Preparation of ZnO Nanorods/Graphene Composite Anodes for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
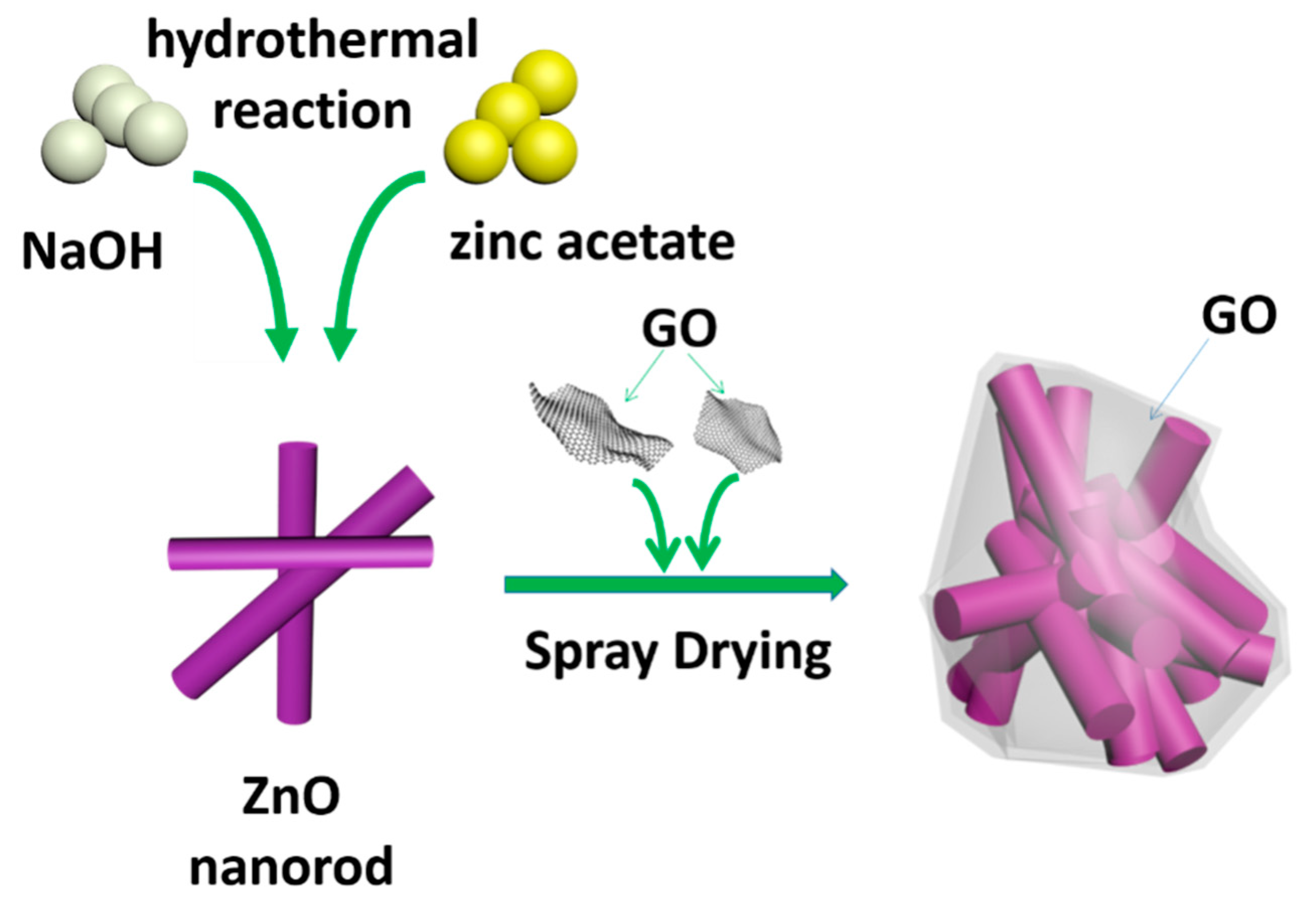
2. Materials and Methods
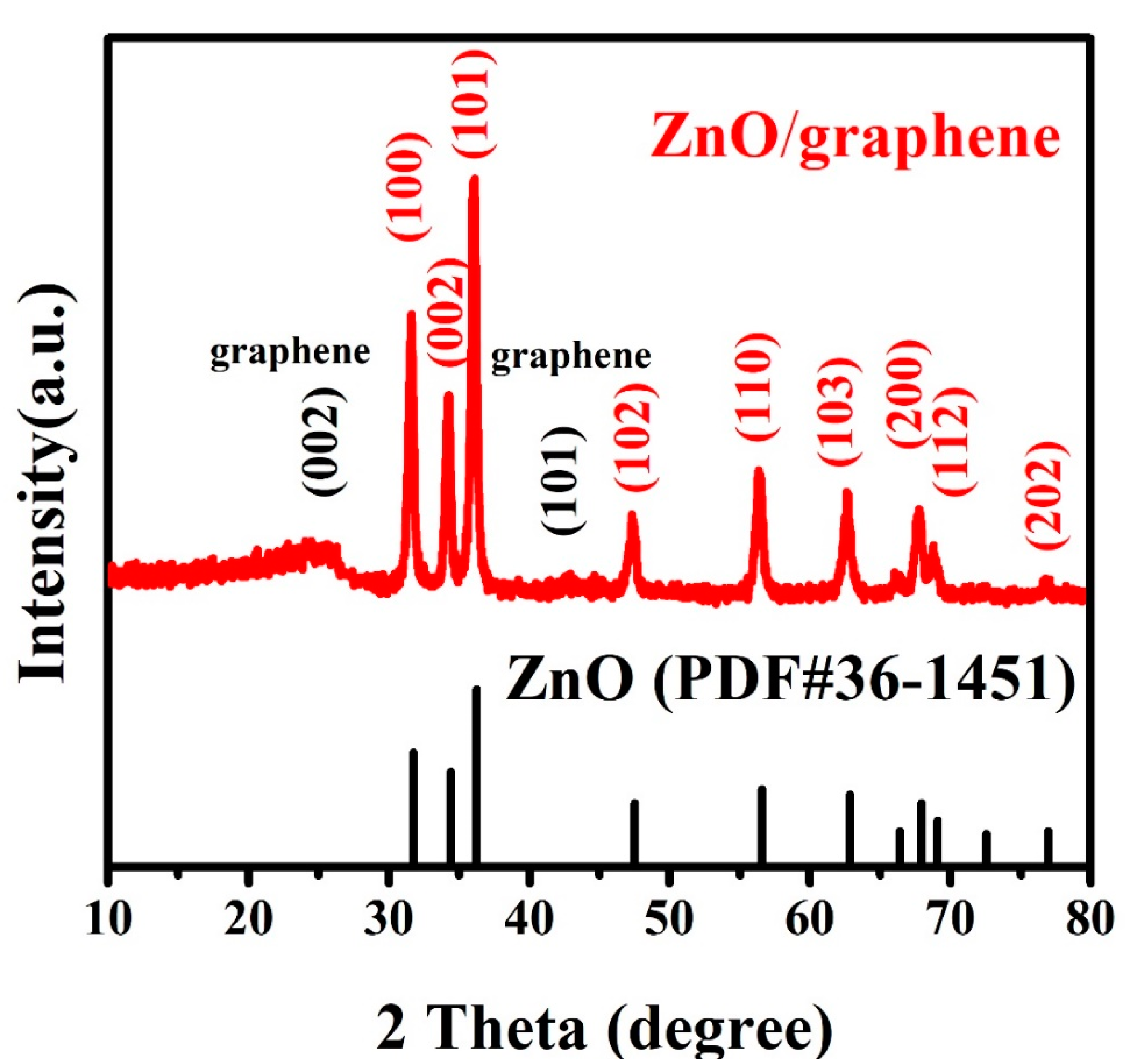
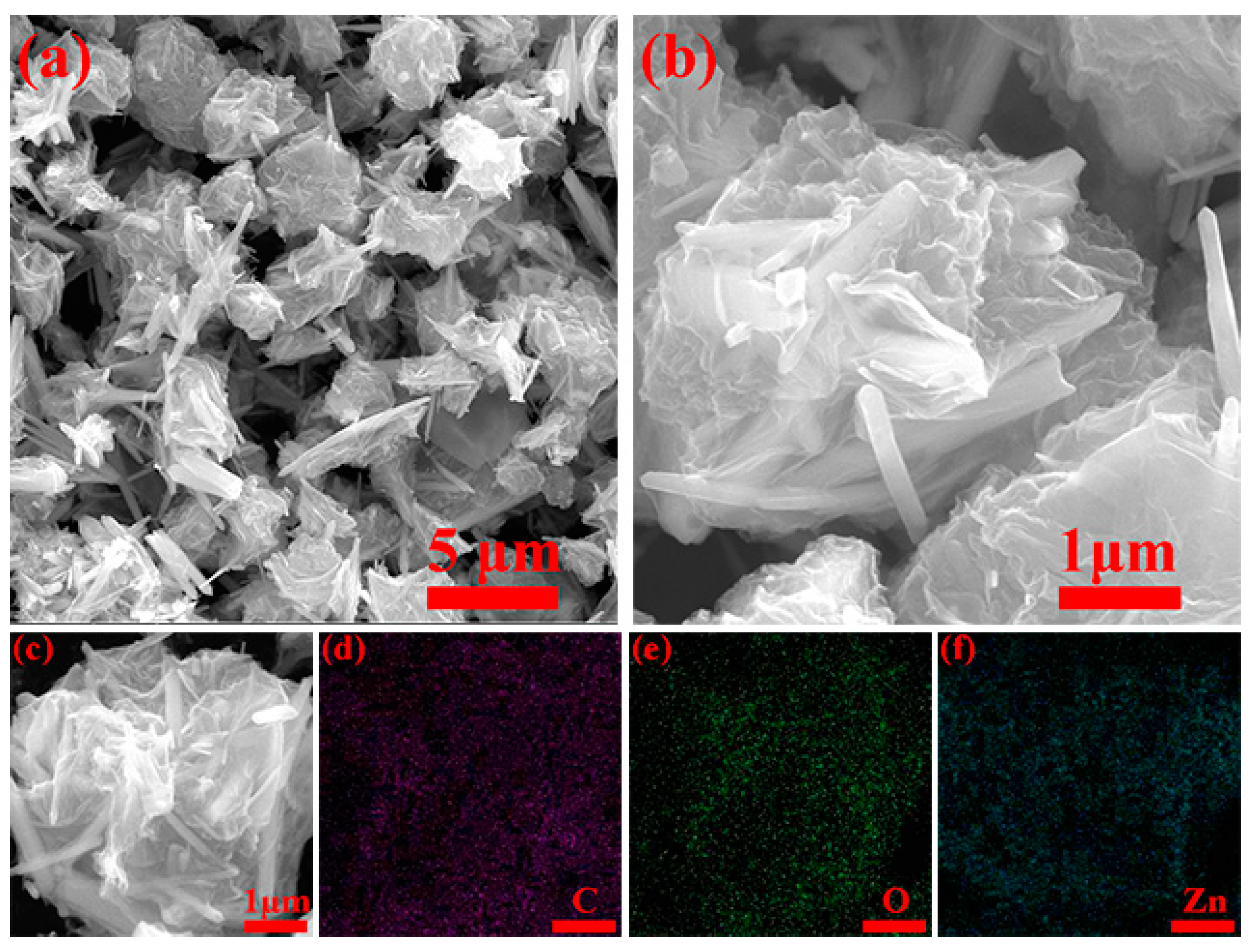
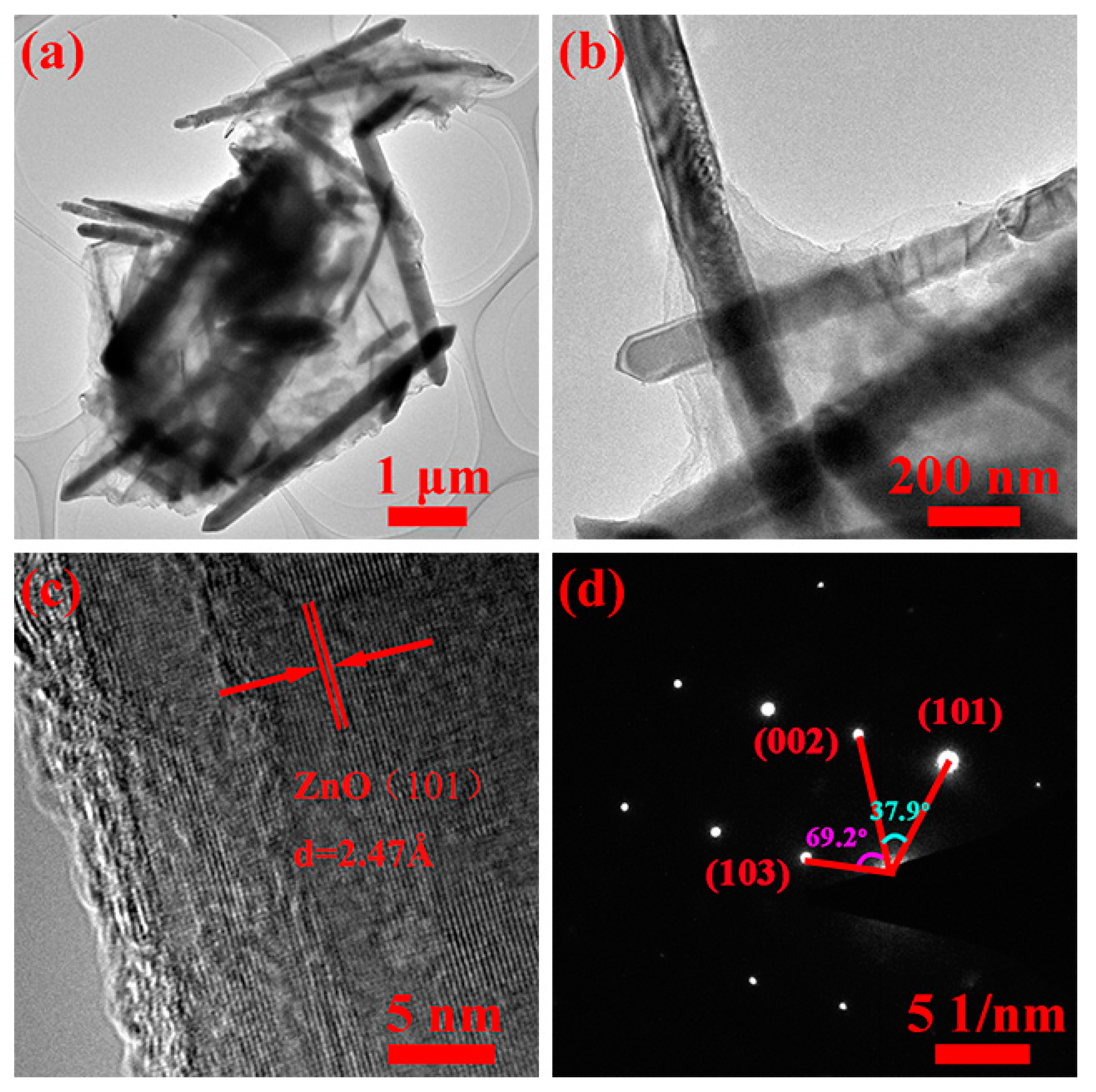
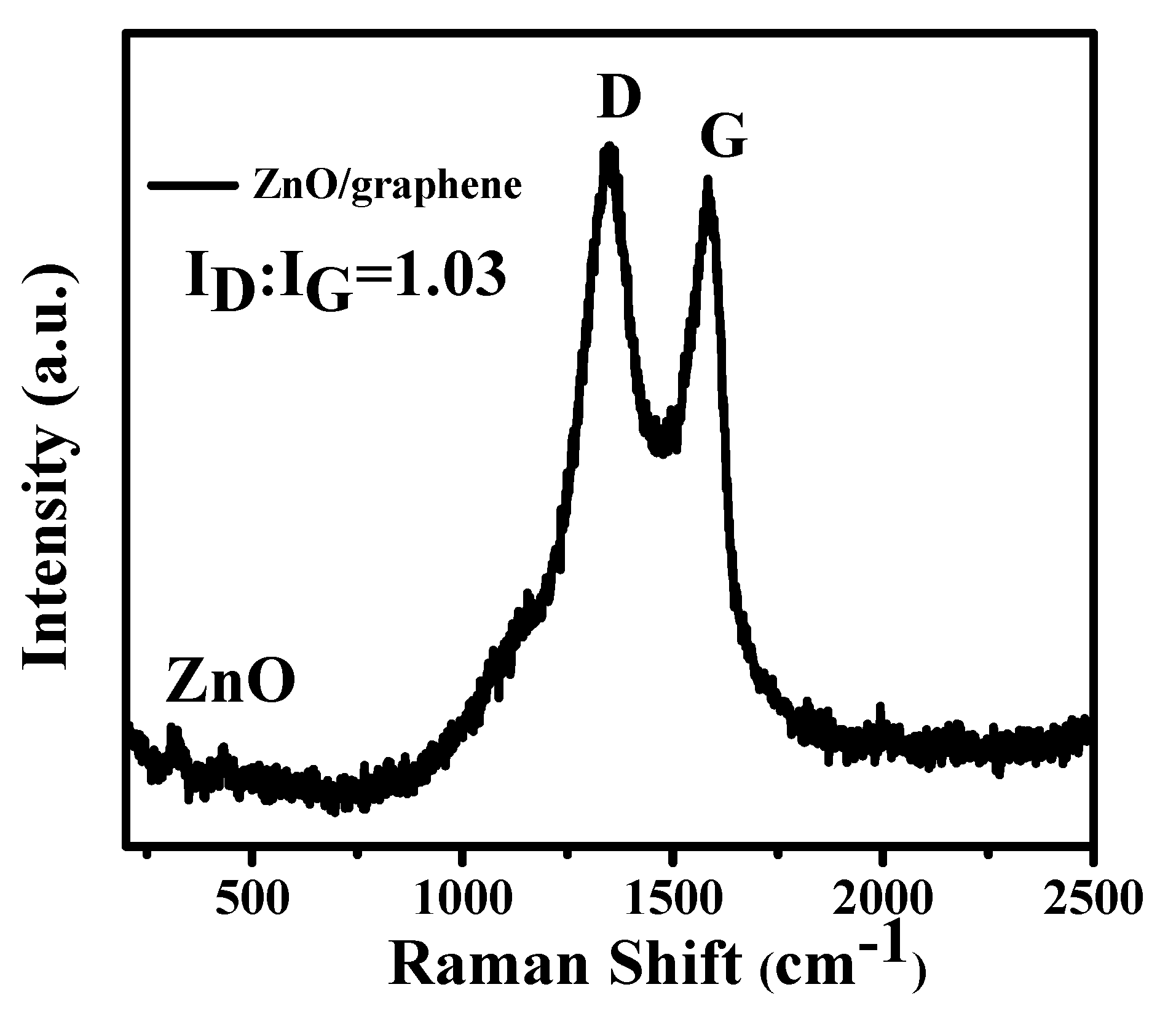
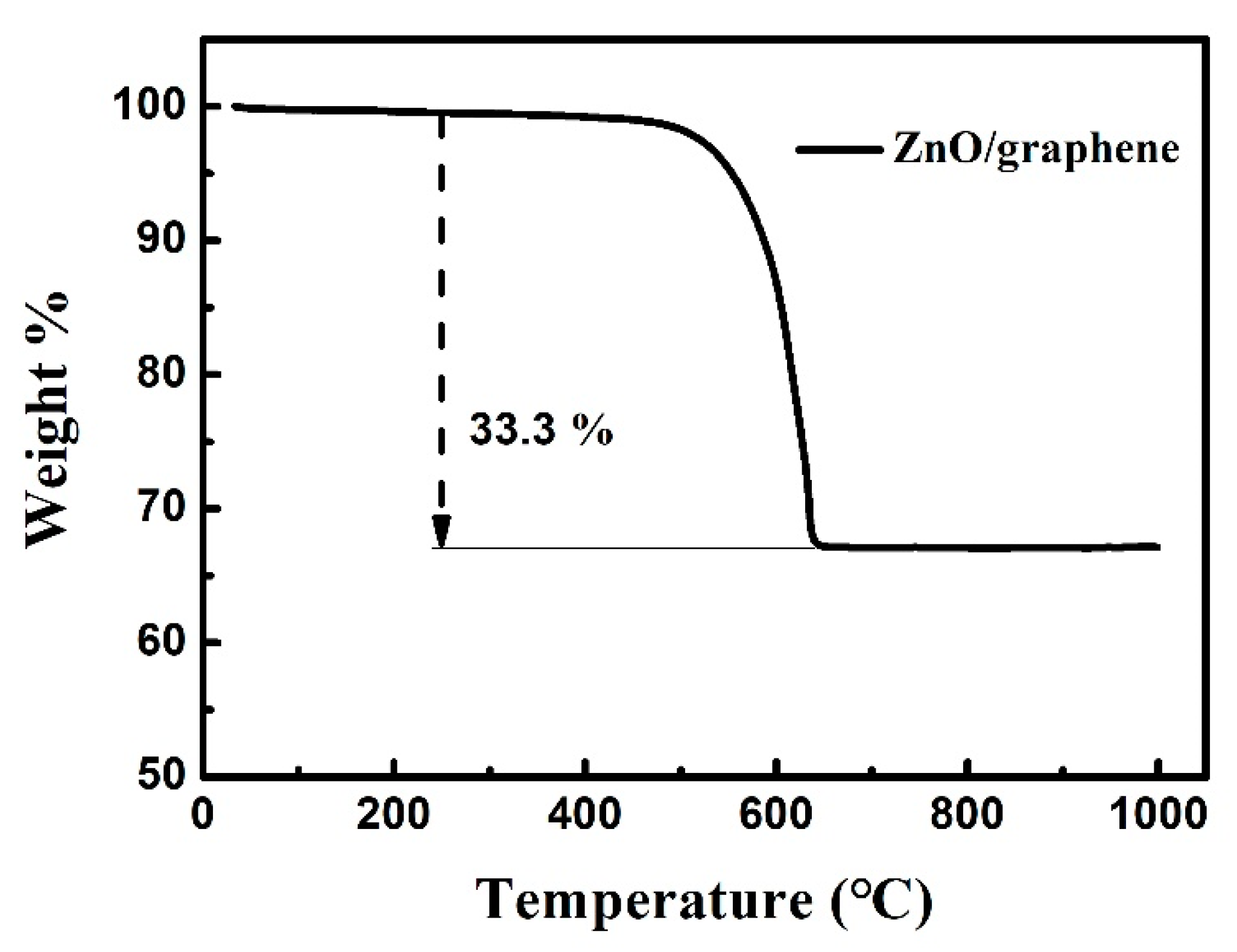
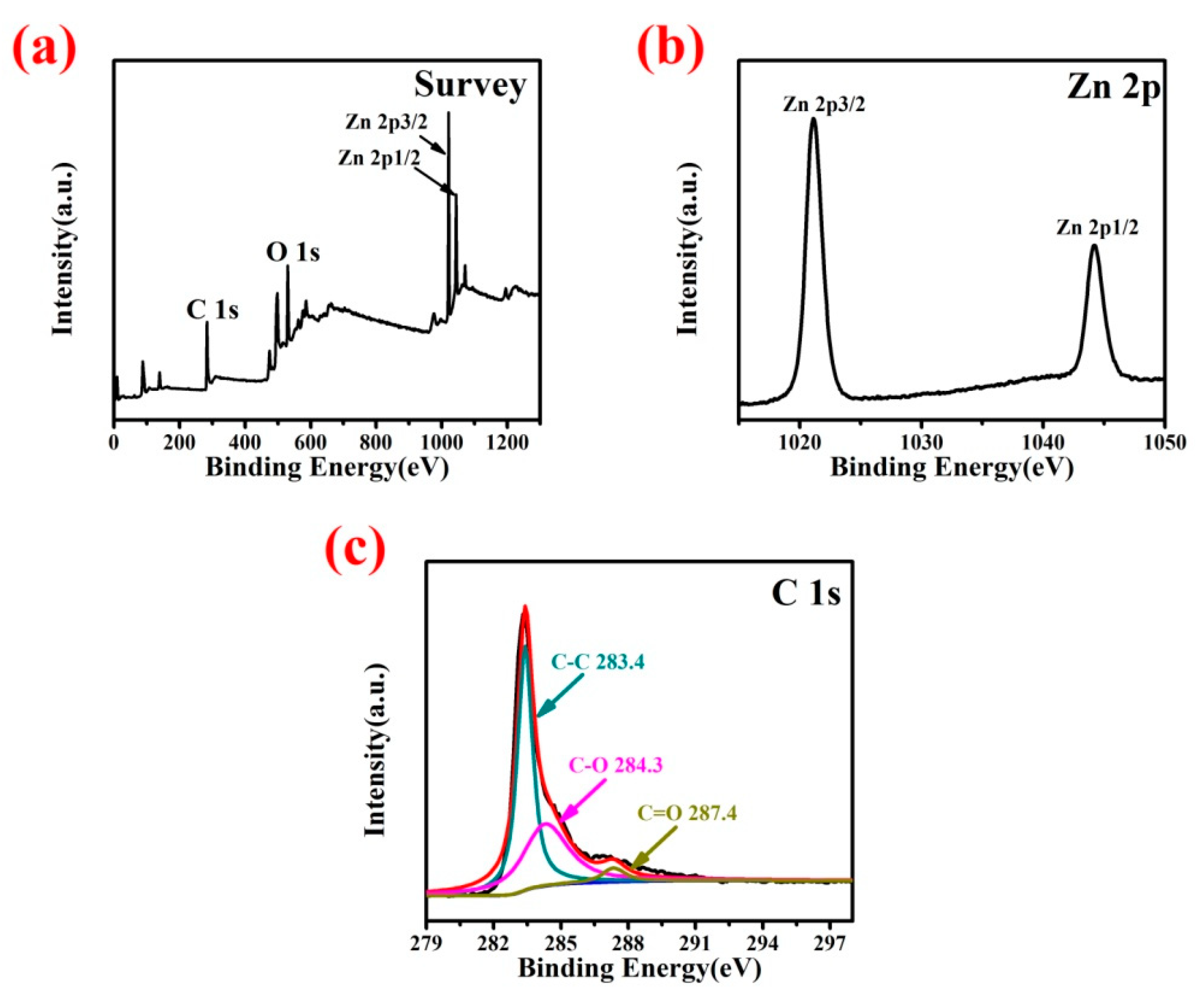
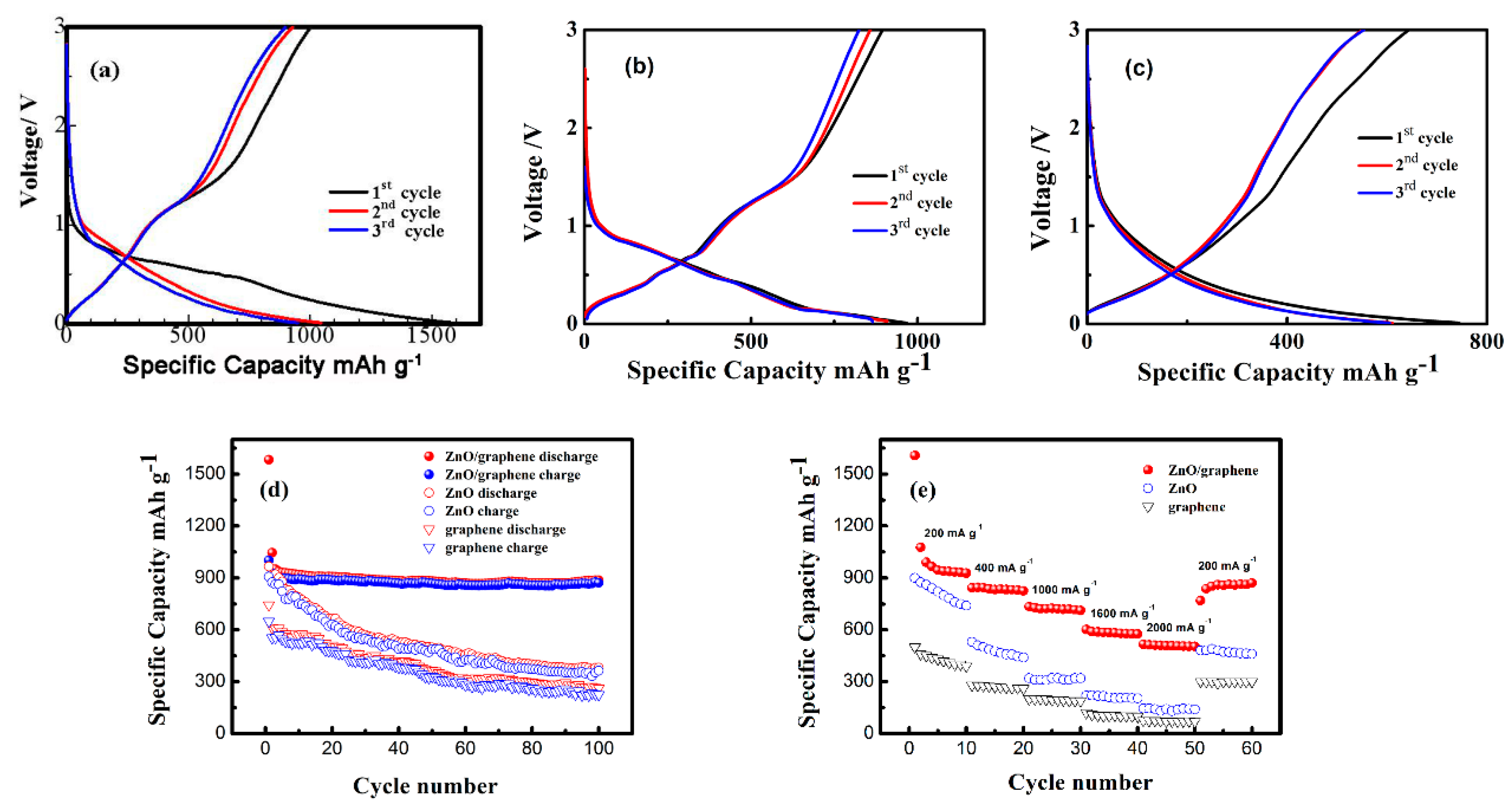
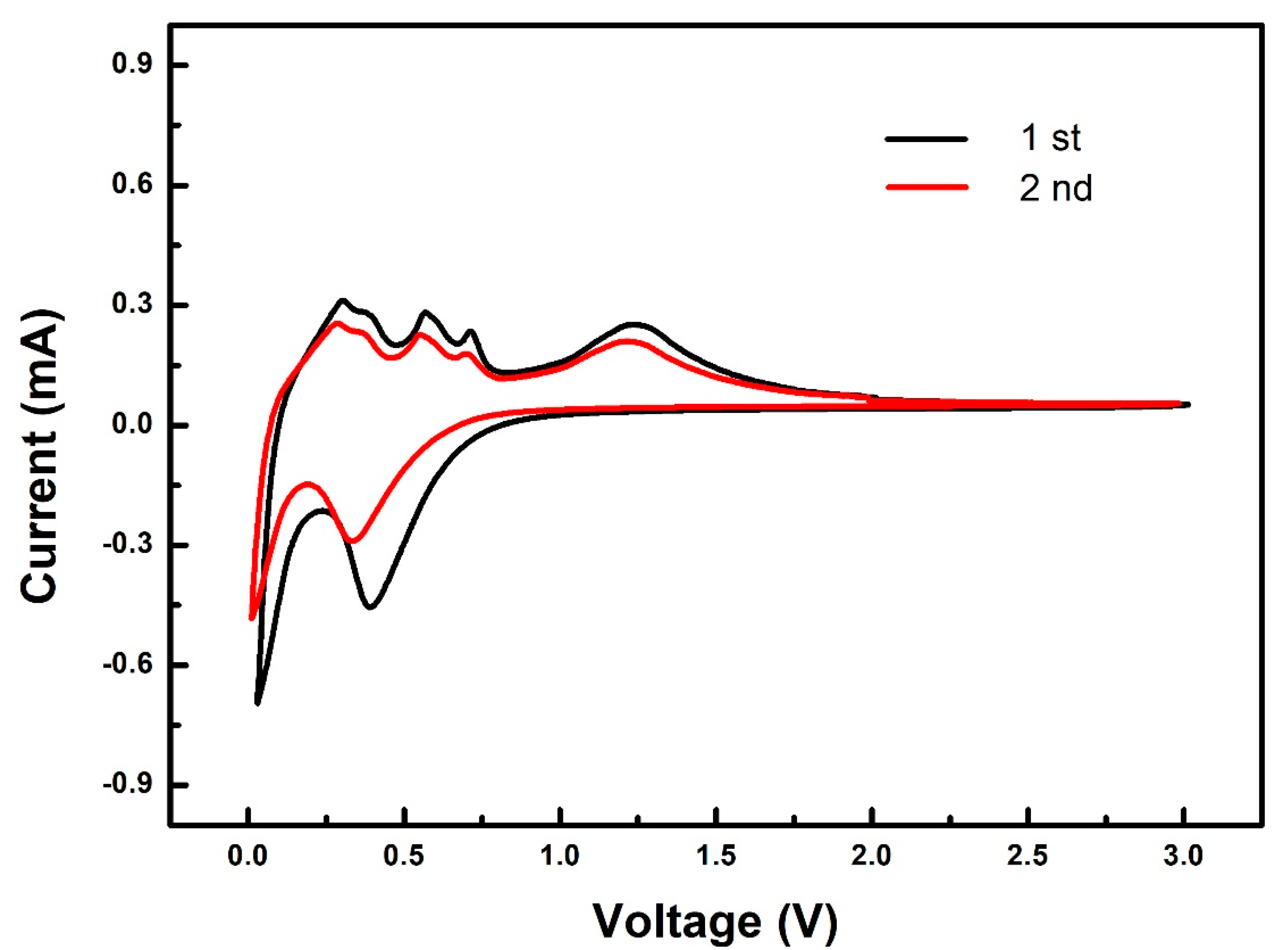
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Wei, Y.; Zhang, Y.; Zhang, C.; Wang, G.; Zhao, Y.; Yin, F.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, B.H. Electrochemical performance of activated carbon nanofiber with ZnO nanoparticles for Li-ion battery. Synth. Met. 2015, 210, 386–391. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, Z.; Zhang, Y.; Wang, X.; Bakenov, Z.; Yin, F. Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries. Nanomaterials 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Haag, J.M.; Pattanaik, G.; Durstock, M.F. Nanostructured 3D Electrode Architectures for High-Rate Li-Ion Batteries. Adv. Mater. 2013, 25, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Zhang, Y.; Zhang, C. Facile synthesis of carbon-coated Fe3O4, core–shell nanoparticles as anode materials for lithium-ion batteries. J. Nanopart. Res. 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene–carbon nanotube–Mn3O4, mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloy. Compd. 2016, 699, 106–111. [Google Scholar] [CrossRef]
- Quartarone, E.; Dall’Asta, V.; Resmini, A.; Tealdi, C.; Tredici, G.; Tamburini, U.A.; Mustarelli, P. Graphite-coated ZnO nanosheets as high-capacity, highly stable, and binder-free anodes for lithium-ion batteries. J. Power Sources 2016, 320, 314–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Li, H.; Zhao, Y.; Yin, F.; Wang, X. Simple fabrication of free-standing ZnO/graphene/carbon nanotube composite anode for lithium-ion batteries. Mater. Lett. 2016, 184, 235–238. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Zhang, Y.; Xie, H.; Yin, F. One-pot Synthesis of radial ZnO microparticles deposited on graphene nanosheets as the Anode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2016, 11, 3179–3189. [Google Scholar] [CrossRef]
- Belliard, F.; Irvine, J.T.S. Electrochemical performance of ball-milled ZnO–SnO2, systems as anodes in lithium-ion battery. J. Power Sources 2001, 97, 219–222. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Wang, G.; Bakenov, Z. Synthesis and electrochemical investigation of highly dispersed ZnO nanoparticles as anode material for lithium-ion batteries. Ionics 2016, 22, 1387–1393. [Google Scholar] [CrossRef]
- Ahmad, M.; Shi, Y.; Nisar, A.; Sun, H.; Shen, W.; Wei, M.; Zhu, J. Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance Li-ion battery anodes. J. Mater. Chem. 2011, 21, 7723–7729. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Liu, Y.; Zheng, P.; Yuan, X.; Guo, S. Al doped-ZnO nanoparticles implanted in reduced graphene oxide with improved electrochemical properties for lithium ion batteries. Mater. Lett. 2016, 165, 165–168. [Google Scholar] [CrossRef]
- Oschmann, B.; Tahir, M.N.; Mueller, F.; Bresser, D.; Lieberwirth, I.; Tremel, W.; Passerini, S.; Zentel, R. Precursor Polymers for the Carbon Coating of Au@ZnO Multipods for Application as Active Material in Lithium-Ion Batteries. Macromol. Rapid Commun. 2015, 36, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Mu, D.; Chen, S.; Wu, B.; Wu, F. Enhanced electrochemical performance of ZnO-loaded/porous carbon composite as anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, Y.; Zhao, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Bakenov, Z. Simple one-pot synthesis of hexagonal ZnO nanoplates as anode material for lithium-ion batteries. J. Nanomater. 2016, 2016, 4675960. [Google Scholar] [CrossRef]
- Park, K.; Xia, F.; Kim, S.; Song, T.; Paik, U.; Park, W. Facile Synthesis of Ultrathin ZnO Nanotubes with Well-Organized Hexagonal Nanowalls and Sealed Layouts: Applications for Lithium Ion Battery Anodes. J. Phys. Chem. C 2013, 117, 1037–1043. [Google Scholar] [CrossRef]
- Xiao, L.; Mei, D.; Cao, M.; Qu, D.; Deng, B. Effects of structural patterns and degree of crystallinity on the performance of nanostructured ZnO as anode material for lithium-ion batteries. J. Alloy. Compd. 2015, 627, 455–462. [Google Scholar] [CrossRef]
- Li, F.; Yang, L.; Xu, G.; Huang, X.; Yang, X.; Wei, X.; Ren, Z.; Shen, G.; Han, G. Hydrothermal self-assembly of hierarchical flower-like ZnO nanospheres with nanosheets and their application in Li-ion batteries. J. Alloy. Compd. 2013, 577, 663–668. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Guo, X.; Mao, S.; Chen, J. Decorating in situ ultrasmall tin particles on crumpled N-doped graphene for lithium-ion batteries with a long life cycle. J. Power Sources 2016, 328, 482–491. [Google Scholar] [CrossRef]
- Zhao, Y.; Bakenova, Z.; Zhang, Y.; Peng, H.; Xie, H.; Bakenov, Z. High performance sulfur/nitrogen-doped graphene cathode for lithium/sulfur batteries. Ionics 2015, 21, 1925–1930. [Google Scholar] [CrossRef]
- Shobukawa, H.; Alvarado, J.; Yang, Y.; Meng, Y.S. Electrochemical performance and interfacial investigation on Si composite anode for lithium ion batteries in full cell. J. Power Sources 2017, 359, 173–181. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Hou, Y. Nickel sulfide/nitrogen-doped graphene composites: Phase-controlled synthesis and high performance anode materials for lithium ion batteries. Small 2013, 9, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Zhang, C.; Liu, F.; Zhu, J.; Hou, Y. Hybrid of Co3Sn2@Co nanoparticles and nitrogen-doped graphene as a lithium ion battery anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, C.; Rajamathi, C.R.; Rajamathi, M.; Gautam, U.K.; Wang, X.; Golberg, D.; Bando, Y. N-doped graphene-VO2(B) nanosheet-built 3D flower hybrid for lithium ion battery. ACS Appl. Mater. Inter. 2013, 5, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, Q.; Cheng, Y.; Zhao, J.; Yin, G. Evaluation of ZnO nanorod arrays with dandelion-like morphology as negative electrodes for lithium-ion batteries. Electrochim. Acta 2009, 54, 2851–2855. [Google Scholar] [CrossRef]
- Bak, S.M.; Nam, K.W.; Lee, C.W.; Kim, K.H.; Jung, H.C.; Yang, X.Q.; Kim, K.B. Spinel LiMn2O4/reduced graphene oxide hybrid for high rate lithium ion batteries. J. Mater. Chem. 2011, 21, 17309–17315. [Google Scholar] [CrossRef]
- Yuan, G.; Xiang, J.; Jin, H.; Wu, L.; Jin, Y.; Zhao, Y. Anchoring ZnO Nanoparticles in Nitrogen-Doped Graphene Sheets as a High-Performance Anode Material for Lithium-Ion Batteries. Materials 2018, 11, 96. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tan, T.; Zhao, Y.; Liu, N. Preparation of ZnO Nanorods/Graphene Composite Anodes for High-Performance Lithium-Ion Batteries. Nanomaterials 2018, 8, 966. https://doi.org/10.3390/nano8120966
Zhang J, Tan T, Zhao Y, Liu N. Preparation of ZnO Nanorods/Graphene Composite Anodes for High-Performance Lithium-Ion Batteries. Nanomaterials. 2018; 8(12):966. https://doi.org/10.3390/nano8120966
Chicago/Turabian StyleZhang, Junfan, Taizhe Tan, Yan Zhao, and Ning Liu. 2018. "Preparation of ZnO Nanorods/Graphene Composite Anodes for High-Performance Lithium-Ion Batteries" Nanomaterials 8, no. 12: 966. https://doi.org/10.3390/nano8120966
APA StyleZhang, J., Tan, T., Zhao, Y., & Liu, N. (2018). Preparation of ZnO Nanorods/Graphene Composite Anodes for High-Performance Lithium-Ion Batteries. Nanomaterials, 8(12), 966. https://doi.org/10.3390/nano8120966



