Comparative Study of the ORR Activity and Stability of Pt and PtM (M = Ni, Co, Cr, Pd) Supported on Polyaniline/Carbon Nanotubes in a PEM Fuel Cell
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the PANI-CNT Support
2.2. Synthesis of PtM/PANI-CNT Catalysts
2.3. Preparation of the Carbon Sublayer and Membrane Electrode Assembly (MEA)
2.4. Catalyst and Electrode Characterization
2.5. ORR Activity and Stability Tests
3. Results and Discussion
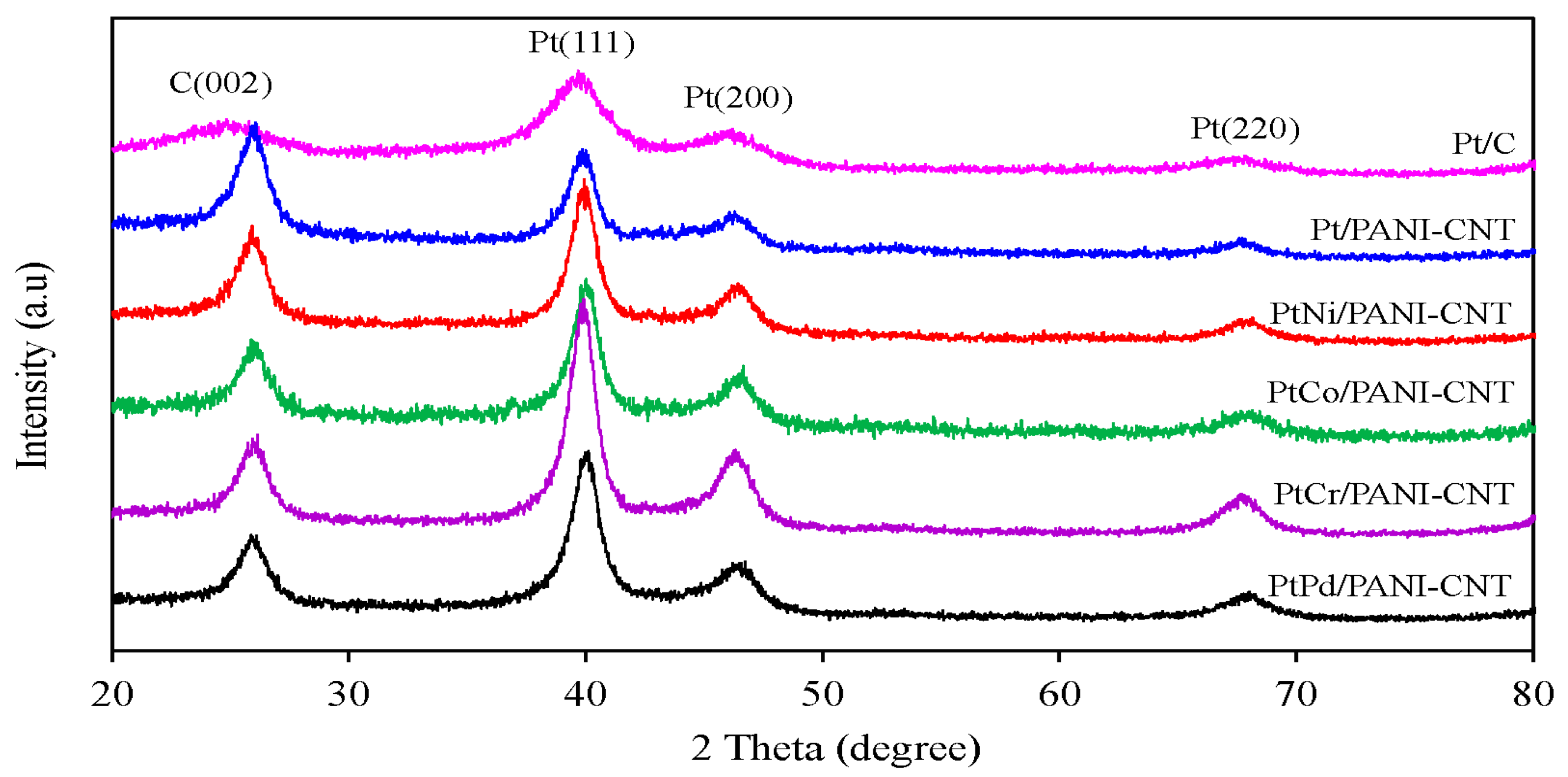
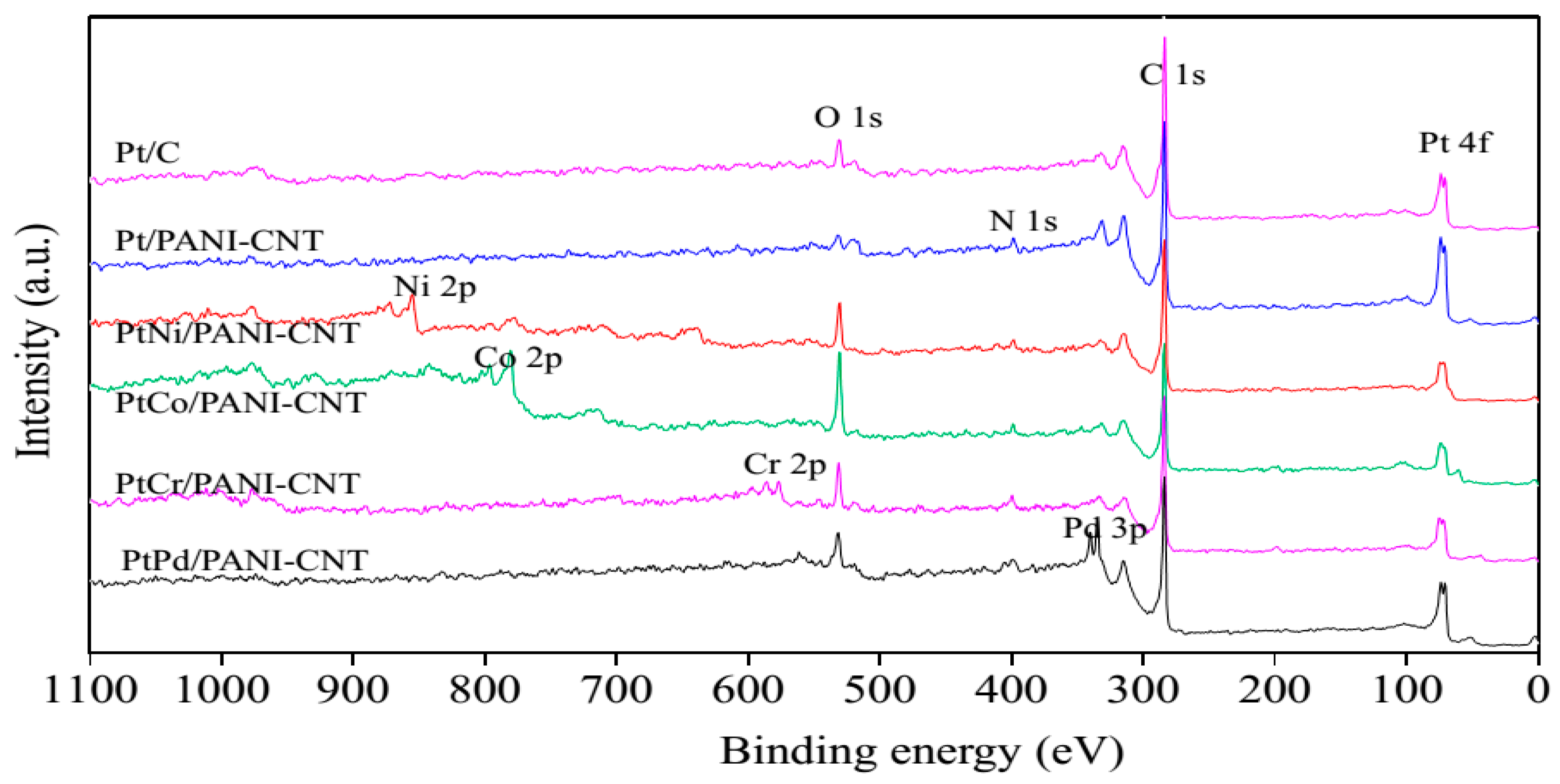
3.1. Property of Pt/PANI-CNT and PtM/PANI-CNT Catalysts
3.2. Electrochemical Properties of Pt/PANI-CNT and PtM/PANI-CNT Electrodes
3.3. ORR Activity Test
3.3.1. ORR Activity in Acid Solution
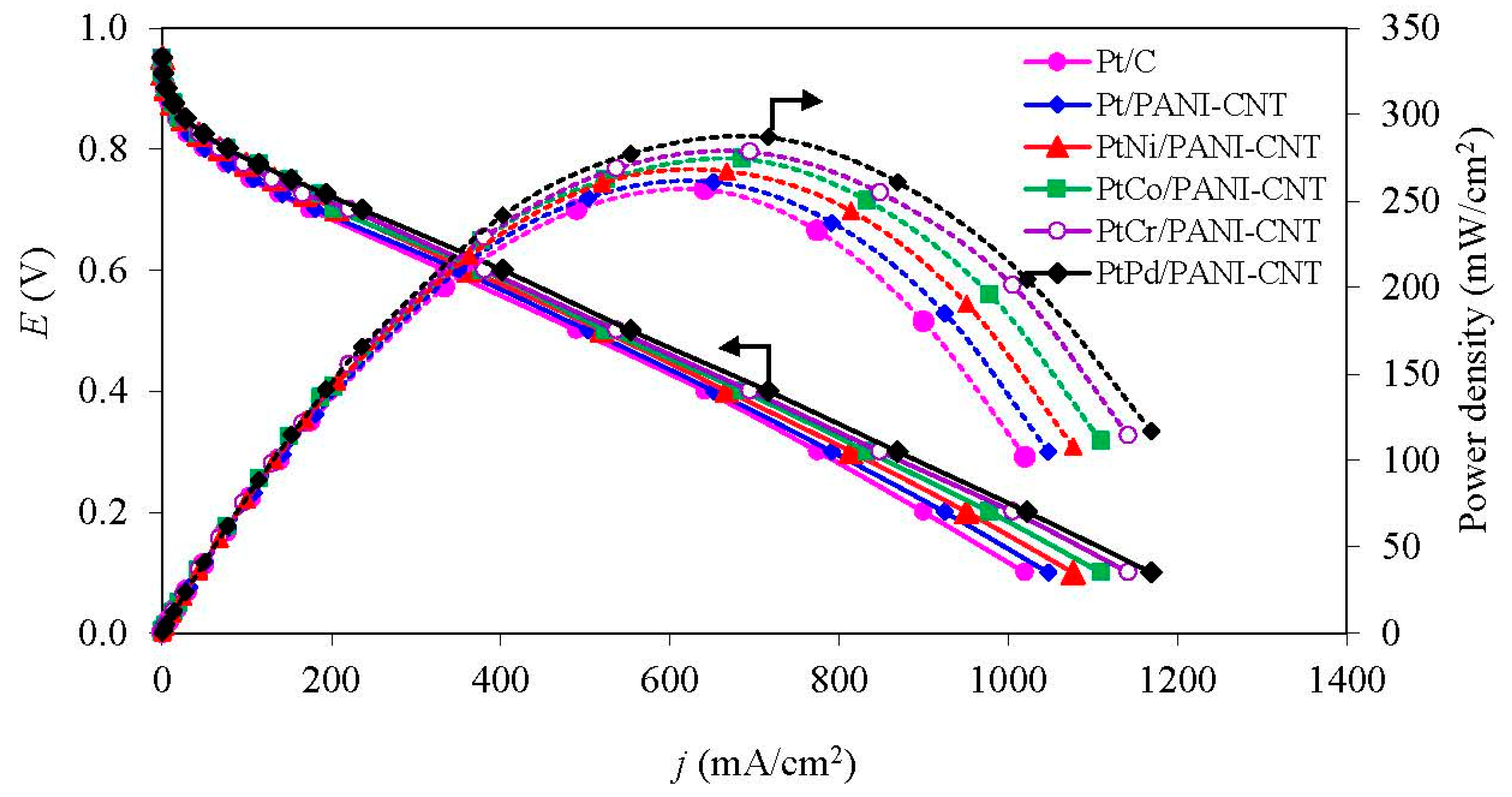
3.3.2. ORR Activity in the PEM Fuel Cell
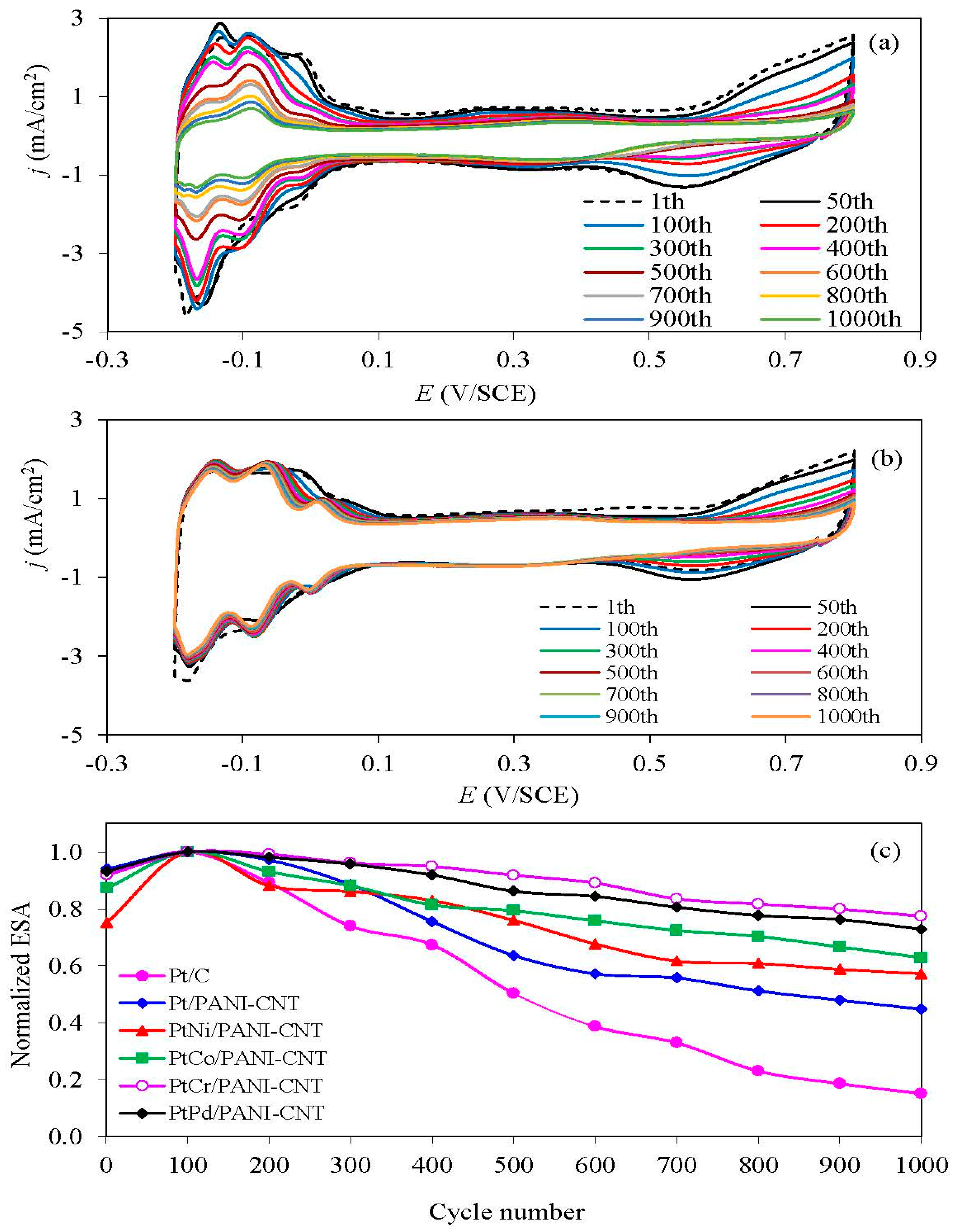
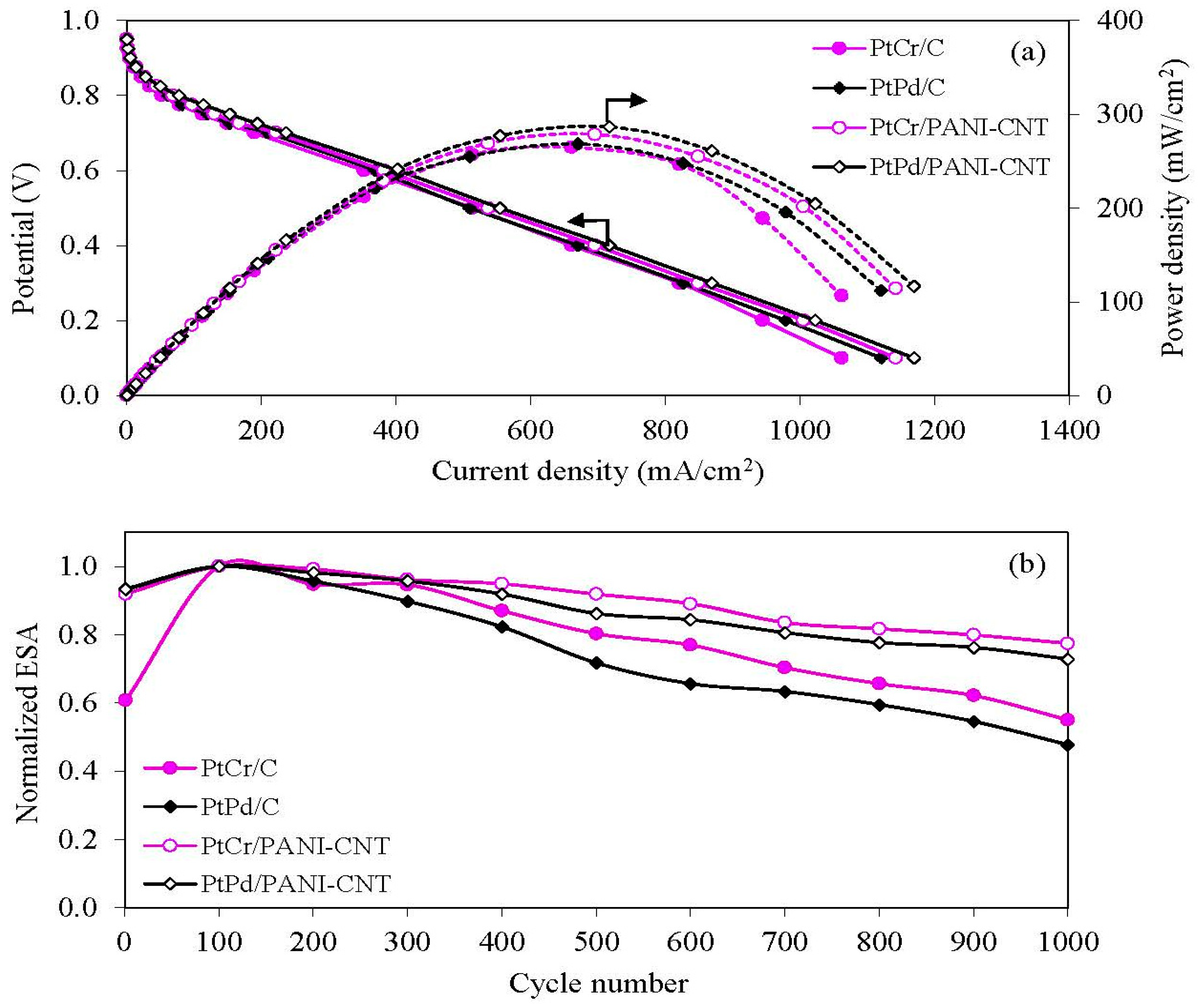
3.4. Stability Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| [Pt] | Pt loading |
| a | lattice parameter |
| b | Tafel slope |
| C | bulk concentration of oxygen molecules |
| D | diffusion coefficient of oxygen |
| Er | reversible potential for the electrode |
| F | Faraday’s constant |
| I | test current |
| j | current density |
| j0 | exchange current density for the ORR |
| jk | kinetic current density |
| l | number of layers and |
| m | parameters related to the mass transport limitation |
| n | parameters related to the mass overpotential |
| ne | number of exchanged electrons in the reaction |
| NS | number of surface atoms |
| NT | total number of atoms |
| QH | charge exchanged |
| qH | charge required for the monolayer adsorption of H2 on Pt surfaces |
| R | total resistance |
| s | probe spacing |
| t | specimen thickness |
| V | measured voltage |
Symbols
| ω | angular velocity |
| ν | kinematic viscosity |
| σ | in-plane conductivity |
References
- Jeon, M.K.; McGinn, P.J. Co-alloying effect of co and Cr with Pt for oxygen electro-reduction reaction. Electrochim. Acta 2012, 64, 147–153. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- Wikander, K.; Ekström, H.; Palmqvist, A.; Lundblad, A.; Holmberg, K.; Lindbergh, G. Alternative catalysts and carbon support material for pemfc. Fuel Cells 2006, 6, 21–25. [Google Scholar] [CrossRef]
- Roen, L.; Paik, C.; Jarvi, T. Electrocatalytic corrosion of carbon support in pemfc cathodes. Electrochem. Solid-State Lett. 2004, 7, A19–A22. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Wang, Z.; Gao, Y. Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235–242. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Ciou, J.-R. Effects of surface chemical states of carbon nanotubes supported Pt nanoparticles on performance of proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 6826–6831. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L. Substrate-enhanced electroless deposition of metal nanoparticles on carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-S.; Kim, K.; Ko, Y.-J.; Kim, H. Effect of chemical oxidation of CNFs on the electrochemical carbon corrosion in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2010, 35, 701–708. [Google Scholar] [CrossRef]
- Kongkanand, A.; Vinodgopal, K.; Kuwabata, S.; Kamat, P.V. Highly dispersed Pt catalysts on single-walled carbon nanotubes and their role in methanol oxidation. J. Phys. Chem. B 2006, 110, 16185–16188. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Fujigaya, T.; Nakashima, N. Design of an assembly of poly(benzimidazole), carbon nanotubes, and Pt nanoparticles for a fuel-cell electrocatalyst with an ideal interfacial nanostructure. Small 2009, 5, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Fujigaya, T.; Okamoto, M.; Nakashima, N. Design of an assembly of pyridine-containing polybenzimidazole, carbon nanotubes and Pt nanoparticles for a fuel cell electrocatalyst with a high electrochemically active surface area. Carbon 2009, 47, 3227–3232. [Google Scholar] [CrossRef]
- Kaewsai, D.; Lin, H.-L.; Liu, Y.-C.; Yu, T.L. Platinum on pyridine-polybenzimidazole wrapped carbon nanotube supports for high temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2016, 41, 10430–10445. [Google Scholar] [CrossRef]
- Oh, H.-S.; Kim, K.; Kim, H. Polypyrrole-modified hydrophobic carbon nanotubes as promising electrocatalyst supports in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 11564–11571. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Liao, H.-Y.; Kuo, P.-L. Aniline as a dispersant and stabilizer for the preparation of Pt nanoparticles deposited on carbon nanotubes. J. Phys. Chem. C 2010, 114, 7933–7939. [Google Scholar] [CrossRef]
- He, D.; Zeng, C.; Xu, C.; Cheng, N.; Li, H.; Mu, S.; Pan, M. Polyaniline-functionalized carbon nanotube supported platinum catalysts. Langmuir 2011, 27, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Adhikary, R.; Datta, J. Proactive role of carbon nanotube-polyaniline conjugate support for Pt nano-particles toward electro-catalysis of ethanol in fuel cell. Int. J. Hydrog. Energy 2017, 42, 25316–25325. [Google Scholar] [CrossRef]
- Mizuhata, M.; Oga, M.; Miyachi, Y.; Deki, S. Preparation of Pt/electro-conductive polymer loaded carbon composite for improvement of electrode durability for fuel cells. ECS Trans. 2008, 6, 75–83. [Google Scholar]
- Chen, S.; Wei, Z.; Qi, X.; Dong, L.; Guo, Y.-G.; Wan, L.; Shao, Z.; Li, L. Nanostructured polyaniline-decorated Pt/C@PANI core-shell catalyst with enhanced durability and activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Manthiram, A. Effect of atomic ordering on the catalytic activity of carbon supported PtM (M = Fe, Co, Ni, and Cu) alloys for oxygen reduction in PEMFCS. J. Electrochem. Soc. 2005, 152, A697–A703. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.; Gonzalez, E.R. The stability of Pt-M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells: A literature review and tests on a Pt-Co catalyst. J. Power Sources 2006, 160, 957–968. [Google Scholar] [CrossRef]
- Chaisubanan, N.; Maniwan, W.; Hunsom, M. Effect of heat-treatment on the performance of PtM/C (M= Cr, Pd, Co) catalysts towards the oxygen reduction reaction in PEM fuel cell. Energy 2017, 127, 454–461. [Google Scholar] [CrossRef]
- Jalan, V.; Taylor, E. Importance of interatomic spacing in catalytic reduction of oxygen in phosphoric acid. Electrochem. Soc. J. 1983, 130, 2299–2302. [Google Scholar] [CrossRef]
- Kaewsai, D.; Piumsomboon, P.; Pruksathorn, K.; Hunsom, M. Synthesis of polyaniline-wrapped carbon nanotube-supported PtCo catalysts for proton exchange membrane fuel cells: Activity and stability tests. RSC Adv. 2017, 7, 20801–20810. [Google Scholar] [CrossRef]
- Thanasilp, S.; Hunsom, M. Preparation of a high-performance Pt-Pd/C-electrocatalyst-coated membrane for ORR in PEM fuel cells via a combined process of impregnation and seeding: Effect of electrocatalyst loading on carbon support. Electrochim. Acta 2011, 56, 1164–1171. [Google Scholar] [CrossRef]
- Shi, W. Preparation of coralline-like nitrogen-doped porous carbon by urea-assisted pyrolysis of low-cost and environmental friendly polyaniline. Environ. Prog. Sustain. Energy 2016, 35, 840–846. [Google Scholar] [CrossRef]
- Trongchuankij, W.; Poochinda, K.; Pruksathorn, K.; Hunsom, M. A study on novel combined processes for preparation of high performance Pt-Co/C electrocatalyst for oxygen reduction in PEM fuel cell. Renew. Energy 2010, 35, 2839–2843. [Google Scholar] [CrossRef]
- Schmidt, T.; Gasteiger, H.; Stäb, G.; Urban, P.; Kolb, D.; Behm, R. Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Vielstich, W.; Yokokawa, H.; Gasteiger, H.A. Handbook of Fuel Cells: Fundamentals Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Fraga, M.; Jordao, E.; Mendes, M.; Freitas, M.; Faria, J.; Figueiredo, J. Properties of carbon-supported platinum catalysts: Role of carbon surface sites. J. Catal. 2002, 209, 355–364. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Badenes, P.; Rodrıguez-Ramos, I. Study of some factors affecting the Ru and Pt dispersions over high surface area graphite-supported catalysts. Appl. Catal. A Gen. 1998, 173, 313–321. [Google Scholar] [CrossRef]
- Sepulveda-Escribano, A.; Coloma, F.; Rodrıguez-Reinoso, F. Platinum catalysts supported on carbon blacks with different surface chemical properties. Appl. Catal. A Gen. 1998, 173, 247–257. [Google Scholar] [CrossRef]
- Van Der Klink, J. NMR spectroscopy as a probe of surfaces of supported metal catalysts. Adv. Catal. 1999, 44, 1–117. [Google Scholar]
- Onoe, T.; Iwamoto, S.; Inoue, M. Synthesis and activity of the Pt catalyst supported on CNT. Catal. Commun. 2007, 8, 701–706. [Google Scholar] [CrossRef]
- Rahsepar, M.; Pakshir, M.; Kim, H. Synthesis of multiwall carbon nanotubes with a high loading of Pt by a microwave-assisted impregnation method for use in the oxygen reduction reaction. Electrochim. Acta 2013, 108, 769–775. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile fabrication of platinum-cobalt alloy nanoparticles with enhanced electrocatalytic activity for a methanol oxidation reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yan, X.; Chen, J.; Xu, S.; Xue, Q. PtFe nanotubes/graphene hybrid: Facile synthesis and its electrochemical properties. Int. J. Hydrog. Energy 2013, 38, 13011–13016. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Li, N.; Yan, Y.; Lou, X.W.D.; Wang, X. One-pot synthesis of Pt-Co alloy nanowire assemblies with tunable composition and enhanced electrocatalytic properties. Angew. Chem. 2015, 127, 3868–3872. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, W.; Wang, W.; Li, J.; Chen, S. Pt-Pd nanodendrites as oxygen reduction catalyst in polymer-electrolyte-membrane fuel cell. Int. J. Hydrog. Energy 2017, 42, 25234–25243. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Herrero, E.; Franaszczuk, K.; Wieckowski, A. Electrochemistry of methanol at low index crystal planes of platinum: An integrated voltammetric and chronoamperometric study. J. Phys. Chem. 1994, 98, 5074–5083. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Zignani, S.C.; Baglio, V.; Sebastián, D.; Saccà, A.; Gatto, I.; Aricò, A.S. Towards highly performing and stable ptni catalysts in polymer electrolyte fuel cells for automotive application. Materials 2017, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]
- He, T.; Kreidler, E.; Xiong, L.; Luo, J.; Zhong, C. Alloy electrocatalysts combinatorial discovery and nanosynthesis. J. Electrochem. Soc. 2006, 153, A1637–A1643. [Google Scholar] [CrossRef]
- Knupp, S.L.; Li, W.; Paschos, O.; Murray, T.M.; Snyder, J.; Haldar, P. The effect of experimental parameters on the synthesis of carbon nanotube/nanofiber supported platinum by polyol processing techniques. Carbon 2008, 46, 1276–1284. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
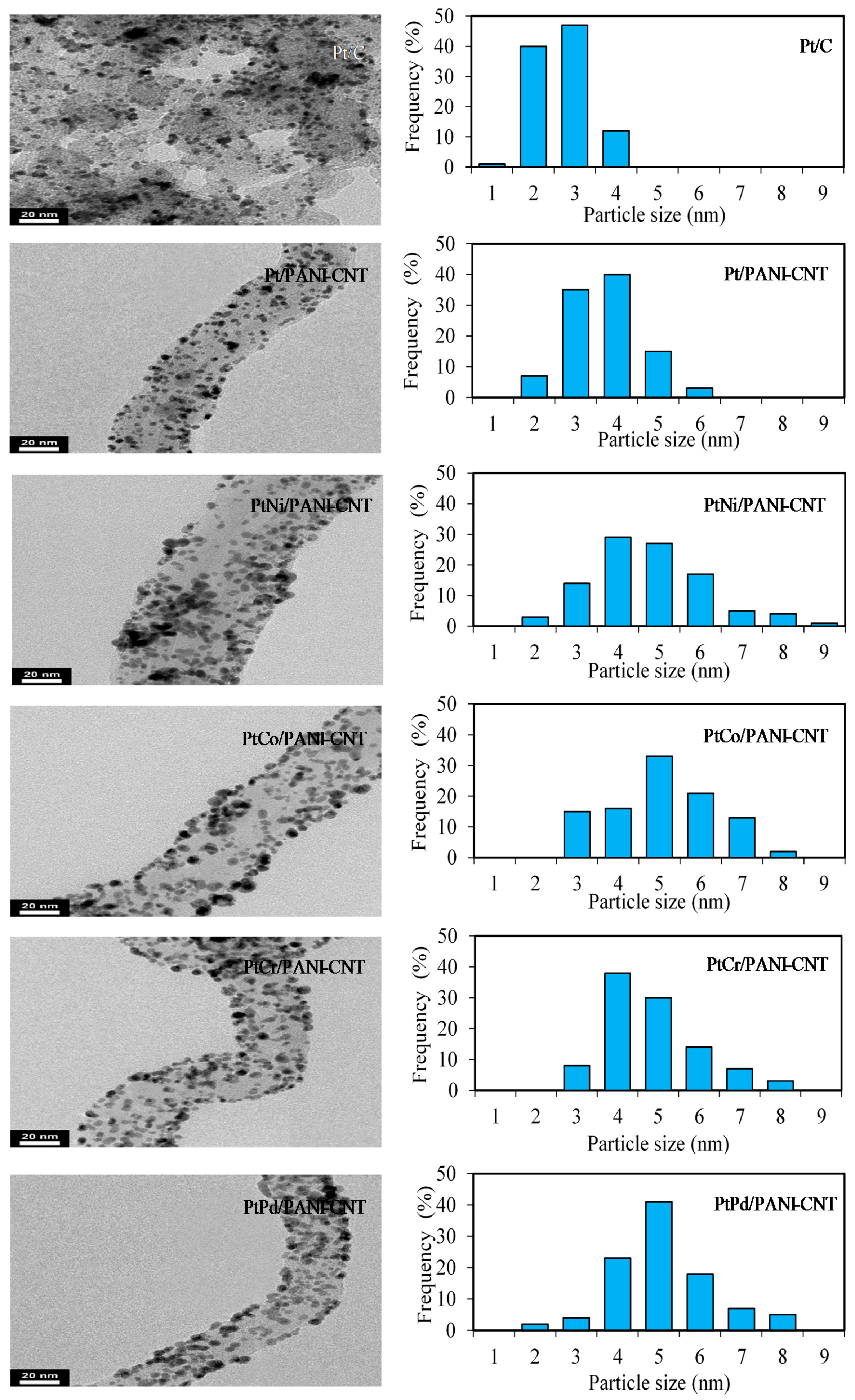
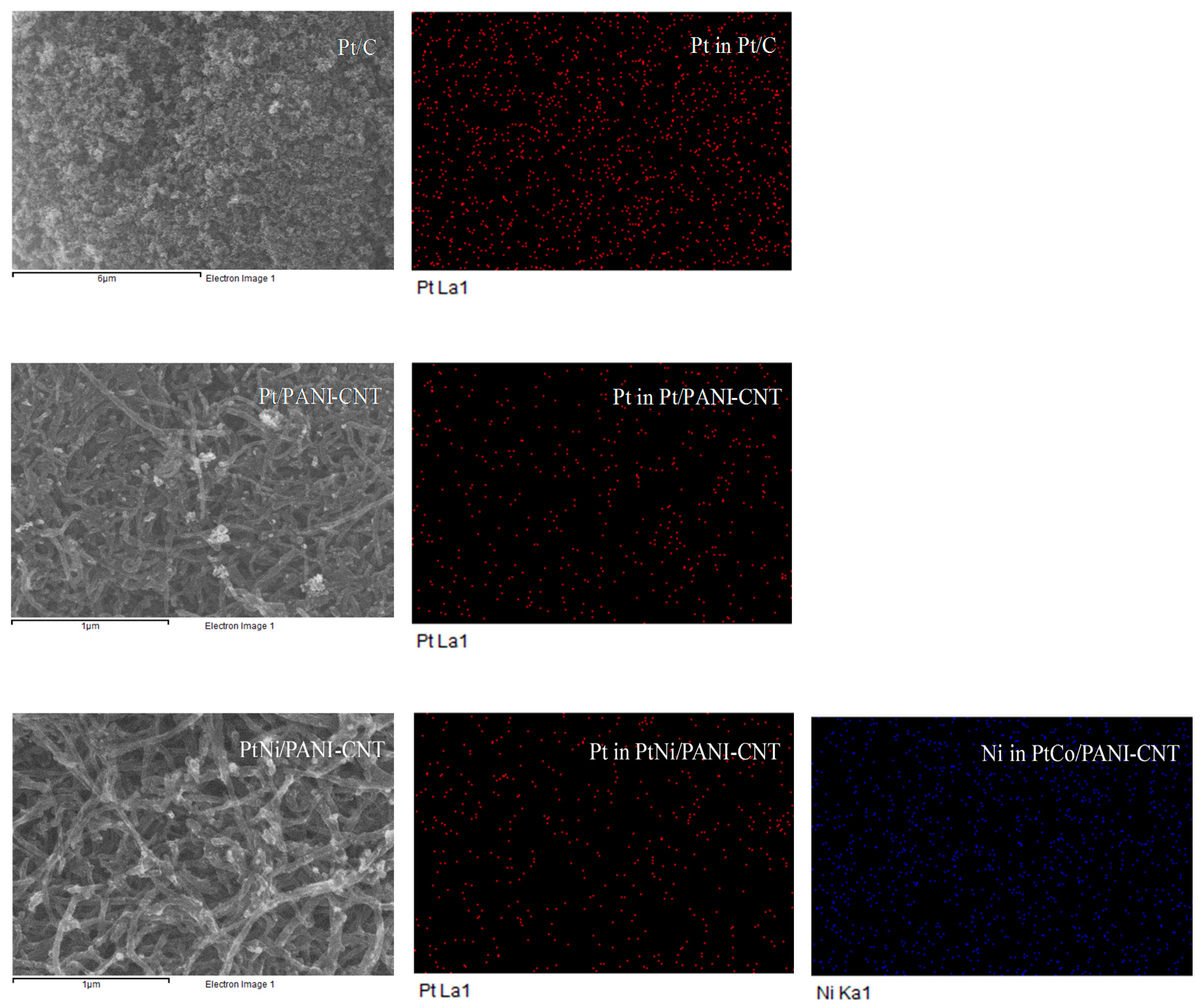
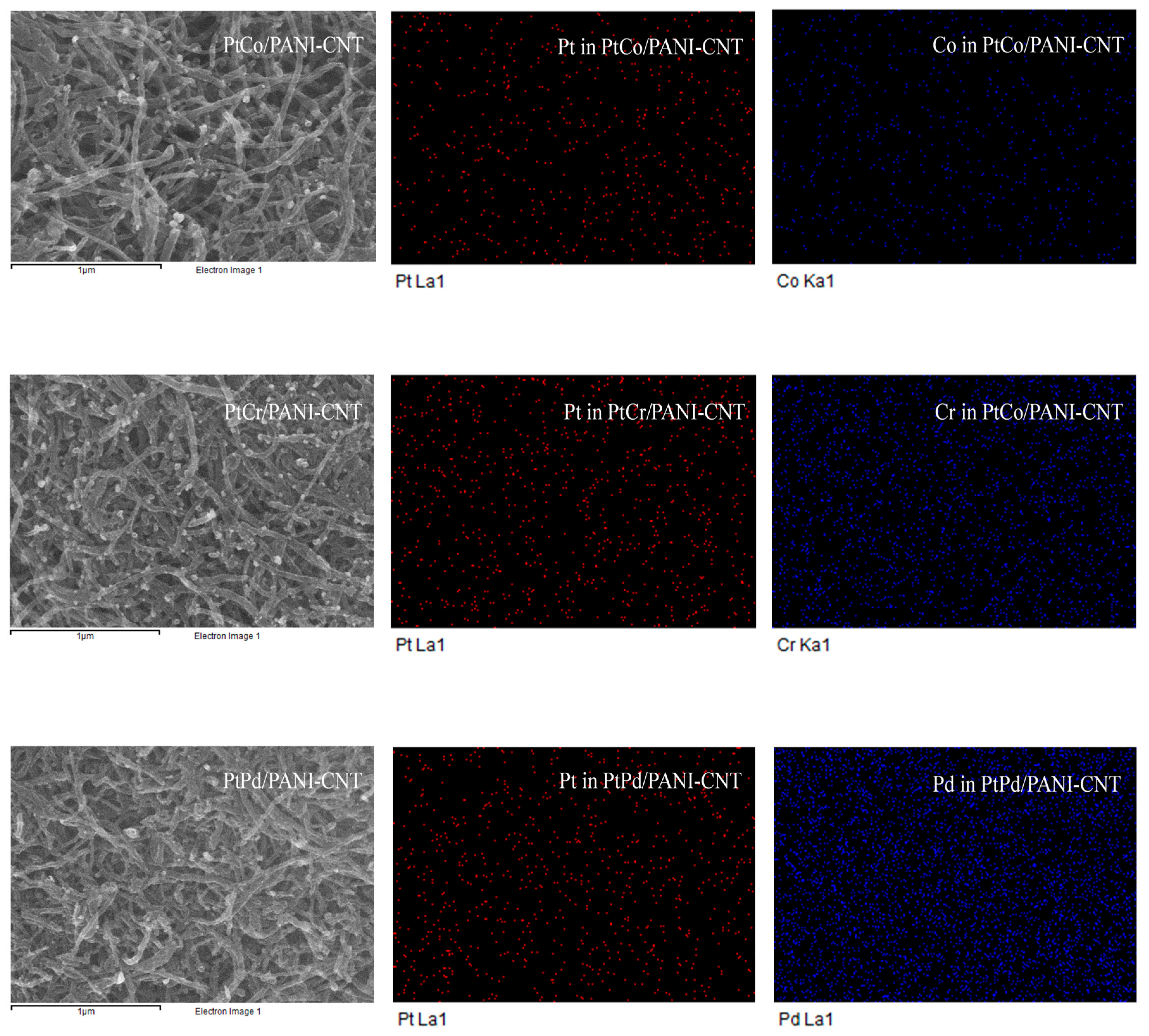
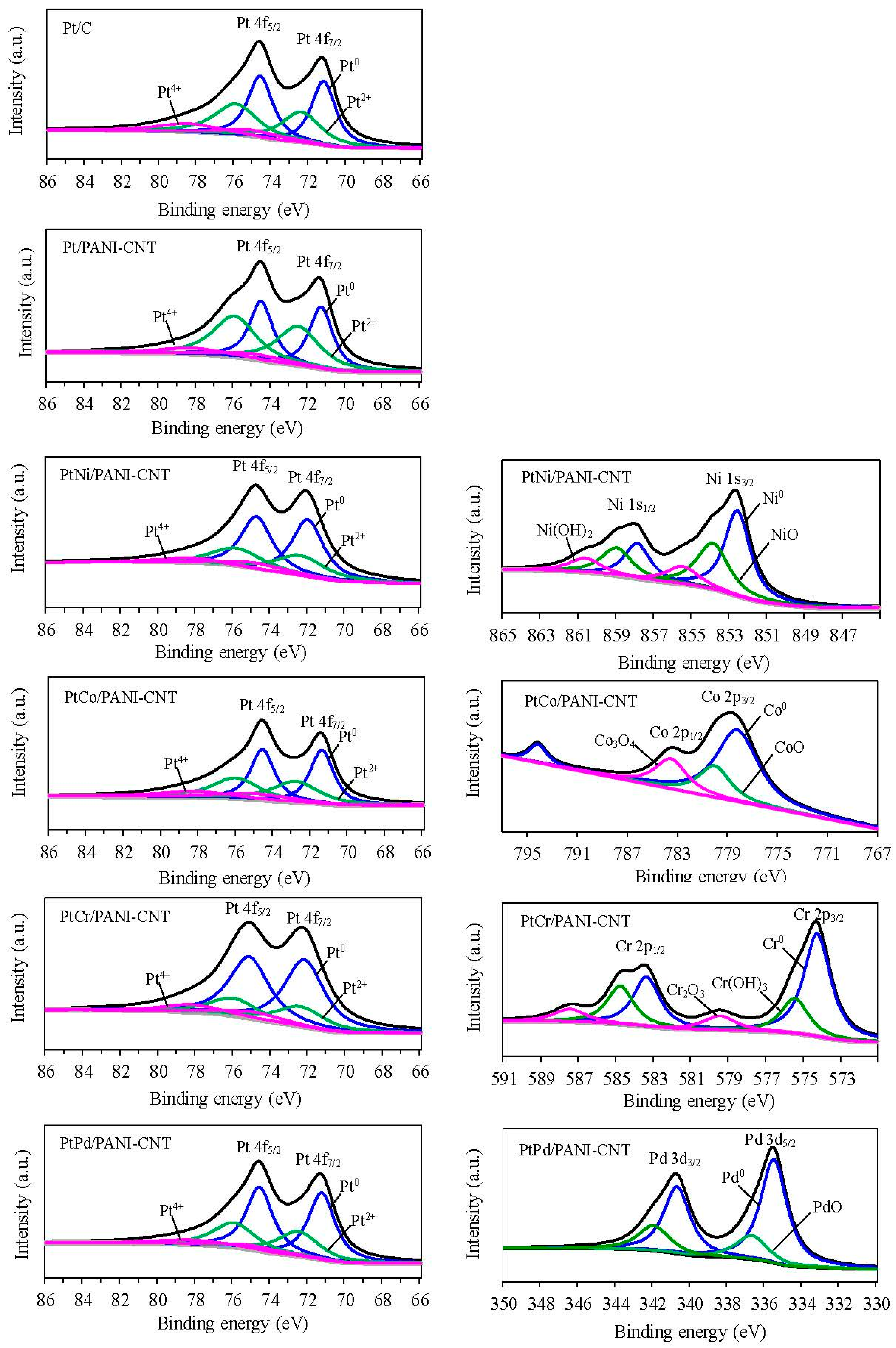
| Catalyst | Lattice Parameter (nm) | Crystallite Size (nm) | Particle Size (nm) | Dispersion (NS/NT) (%) a | Pt:M (Atomic Ratio) b | Metal Loading (wt %) b | Electrode Conductivity (S/cm) | ESA (m2/gPt) |
|---|---|---|---|---|---|---|---|---|
| Pt/C | 0.3924 | 3.43 | 3.69 ± 0.56 | 32.76 | 100.00:0 | 17.4 | 18.21 ± 0.001 | 72.44 |
| Pt/PANI-CNT | 0.3923 | 6.29 | 4.75 ± 0.85 | 26.14 | 100.00:0 | 16.4 | 22.80 ± 0.001 | 69.77 |
| PtNi/PANI-CNT | 0.3909 | 6.76 | 5.80 ± 1.36 | 21.73 | 72.84:27.16 | 15.2 | 22.54 ± 0.001 | 34.44 |
| PtCo/PANI-CNT | 0.3899 | 6.58 | 6.08 ± 1.29 | 20.86 | 74.63:25.37 | 16.6 | 22.61 ± 0.001 | 44.87 |
| PtCr/PANI-CNT | 0.3909 | 6.72 | 5.82 ± 1.15 | 21.71 | 72.56:27.44 | 17.8 | 22.01 ± 0.003 | 66.88 |
| PtPd/PANI-CNT | 0.3901 | 6.33 | 6.06 ± 1.16 | 20.88 | 71.32:28.68 | 17.5 | 22.57 ± 0.002 | 68.36 |
| Catalyst | Pt Species | B.E. (eV) | Relative Peak Area (%) |
|---|---|---|---|
| Pt/C | Pt0 | 71.2 | 46.94 |
| Pt2+ | 72.4 | 38.53 | |
| Pt4+ | 75.0 | 14.53 | |
| Pt/PANI-CNT | Pt0 | 71.3 | 51.14 |
| Pt2+ | 72.5 | 38.76 | |
| Pt4+ | 74.9 | 10.10 | |
| PtNi/PANI-CNT | Pt0 | 72.0 | 56.82 |
| Pt2+ | 72.4 | 31.69 | |
| Pt4+ | 74.9 | 11.49 | |
| Ni0 | 852.6 | 48.37 | |
| NiO | 853.9 | 35.99 | |
| Ni(OH)2 | 855.5 | 15.64 | |
| PtCo/PANI-CNT | Pt0 | 71.0 | 57.54 |
| Pt2+ | 72.4 | 31.48 | |
| Pt4+ | 74.9 | 11.98 | |
| Co | 778.2 | 54.67 | |
| CoO | 783.5 | 11.98 | |
| Co3O4 | 780 | 33.35 | |
| PtCr/PANI-CNT | Pt0 | 72.2 | 57.81 |
| Pt2+ | 72.7 | 31.75 | |
| Pt4+ | 74.9 | 10.44 | |
| Cr | 574.3 | 59.37 | |
| Cr(OH)3 | 575.5 | 28.49 | |
| Cr2O3 | 579.5 | 12.15 | |
| PtPd/PANI-CNT | Pt0 | 71.3 | 60.12 |
| Pt2+ | 72.5 | 30.80 | |
| Pt4+ | 75.0 | 9.08 | |
| Pd | 335.5 | 74.86 | |
| PdO | 336.7 | 25.14 |
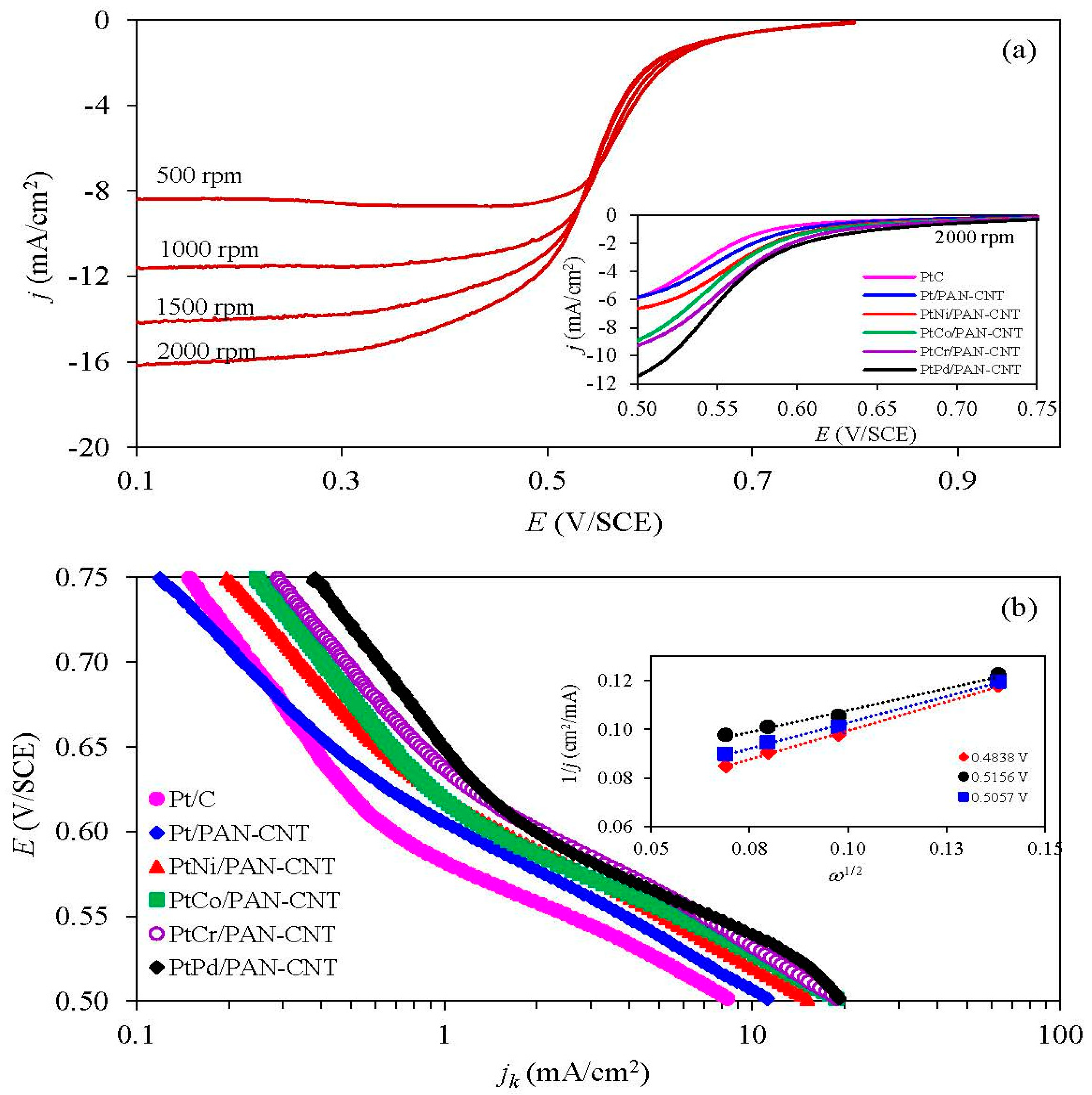
| Catalyst | Parameters Obtained from the NLLS Model | |||||||
|---|---|---|---|---|---|---|---|---|
| j0.9 V (mA/cm2) | j0.6 V (mA/cm2) | −b (mV/dec) | j0 (mA/cm2) | R (Ω cm2) | n (cm2/mA) | m (mV) | R2 | |
| Pt/C | 3.2 | 334 | 57.7 | 2.30 × 10−5 | 0.5400 | 0.0036 | 2.80 | 0.9998 |
| Pt/PANI-CNT | 3.6 | 350 | 57.0 | 2.47 × 10−5 | 0.5225 | 0.0034 | 3.55 | 0.9995 |
| PtNi/PANI-CNT | 4.4 | 364 | 57.0 | 2.68 × 10−5 | 0.5200 | 0.0032 | 3.40 | 0.9999 |
| PtCo/PANI-CNT | 4.8 | 378 | 56.0 | 2.65 × 10−5 | 0.5190 | 0.0031 | 3.24 | 0.9996 |
| PtCr/PANI-CNT | 5.2 | 382 | 57.5 | 4.38 × 10−5 | 0.5175 | 0.0029 | 3.20 | 0.9997 |
| PtPd/PANI-CNT | 5.4 | 402 | 57.5 | 5.14 × 10−5 | 0.5150 | 0.0028 | 3.60 | 0.9995 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewsai, D.; Hunsom, M. Comparative Study of the ORR Activity and Stability of Pt and PtM (M = Ni, Co, Cr, Pd) Supported on Polyaniline/Carbon Nanotubes in a PEM Fuel Cell. Nanomaterials 2018, 8, 299. https://doi.org/10.3390/nano8050299
Kaewsai D, Hunsom M. Comparative Study of the ORR Activity and Stability of Pt and PtM (M = Ni, Co, Cr, Pd) Supported on Polyaniline/Carbon Nanotubes in a PEM Fuel Cell. Nanomaterials. 2018; 8(5):299. https://doi.org/10.3390/nano8050299
Chicago/Turabian StyleKaewsai, Duanghathai, and Mali Hunsom. 2018. "Comparative Study of the ORR Activity and Stability of Pt and PtM (M = Ni, Co, Cr, Pd) Supported on Polyaniline/Carbon Nanotubes in a PEM Fuel Cell" Nanomaterials 8, no. 5: 299. https://doi.org/10.3390/nano8050299
APA StyleKaewsai, D., & Hunsom, M. (2018). Comparative Study of the ORR Activity and Stability of Pt and PtM (M = Ni, Co, Cr, Pd) Supported on Polyaniline/Carbon Nanotubes in a PEM Fuel Cell. Nanomaterials, 8(5), 299. https://doi.org/10.3390/nano8050299




