Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application
Abstract
:1. Introduction
2. Results and Discussion
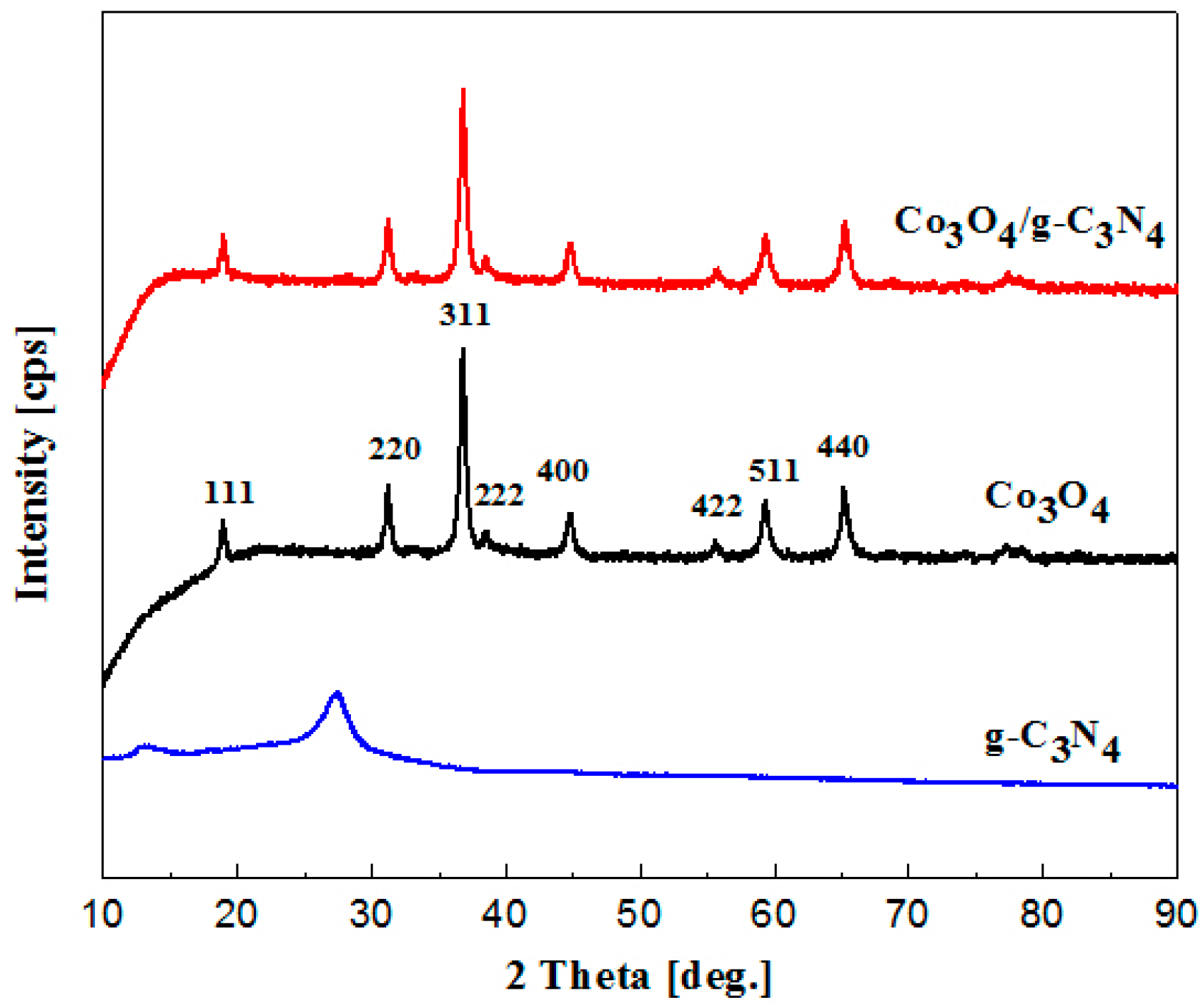
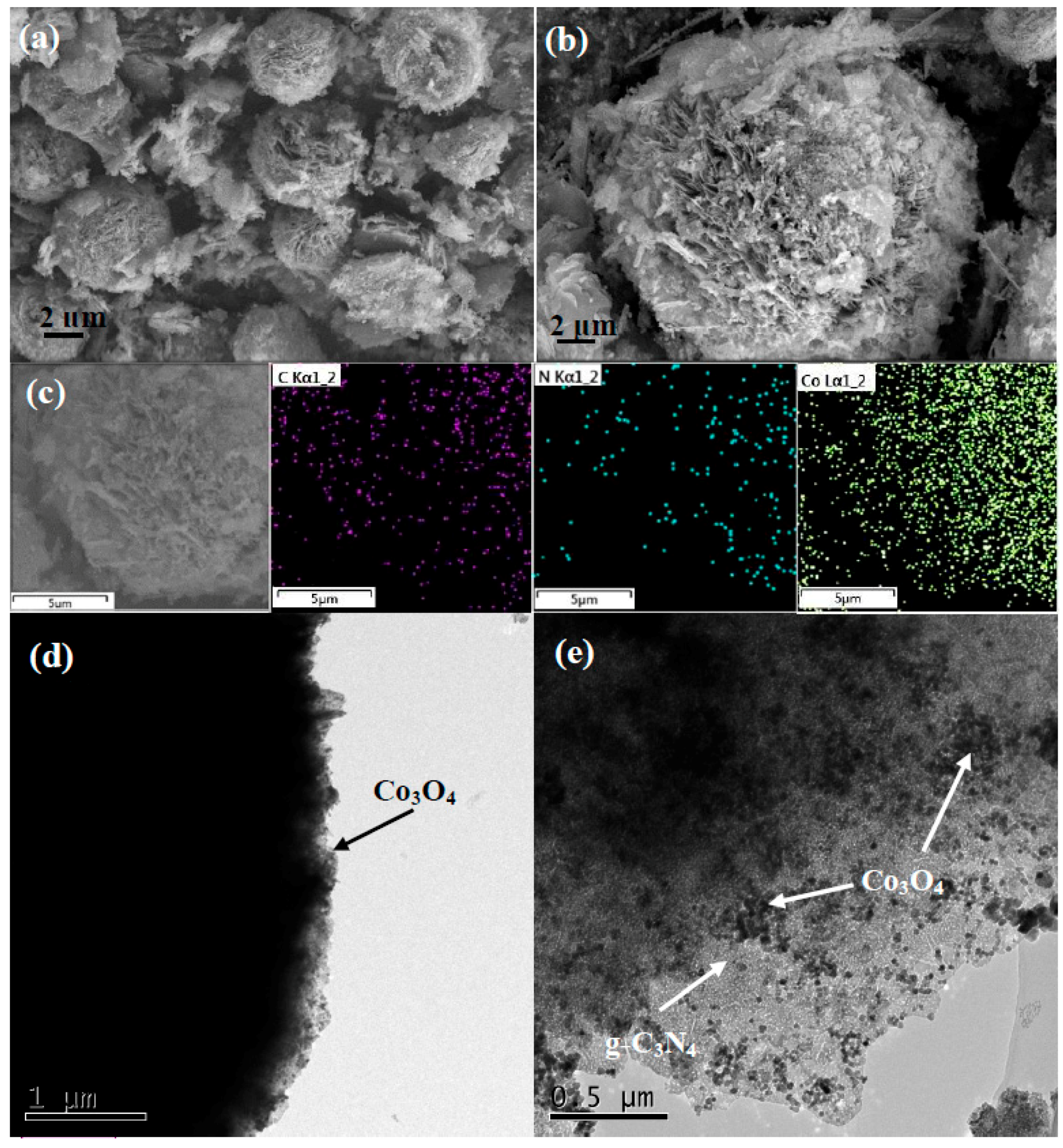
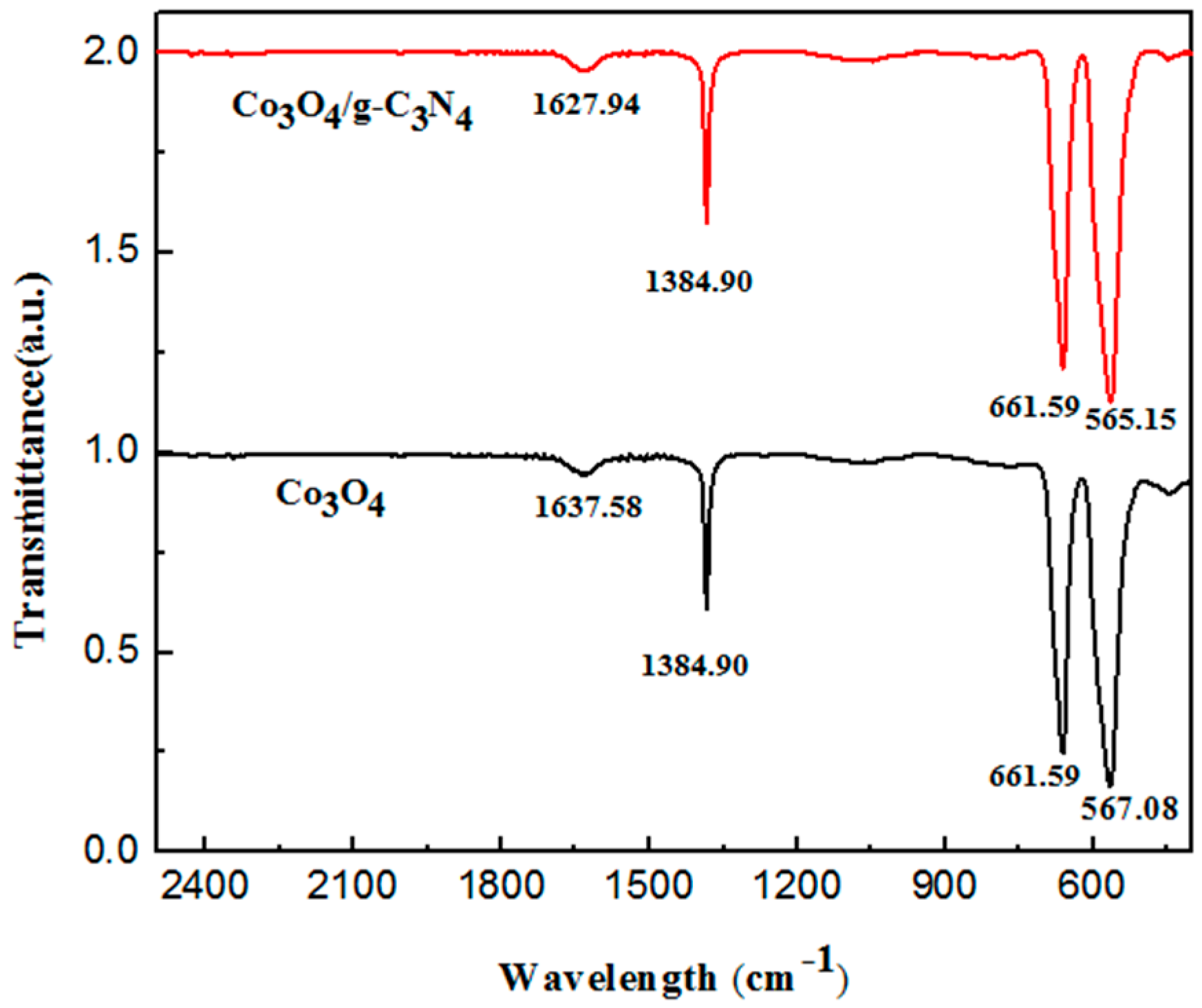
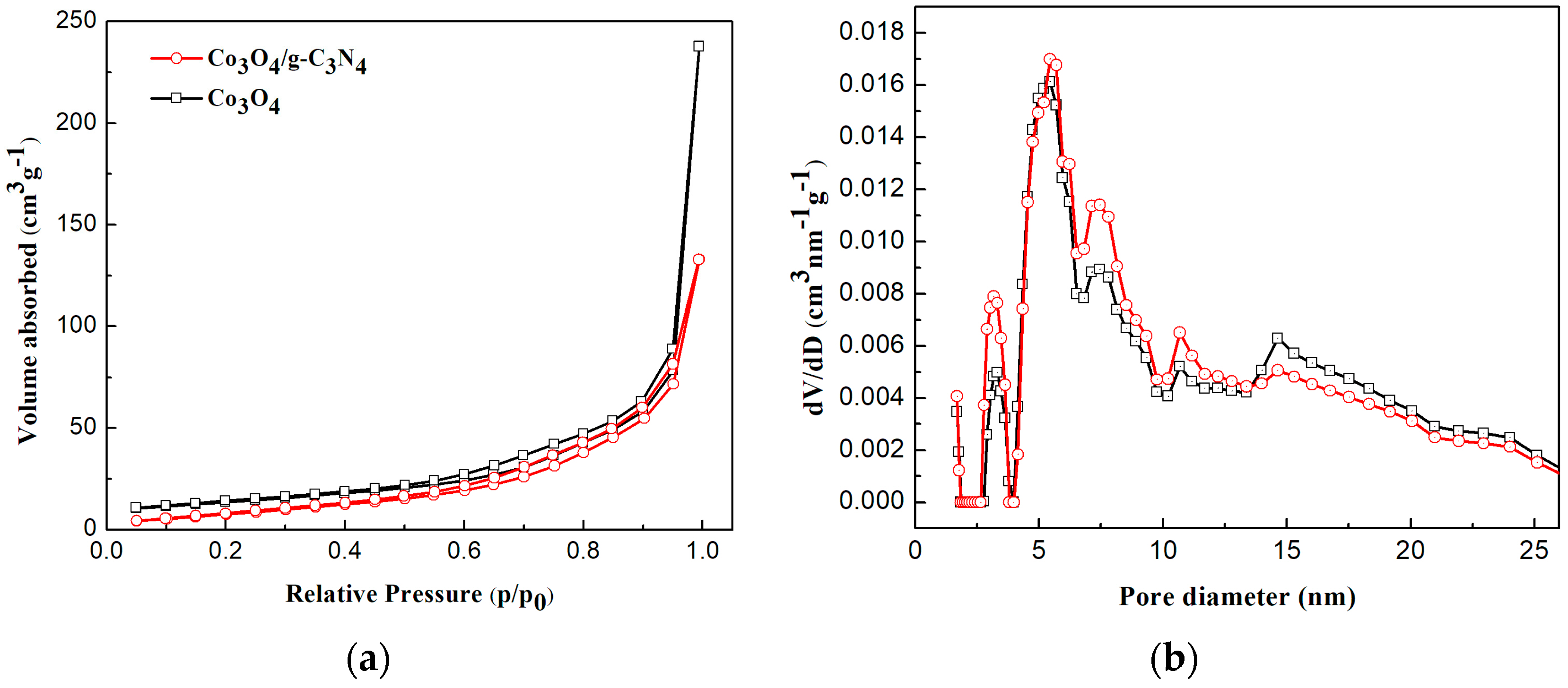
2.1. Sample Characterization
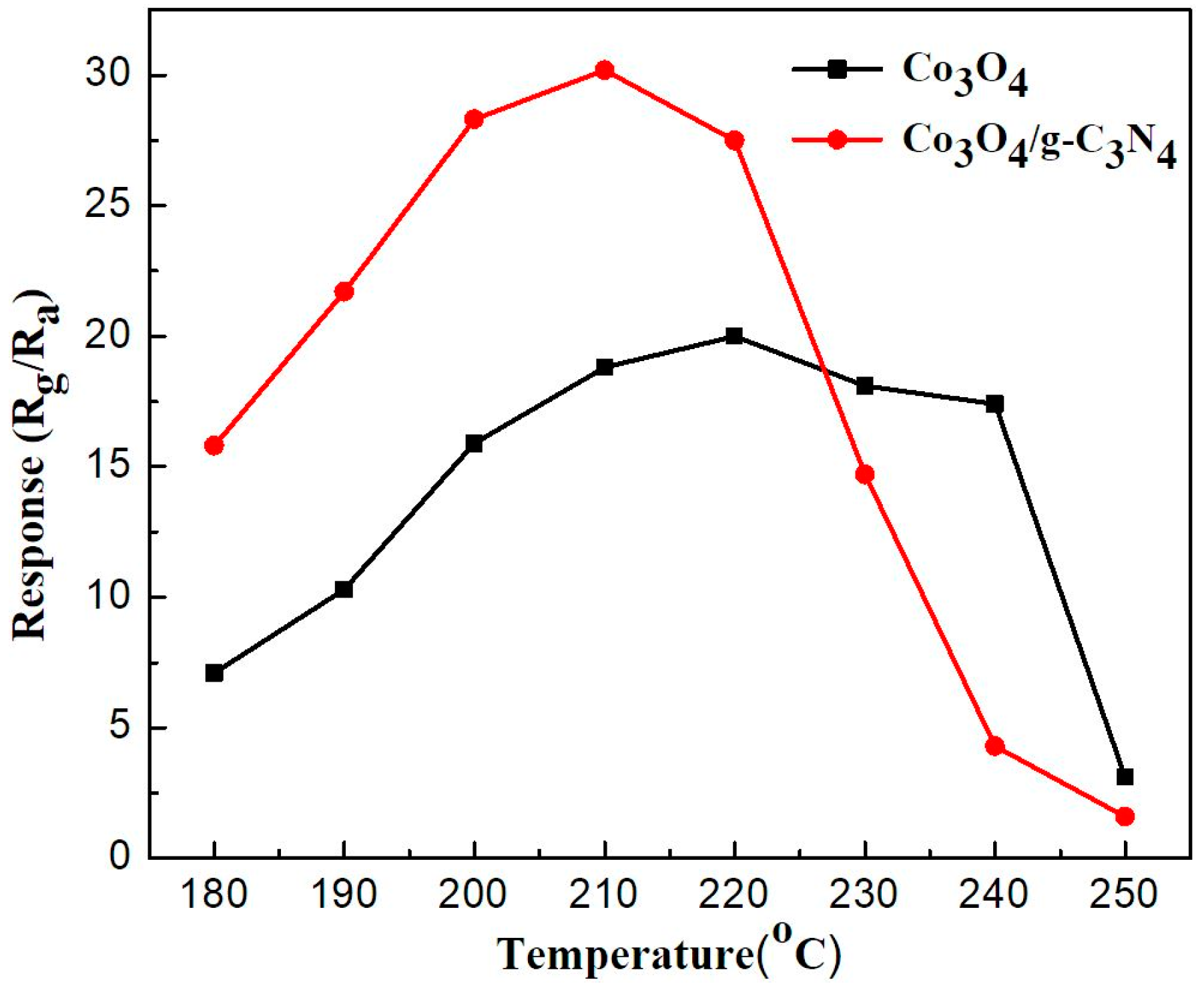
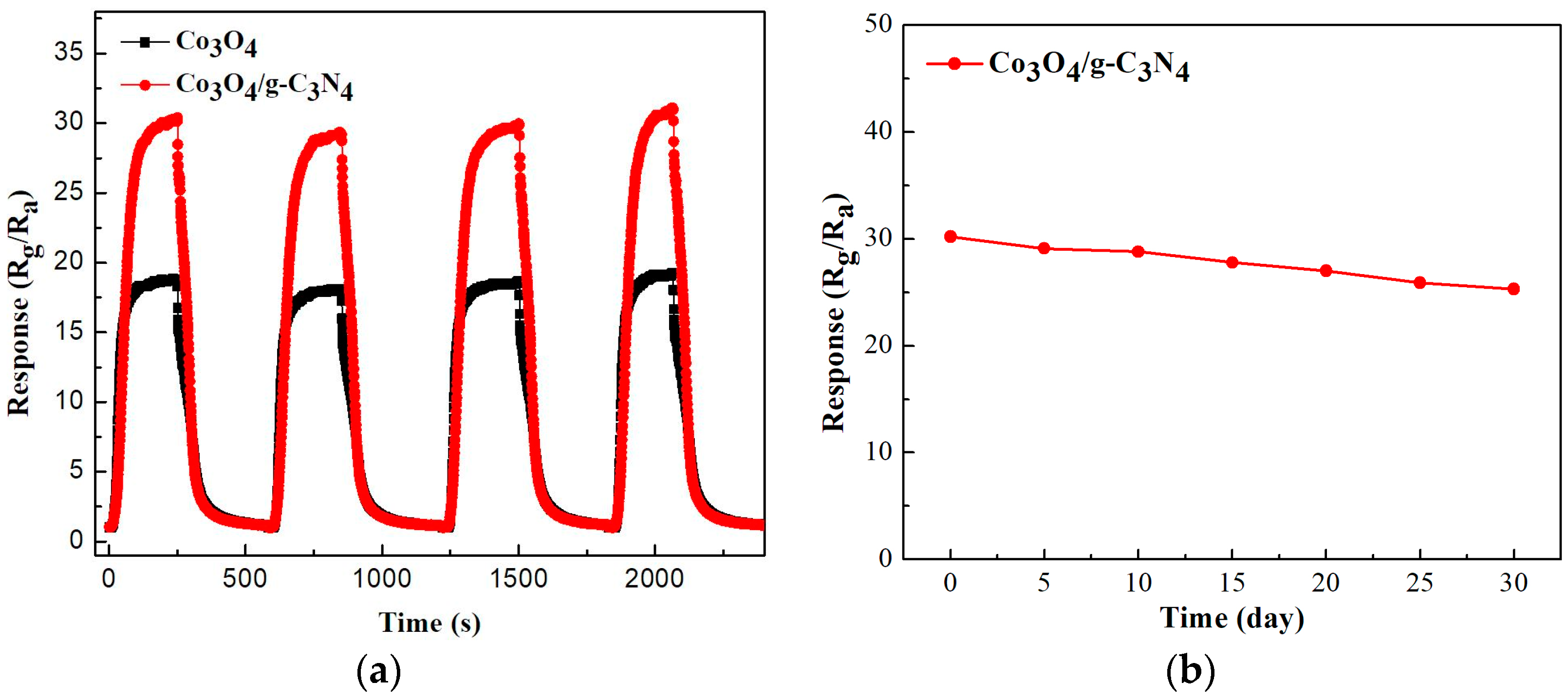
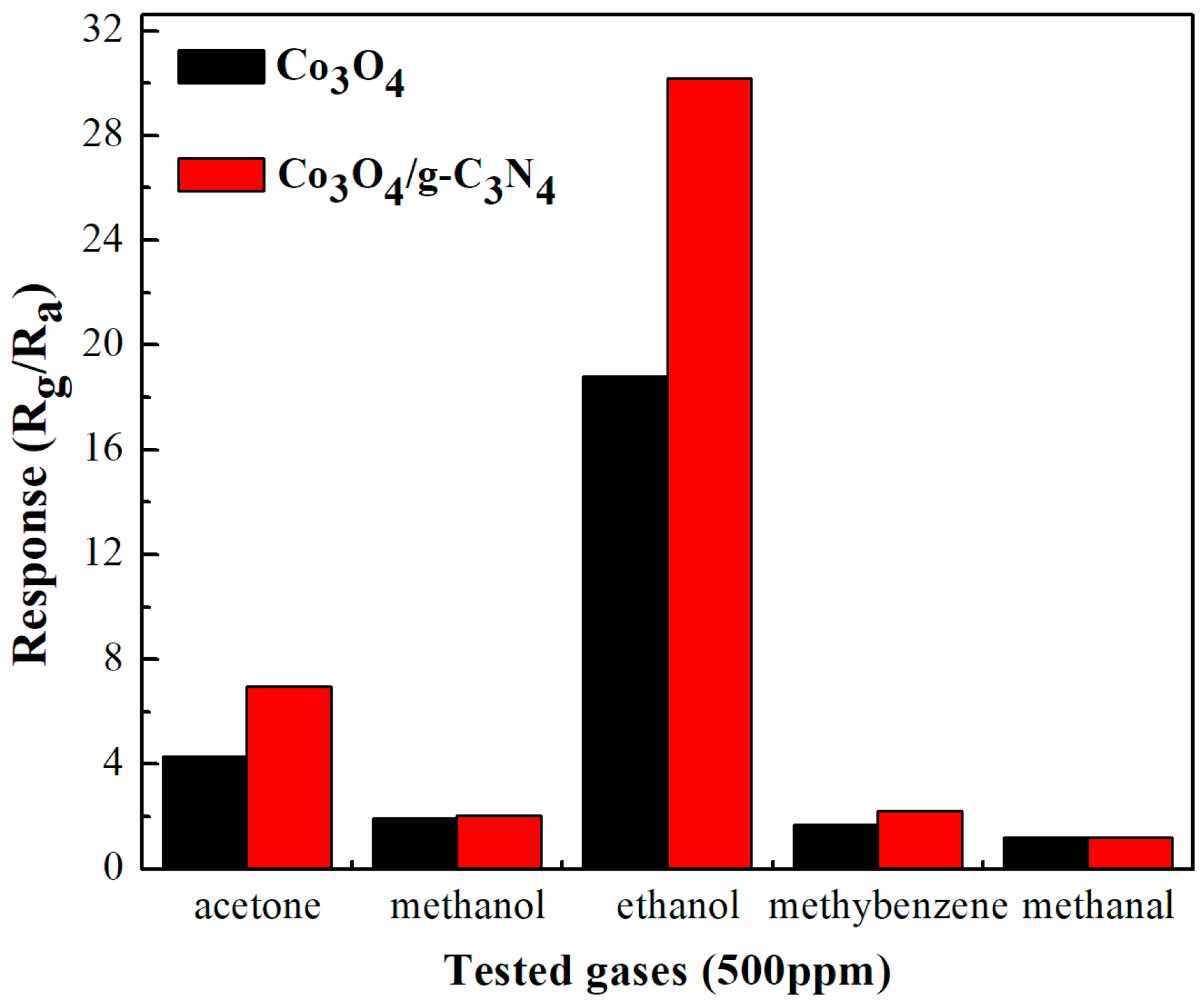
2.2. Gas-Sensing Performance
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterizations
3.3. Gas Sensor Fabrication and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konova, P.; Naydenov, A.; Venkov, C.; Mehandjiev, D.; Andreeva, D.; Tabakova, T. Activity and deactivation of Au/TiO2, catalyst in CO oxidation. J. Mol. Catal. A Chem. 2004, 213, 235–240. [Google Scholar] [CrossRef]
- Zeng, B.R.; Zhang, L.C.; Wu, L.Q.; Su, Y.Y.; Lv, Y. Enclosed hollow tubular ZnO: Controllable synthesis and their high performance cataluminescence gas sensing of H2S. Sens. Actuators B 2017, 242, 1086–1094. [Google Scholar] [CrossRef]
- Kumar, R.; Aldossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, G.W. Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Göpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, I.S.; Kim, S.J.; Lee, C.Y.; Lee, J.H. CuO nanowire gas sensors for air quality control in automotive cabin. Sens. Actuators B 2008, 135, 298–303. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Nguyen, H.; Elsafty, S.A. Meso-and macroporous Co3O4 nanorods for effective VOC gas sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C.; Gong, J.; Li, Q.; Li, G. α-Fe2O3 nanorings prepared by a microwave-assisted hydrothermal process and their sensing properties. Adv. Mater. 2007, 19, 2324–2329. [Google Scholar] [CrossRef]
- Dirksen, J.A.; Duval, K.; Ring, T.A. NiO thin-film formaldehyde gas sensor. Sens. Actuators B 2001, 80, 106–115. [Google Scholar] [CrossRef]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. Ordered mesoporous In2O3: Synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar] [CrossRef]
- Li, X.L.; Lou, T.J.; Sun, X.M.; Li, Y.D. Highly sensitive WO3 hollow-sphere gas sensors. Inorg. Chem. 2004, 43, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Garciasanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal effects associated with the Raman spectroscopy of WO3 gas-sensor materials. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.C.; Cao, J.L.; Sun, G.; Zhang, Z.Y. Synthesis of Co3O4 nanoparticles via the CTAB-assisted method. Mater. Lett. 2011, 65, 222–224. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.A.; Potdar, H.S. Highly sensitive and fast responding CO sensor based on Co3O4 nanorods. Talanta 2010, 81, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Gavagnin, M.; Maccato, C.; Sada, C.; Sberveglieri, G.; et al. Plasma enhanced-CVD of undoped and fluorine-doped Co3O4 nanosystems for novel gas sensors. Sens. Actuators B 2011, 160, 79–86. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Gao, Z.F.; Liu, C.; Zhang, Y.H.; Tu, Z.Q.; Yang, X.P.; Yang, F.; Wen, Z.; Zhu, L.P.; Liu, R.; et al. Synthesis of TiO2 decorated Co3O4 acicular nanowire arrays and their application as an ethanol sensor. J. Mater. Chem. A 2015, 3, 2794–2801. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gueorguiev, G.K.; Broitman, E.; Furlan, A.; Stafström, S.; Hultman, L. Dangling bond energetics in carbon nitride and phosphorus carbide thin films with fullerene-like and amorphous structure. Chem. Phys. Lett. 2009, 482, 110–113. [Google Scholar] [CrossRef]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water adsorption on fullerene-like carbon nitride overcoats. Thin Solid Films 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.L.; Qin, C.; Zhang, B.; Sun, G.; Zhang, Z.Y. Synthesis and enhanced ethanol gas sensing properties of the g-C3N4 nanosheets-decorated tin oxide flower-like nanorods composite. Nanomaterials 2017, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Hu, Y.; Zhao, J.J.; Zeng, L.L.; Tao, X.M.; Chen, W. Holey reduced graphene oxide nanosheets for high performance room temperature gas sensing. J. Mater. Chem. A 2014, 2, 17415–17420. [Google Scholar] [CrossRef]
- Cao, J.L.; Qin, C.; Wang, Y.; Zhang, H.L.; Zhang, B.; Gong, Y.X.; Wang, X.D.; Sun, G.; Bala, H.; Zhang, Z.Y. Synthesis of g-C3N4 nanosheet modified SnO2 composites with improved performance for ethanol gas sensing. RSC Adv. 2017, 7, 25504–25511. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.G.; Wang, X.Y.; Yu, J.; Wang, J.; Tang, Z.N.; Akbar, S.A. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B 2013, 188, 902–908. [Google Scholar] [CrossRef]
- Cao, J.L.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.X.; Zhang, H.L.; Sun, G.; Bala, H.; Zhang, Z.Y. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X. Functional carbon nitride materials-design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; Mcmillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.J.; Zhao, M.G.; Fan, S.S.; Ma, Y.; Liang, J.G.; Wang, X.T.; Song, Y.W.; Chen, S.G. Preparing Co3O4 urchin-like hollow microspheres self-supporting architecture for improved glucose biosensing performance. Sens. Actuators B 2016, 235, 162–169. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Moharram, A.H.; Abdelrahim, M.A. Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal-organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Ryu, W.H.; Kim, S.J.; Cho, H.J.; Kim, I.D. Bi-functional co-sensitization of graphene oxide sheets and Ir nanoparticles on p-type Co3O4 nanofibers for selective acetone detection. J. Mater. Chem. B 2014, 2, 7160–7167. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.B.; Lu, L.; Zeng, H.C. Preparation of nanocomposites of metals, metal oxides, and carbon nanotubes via self-assembly. J. Am. Chem. Soc. 2007, 129, 9401–9409. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, J.W.; Liang, S.M.; Bi, H.P.; Han, Q.F.; Liu, X.H.; Wang, X. Reduced graphene oxide decorated with CuO–ZnO hetero-junctions: Towards high selective gas-sensing property to acetone. J. Mater. Chem. A 2014, 2, 18635–18643. [Google Scholar] [CrossRef]
- Mehrabadi, Z.S.; Ahmadpour, A.; Shahtahmasebi, N.; Mohagheghi, M.M.B. Synthesis and characterization of Cu doped cobalt oxide nanocrystals as methane gas sensors. Phys. Scr. 2011, 84, 015801. [Google Scholar] [CrossRef]
- Cao, J.L.; Gong, Y.X.; Wang, Y.; Zhang, B.; Zhang, H.L.; Sun, G.; Bala, H.; Zhang, Z.Y. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Cao, J.L.; Li, G.J.; Zhang, Z.Y. Cobalt oxide decorated flower-like g-C3N4 hybrid nanomaterials for carbon monoxide oxidation. Surf. Rev. Lett. 2017, 24, 1750058. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Wang, Y.; Sun, G.; Jia, T.; Jia, L.; Zhang, F.; Lin, L.; Zhang, B.; Cao, J.; Zhang, Z. Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application. Nanomaterials 2018, 8, 132. https://doi.org/10.3390/nano8030132
Gong Y, Wang Y, Sun G, Jia T, Jia L, Zhang F, Lin L, Zhang B, Cao J, Zhang Z. Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application. Nanomaterials. 2018; 8(3):132. https://doi.org/10.3390/nano8030132
Chicago/Turabian StyleGong, Yuxiao, Yan Wang, Guang Sun, Tiekun Jia, Lei Jia, Fengmei Zhang, Long Lin, Baoqing Zhang, Jianliang Cao, and Zhanying Zhang. 2018. "Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application" Nanomaterials 8, no. 3: 132. https://doi.org/10.3390/nano8030132
APA StyleGong, Y., Wang, Y., Sun, G., Jia, T., Jia, L., Zhang, F., Lin, L., Zhang, B., Cao, J., & Zhang, Z. (2018). Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application. Nanomaterials, 8(3), 132. https://doi.org/10.3390/nano8030132






