; Golberg, D. Single-Crystalline ZnS Nanobelts as Ultraviolet-Light Sensors. Adv. Mater. 2009, 21, 2034–2039. [Google Scholar] [CrossRef]
- Lou, Z.; Shen, G. Flexible Photodetectors Based on 1D Inorganic Nanostructures. Adv. Sci. 2016, 3, 1500287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, L.; Zhang, Q.; Wu, J.; Long, Y.; Fan, Z. All-printable band-edge modulated ZnO nanowire photodetectors with ultra-high detectivity. Nat. Commun. 2014, 5, 4007. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, J.; Sun, B.; Blakesley, J.C.; Greenham, N.C. Solution-processed ultraviolet photodetectors based on colloidal ZnO nanoparticles. Nano Lett. 2008, 8, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, L.; Xu, J.; Liao, M.; Wu, L.; Fang, X. ZnO hollow-sphere nanofilm-based high-performance and low-cost photodetector. Small 2011, 7, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Sheu, J.K.; Lai, W.C.; Su, Y.K.; Chang, S.J.; Kao, C.J.; Tun, C.J.; Chen, M.G.; Chang, W.H.; Chi, G.C.; et al. Characterizations of GaN Schottky barrier photodetectors with a highly-resistivity low-temperature GaN cap layer. J. Appl. Phys. 2003, 94, 1753–1757. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Z.; Xia, W.; Yuan, C.; Cheng, J.; Lu, Y. A simple in situ tubular chemical vapor deposition processing of large-scale efficient perovskite solar cells and the research on their novel roll-over phenomenon in J–V curves. J. Mater. Chem. A 2015, 3, 12443–12451. [Google Scholar] [CrossRef]
- Ono, L.K.; Leyden, M.R.; Wang, S.; Qi, Y. Organometal halide perovskite thin films and solar cells by vapor deposition. J. Mater. Chem. A 2016, 4, 6693–6713. [Google Scholar] [CrossRef]
- Lei, Y.; Gu, L.; He, W.; Jia, Z.; Yang, X.; Jia, H.; Zheng, Z. Intrinsic charge carrier dynamics and device stability of perovskite/ZnO mesostructured solar cells in moisture. J. Mater. Chem. A 2016, 4, 5474–5481. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, X.F.; Zhang, Q.; Ha, S.T.; Yuan, Y.W.; Shen, C.; Sum, T.C.; Xiong, Q. Vapor Phase Synthesis of Organometal Halide Perovskite Nanowires for Tunable Room-Temperature Nanolasers. Nano Lett. 2015, 15, 4571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, W.; Liao, M.; Bando, Y.; Golberg, D. Recent advances in solution-processed inorganic nanofilm photodetectors. Cheminform 2014, 45, 1400–1422. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Spina, M.; Szekrenyes, Z.; Kamaras, K.; Gaal, R.; Gachet, D.; Forro, L. Nanowires of methylammonium lead iodide (CH3NH3PbI3) prepared by low temperature solution-mediated crystallization. Nano Lett. 2014, 14, 6761–6766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, S.; Shen, X.; Xu, N.; Tan, Y.; Zhuang, S.; Deng, Y.; Lu, Z.; Wang, Z.; Zhu, J. Direct Conversion of Perovskite Thin Films into Nanowires with Kinetic Control for Flexible Optoelectronic Devices. Nano Lett. 2016, 16, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Bonvin, E.; Sienkiewicz, A.; Nafradi, B.; Forro, L.; Horvath, E. Controlled growth of CH3NH3PbI3 nanowires in arrays of open nanofluidic channels. Sci. Rep. 2016, 6, 19834. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Zhang, J.; Shi, Y.; Huang, Y.; Zhang, B. Self-template-directed synthesis of porous perovskite nanowires at room temperature for high-performance visible-light photodetectors. Angew. Chem. Int. Ed. Engl. 2015, 54, 5693–5696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Veeramalai, C.P.; Chen, W.; Guo, T.; Wu, C.; Kim, T.W. Inkjet-Printed Photodetector Arrays Based on Hybrid Perovskite CH3NH3PbI3 Microwires. ACS Appl. Mater. Interfaces 2017, 9, 11662–11668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Z.; Du, S.; Yuan, F.; Li, Z.; Li, Z.; Dai, Q.; Wang, H.; Luo, S.; Zhang, S. Solution-processed photodetectors based on organic-inorganic hybrid perovskite and nanocrystalline graphite. Nanotechnology 2016, 27, 175201. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, X.; Liang, L.; Bao, J.; Li, S.; Yang, W.; Xie, Y. High-Performance Flexible Broadband Photodetector Based on Organolead Halide Perovskite. Adv. Funct. Mater. 2014, 24, 7373–7380. [Google Scholar] [CrossRef]
- He, M.; Chen, Y.; Liu, H.; Wang, J.; Fang, X.; Liang, Z. Chemical decoration of CH3NH3PbI3 perovskites with graphene oxides for photodetector applications. Chem. Commun. 2015, 51, 9659–9661. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Dong, D.; Qiao, K.; Bu, L.; Li, B.; Yang, D.; Wang, H.E.; Cheng, Y.; Zhao, Z.; Tang, J.; Song, H. Growth, patterning and alignment of organolead iodide perovskite nanowires for optoelectronic devices. Nanoscale 2015, 7, 4163–4170. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Adinolfi, V.; Comin, R.; Abdelhady, A.L.; Peng, W.; Dursun, I.; Yuan, M.; Hoogland, S.; Sargent, E.H.; Bakr, O.M. Planar-integrated single-crystalline perovskite photodetectors. Nat. Commun. 2015, 6, 8724. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.B.; Lai, M.; Eaton, S.W.; Yu, Y.; Lin, E.; Dou, L.; Fu, A.; Yang, P. Growth and Anion Exchange Conversion of CH3NH3PbX3 Nanorod Arrays for Light-Emitting Diodes. Nano Lett. 2015, 15, 5519–5524. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Meng, F.; Rowley, M.B.; Thompson, B.J.; Shearer, M.J.; Ma, D.; Hamers, R.J.; Wright, J.C.; Jin, S. Solution Growth of Single Crystal Methylammonium Lead Halide Perovskite Nanostructures for Optoelectronic and Photovoltaic Applications. J. Am. Chem. Soc. 2015, 137, 5810. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Luo, J.; Franckevičius, M.; Pellet, N.; Gao, P.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M.; Park, N.G. Nanowire Perovskite Solar Cell. Nano Lett. 2015, 15, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, X.; Dong, D.; Li, B.; Yang, D.; Yuan, S.; Qiao, K.; Cheng, Y.B.; Tang, J.; Song, H. Flexible and Semitransparent Organolead Triiodide Perovskite Network Photodetector Arrays with High Stability. Nano Lett. 2015, 15, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gangishetty, M.K.; Kelly, T.L. Effect of CH3NH3PbI3 thickness on device efficiency in planar heterojunction perovskite solar cells. J. Mater. Chem. A 2014, 2, 19873–19881. [Google Scholar] [CrossRef]
- Hao, Q.; Chu, Y.; Zheng, X.; Liu, Z.; Liang, L.; Qi, J.; Zhang, X.; Liu, G.; Liu, H.; Chen, H.; Liu, C. Preparation of planar CH3NH3PbI3 thin films with controlled size using 1-ethyl-2-pyrrolidone as solvent. J. Alloys Compd. 2016, 671, 11–16. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.D.; Hernandez-Ramirez, F.; Jimenez-Diaz, R.; Manzanares, M.; Andreu, T.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. The effects of electron-hole separation on the photoconductivity of individual metal oxide nanowires. Nanotechnology 2008, 19, 465501. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Fang, Y.; Chae, J.; Dai, J.; Xiao, Z.; Dong, Q.; Yuan, Y.; Centrone, A.; Zeng, X.C.; Huang, J. High-gain and low-driving-voltage photodetectors based on organolead triiodide perovskites. Adv. Mater. 2015, 27, 1912–1918. [Google Scholar] [CrossRef] [PubMed]

| Materials | Photocurrent (nA) | Dark Current (nA) | On/Off Ratio | Bias Voltage(V) | Ref. |
|---|---|---|---|---|---|
| CH3NH3PbI3 single NWs | 115 | 5 | 23 | 2 | [22] |
| CH3NH3PbI3 single NWs | 0.25 | 10−3 | 250 | 1 | [23] |
| CH3NH3PbI3 single NWs | Not Given | Not Given | 13 | 3 | [15] |
| CH3NH3PbI3 thin film | 185 | 5 | 37 | 5 | [19] |
| CH3NH3PbI3 thin film | 1.75*103 | 54 | 324 | 8 | [20] |
| CH3NH3PbI3 thin film | Not Given | Not Given | 23.5 | 5 | [21] |
| CH3NH3PbI3 single NWs | 920 | 1.55 | 600 | 0.1 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, C.; Ren, G.; Li, S.; Bi, C.; Hao, Q.; Liu, H. High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires. Nanomaterials 2018, 8, 318. https://doi.org/10.3390/nano8050318
Zhang X, Liu C, Ren G, Li S, Bi C, Hao Q, Liu H. High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires. Nanomaterials. 2018; 8(5):318. https://doi.org/10.3390/nano8050318
Chicago/Turabian StyleZhang, Xin, Caichi Liu, Gang Ren, Shiyun Li, Chenghao Bi, Qiuyan Hao, and Hui Liu. 2018. "High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires" Nanomaterials 8, no. 5: 318. https://doi.org/10.3390/nano8050318
APA StyleZhang, X., Liu, C., Ren, G., Li, S., Bi, C., Hao, Q., & Liu, H. (2018). High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires. Nanomaterials, 8(5), 318. https://doi.org/10.3390/nano8050318