Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid
Abstract
:1. Introduction
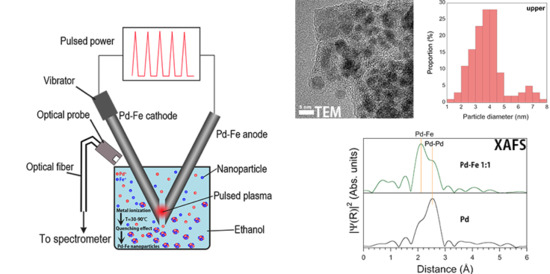
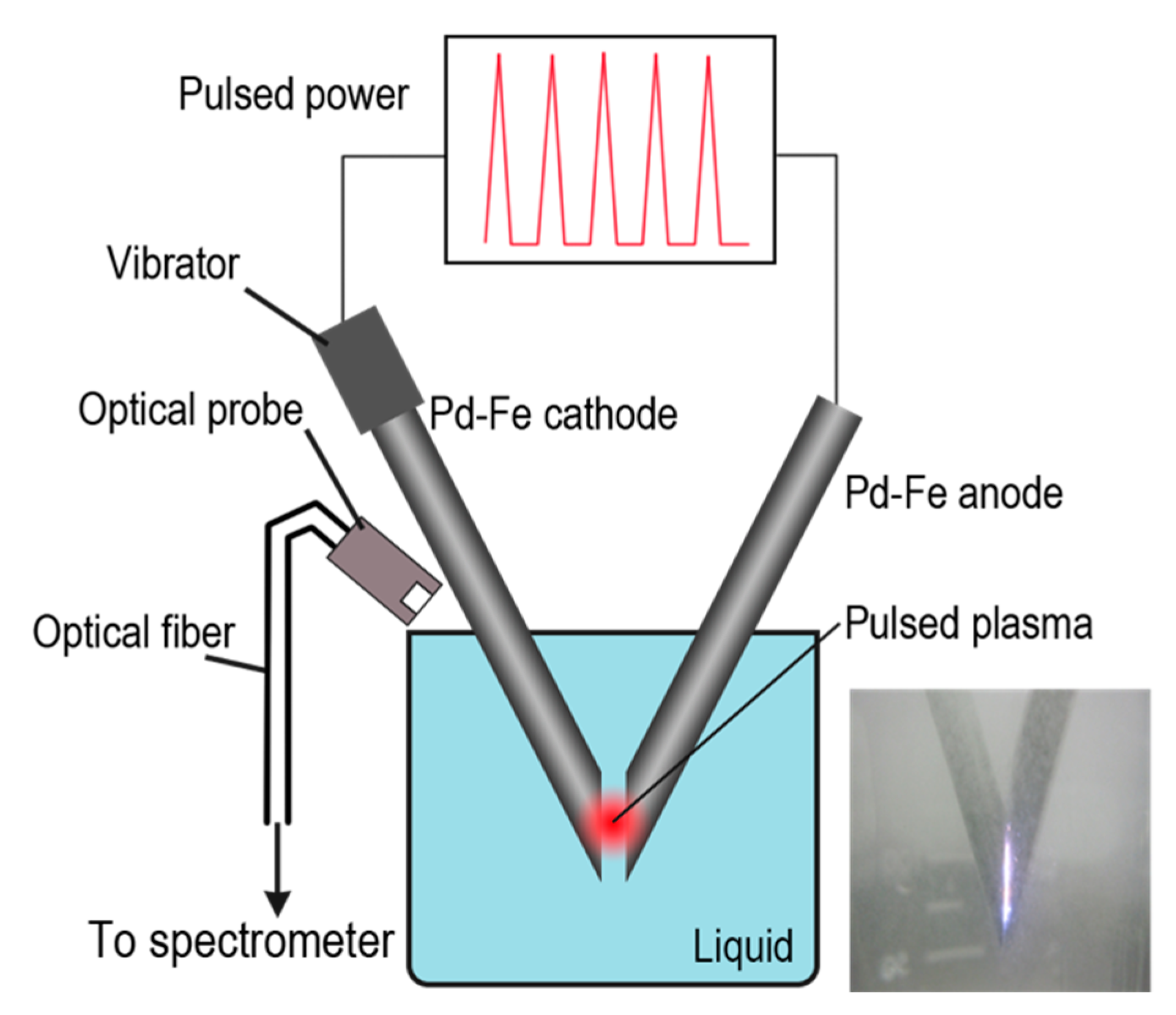
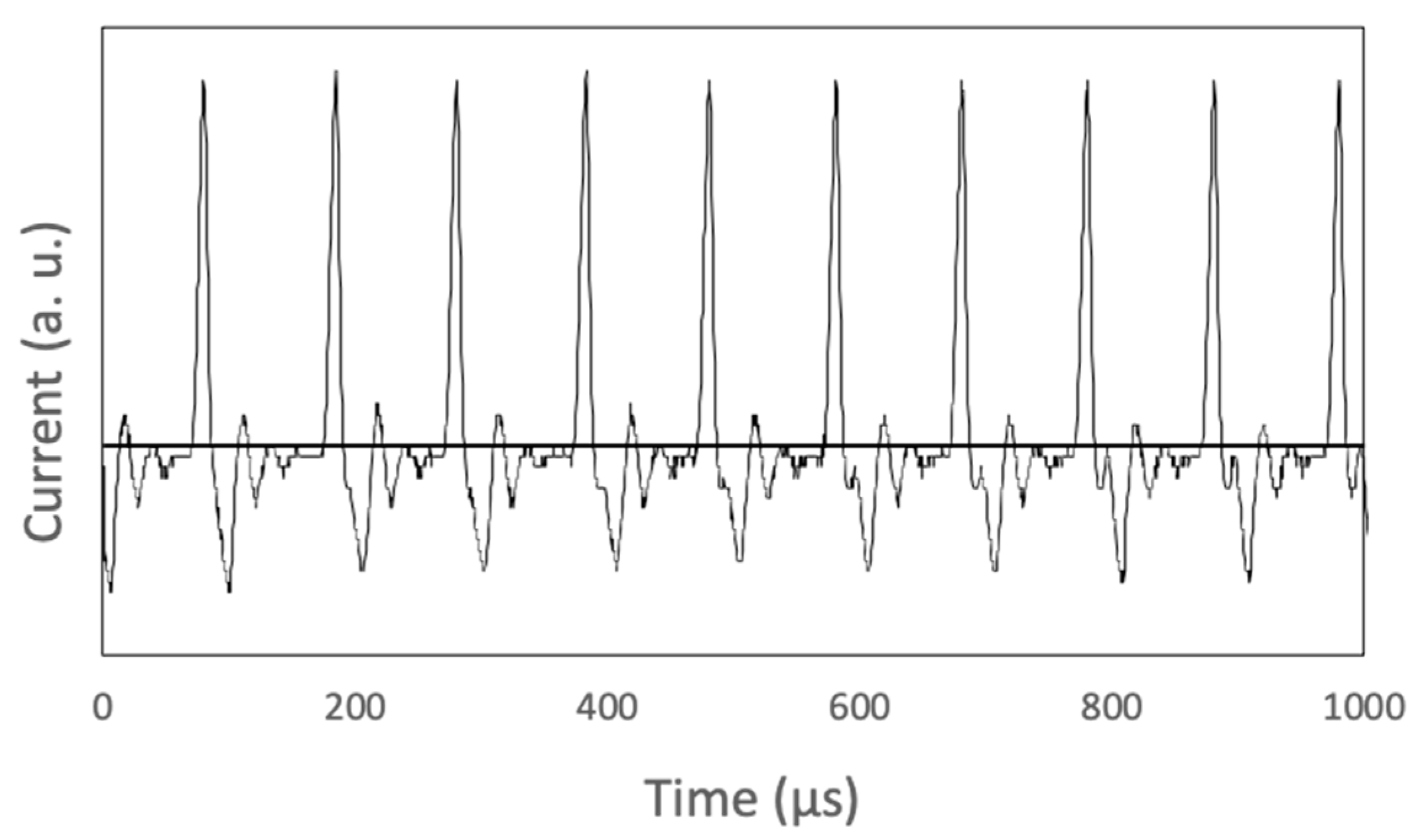
2. Materials and Methods
3. Results and Discussion
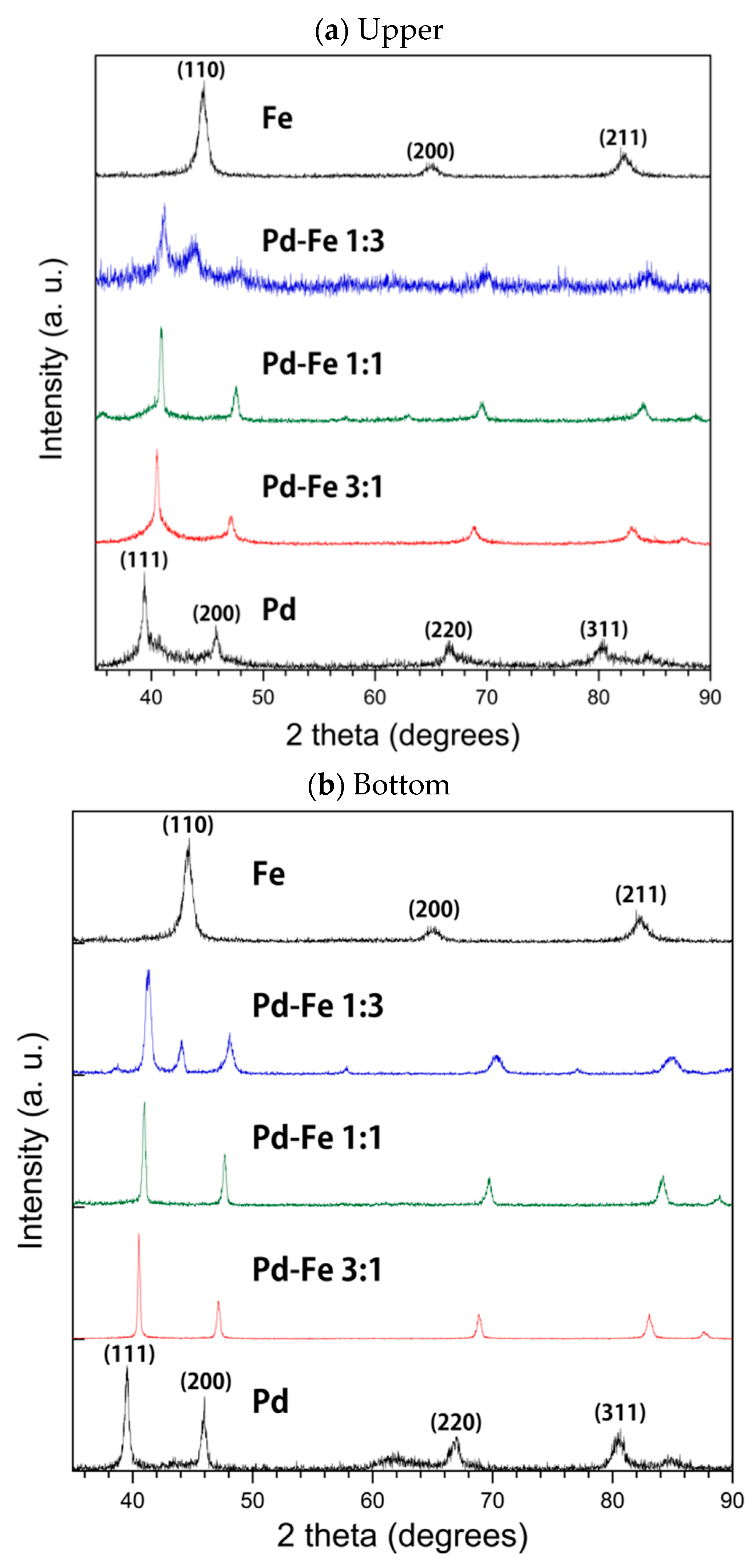
3.1. X-ray Diffraction
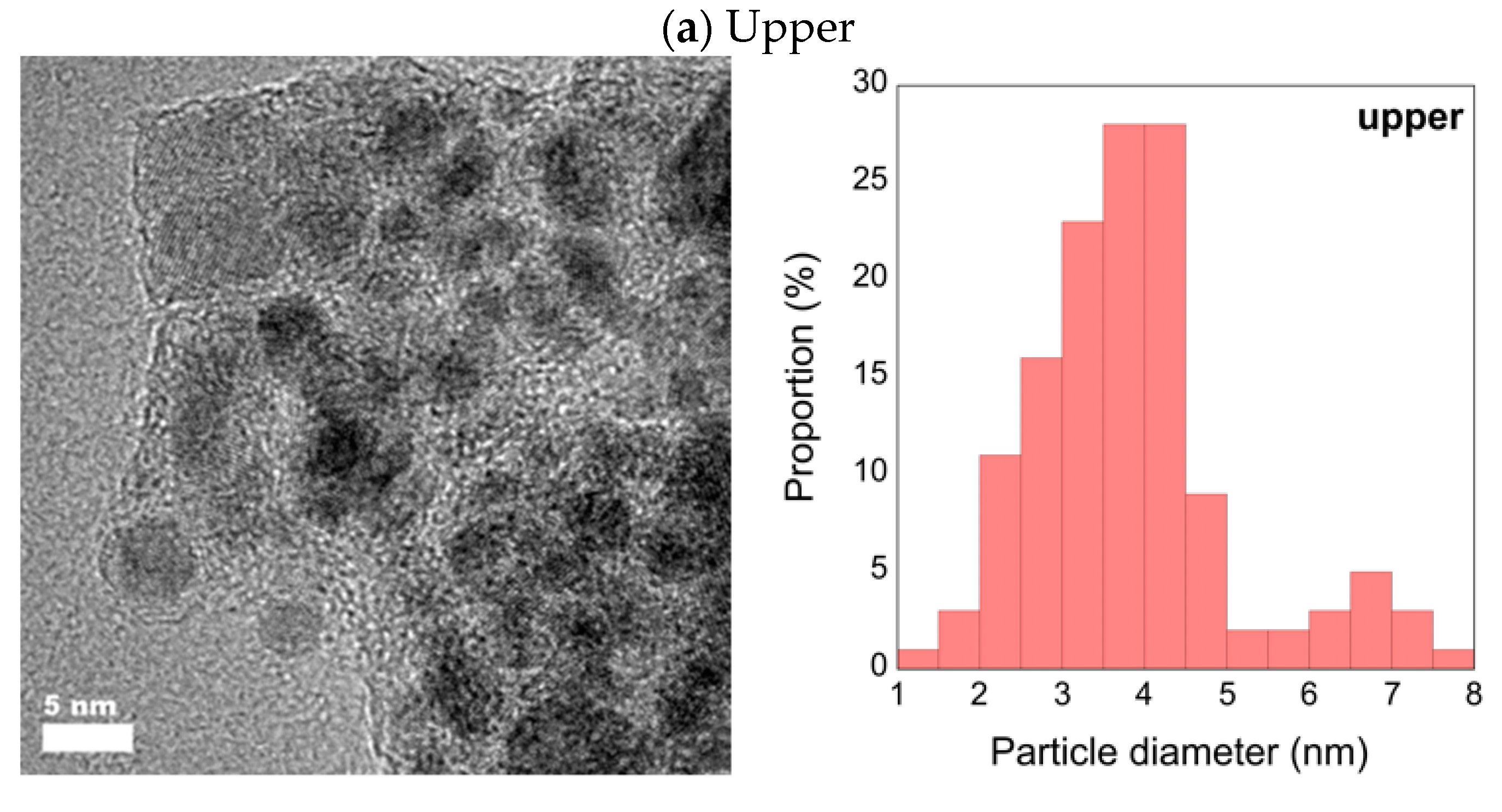
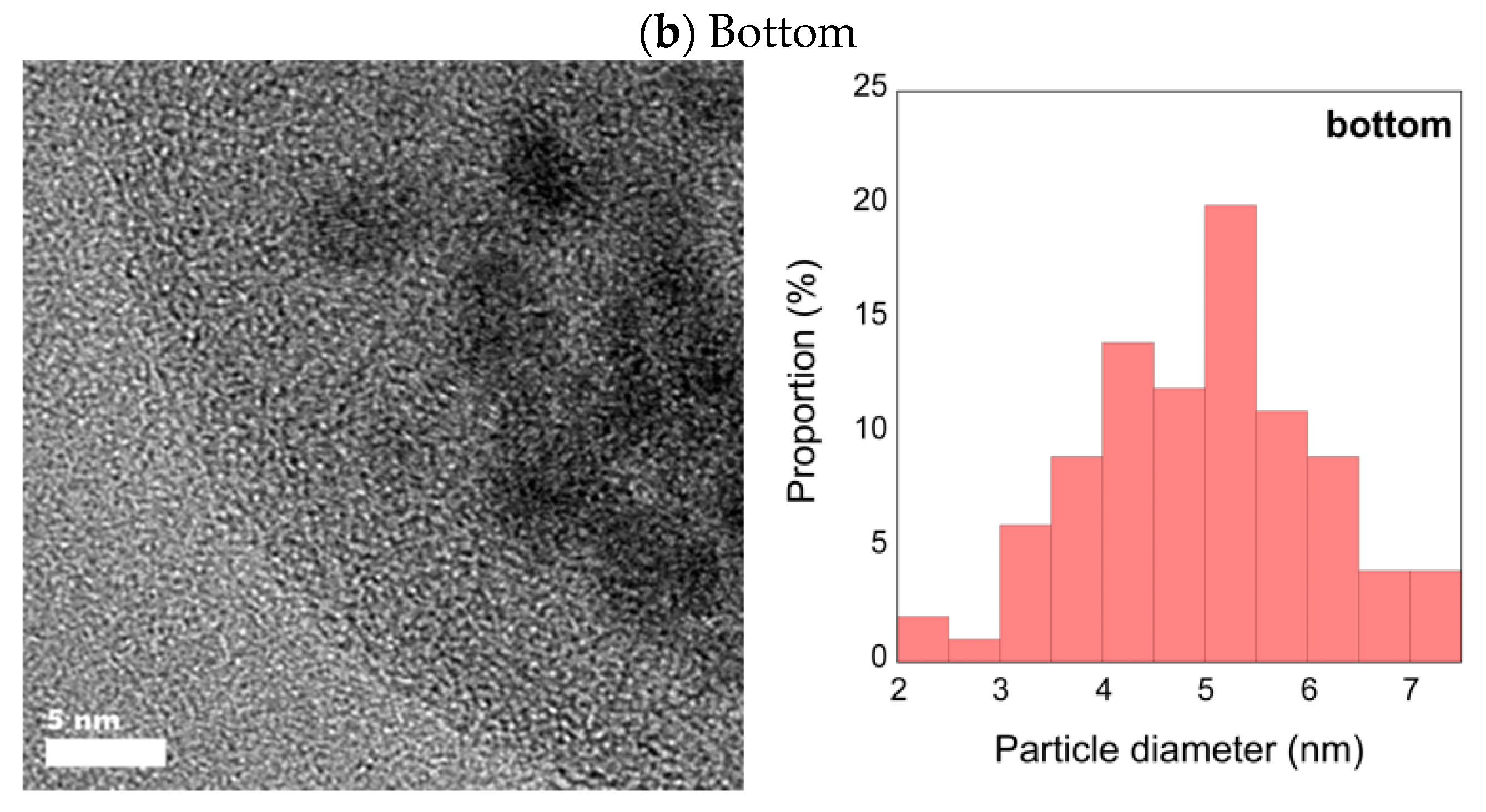
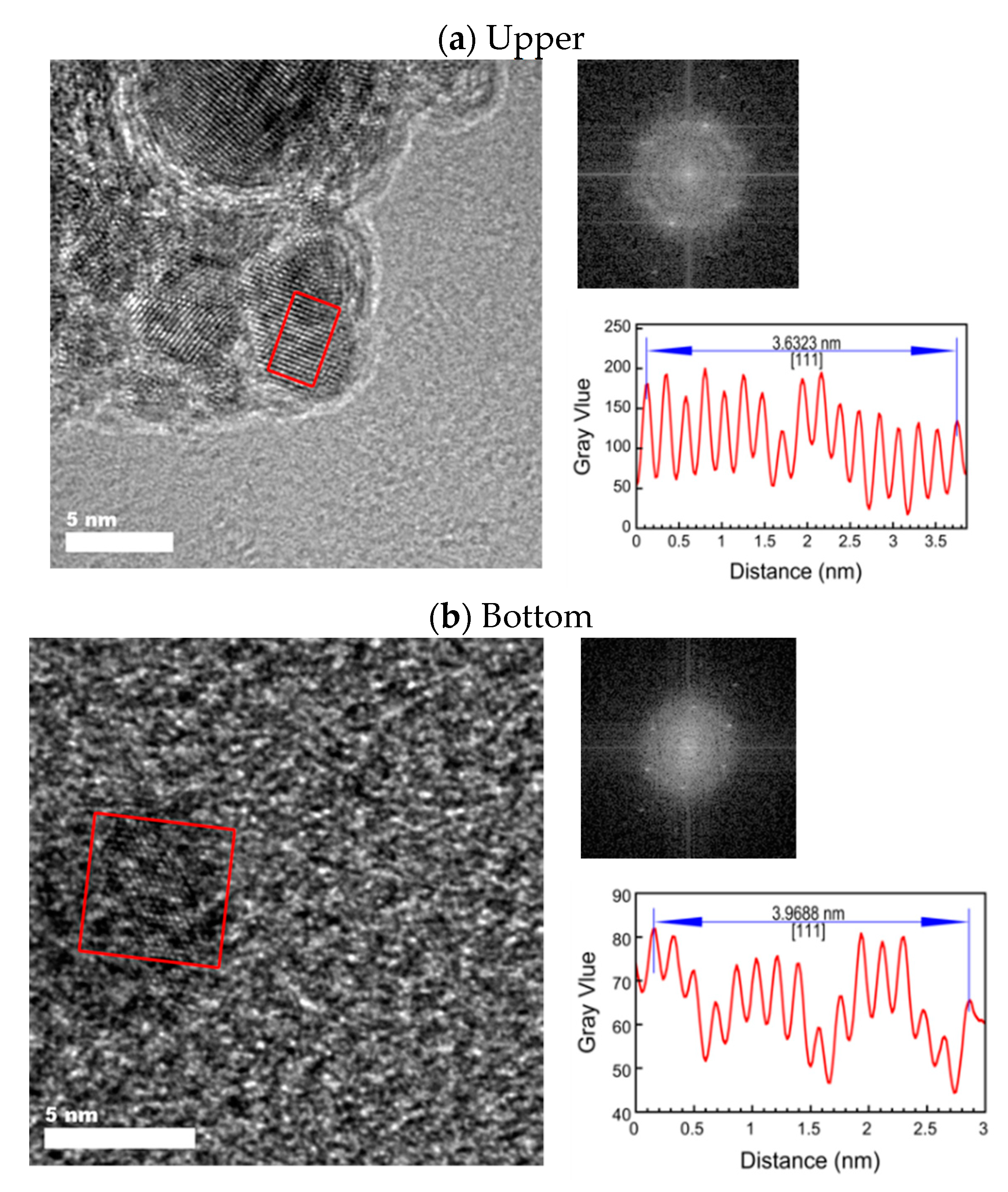
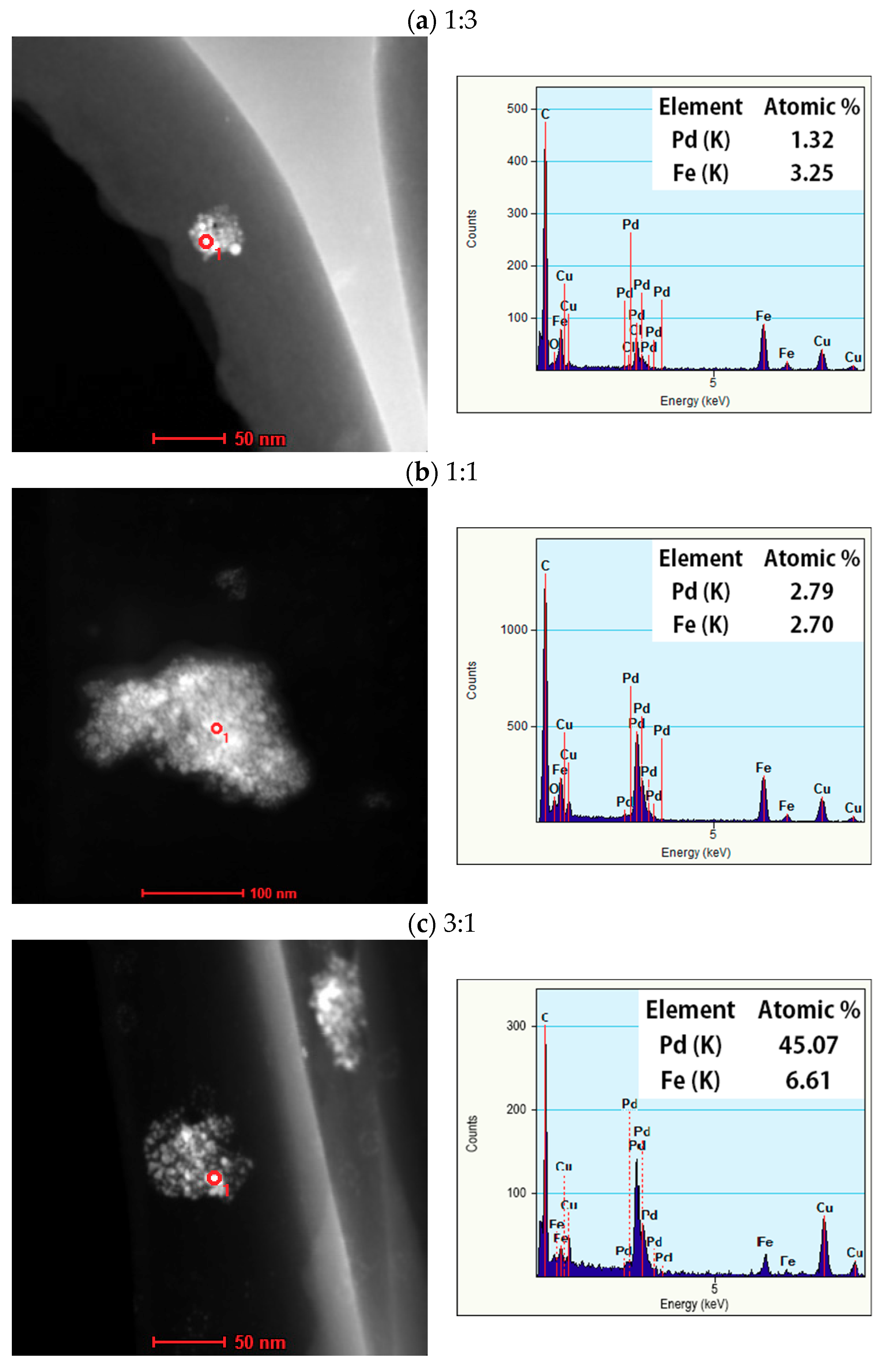
3.2. High-Resolution Transmission Electron Microscopy with Energy Dispersive X-ray Spectrometry
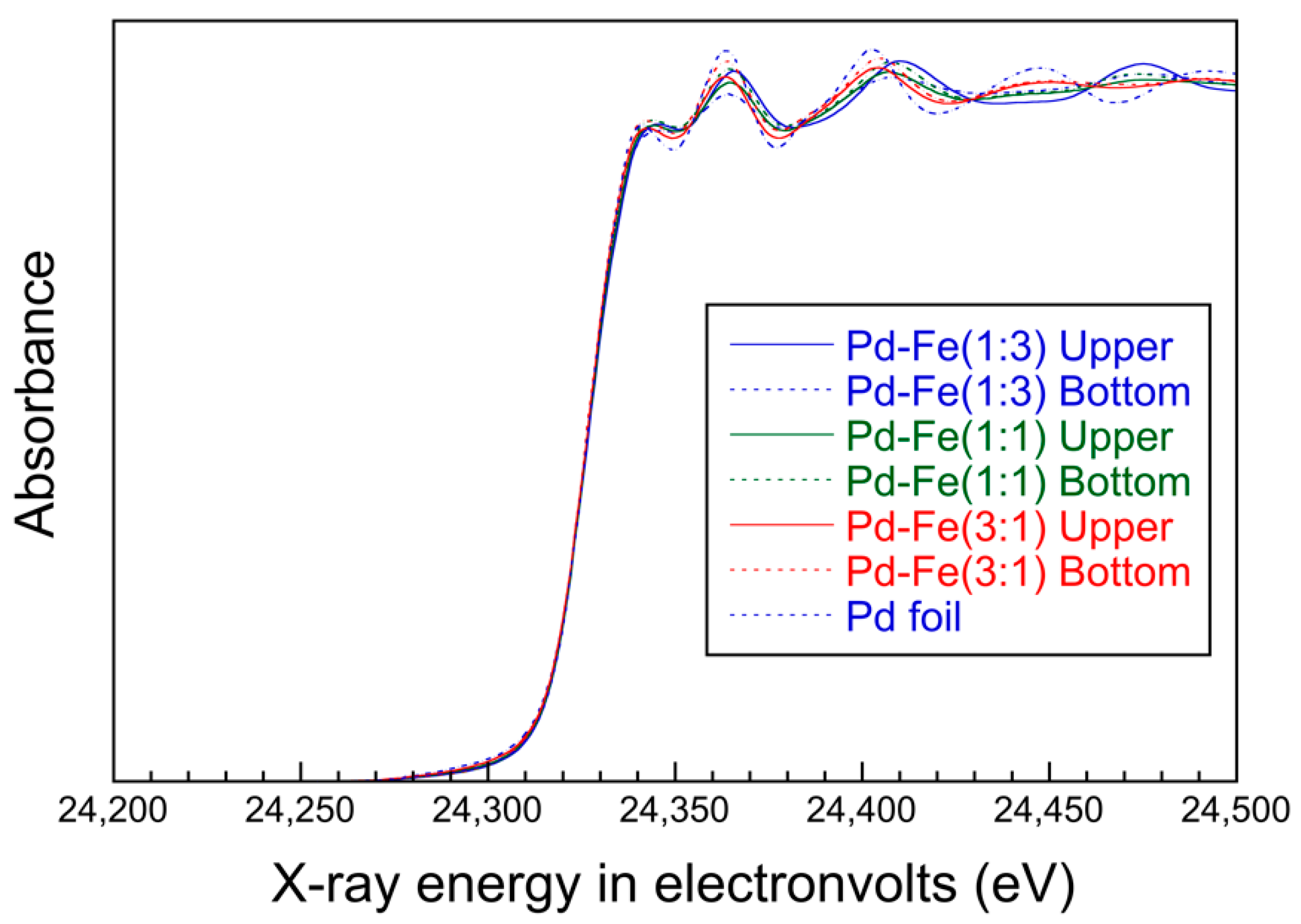
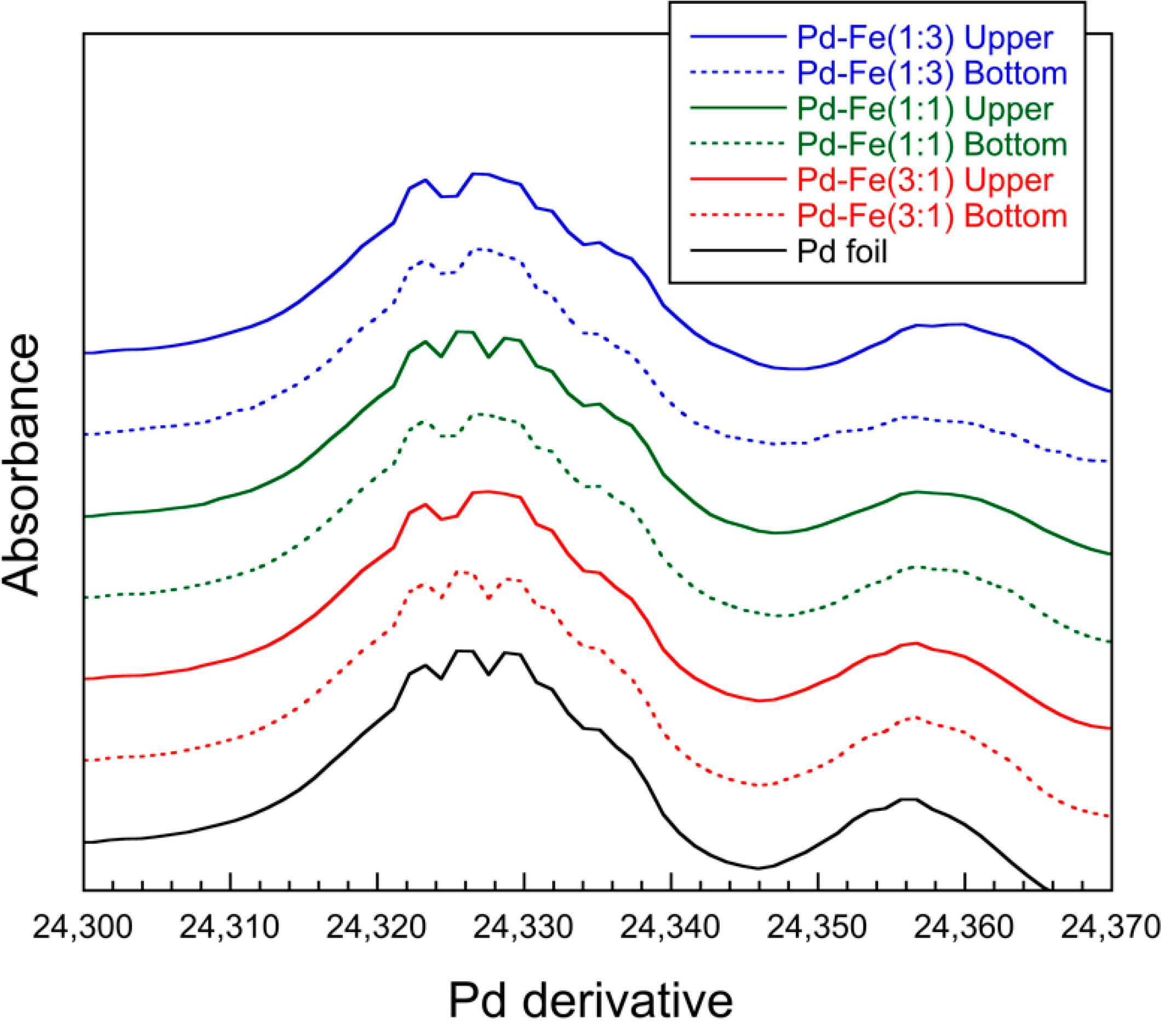
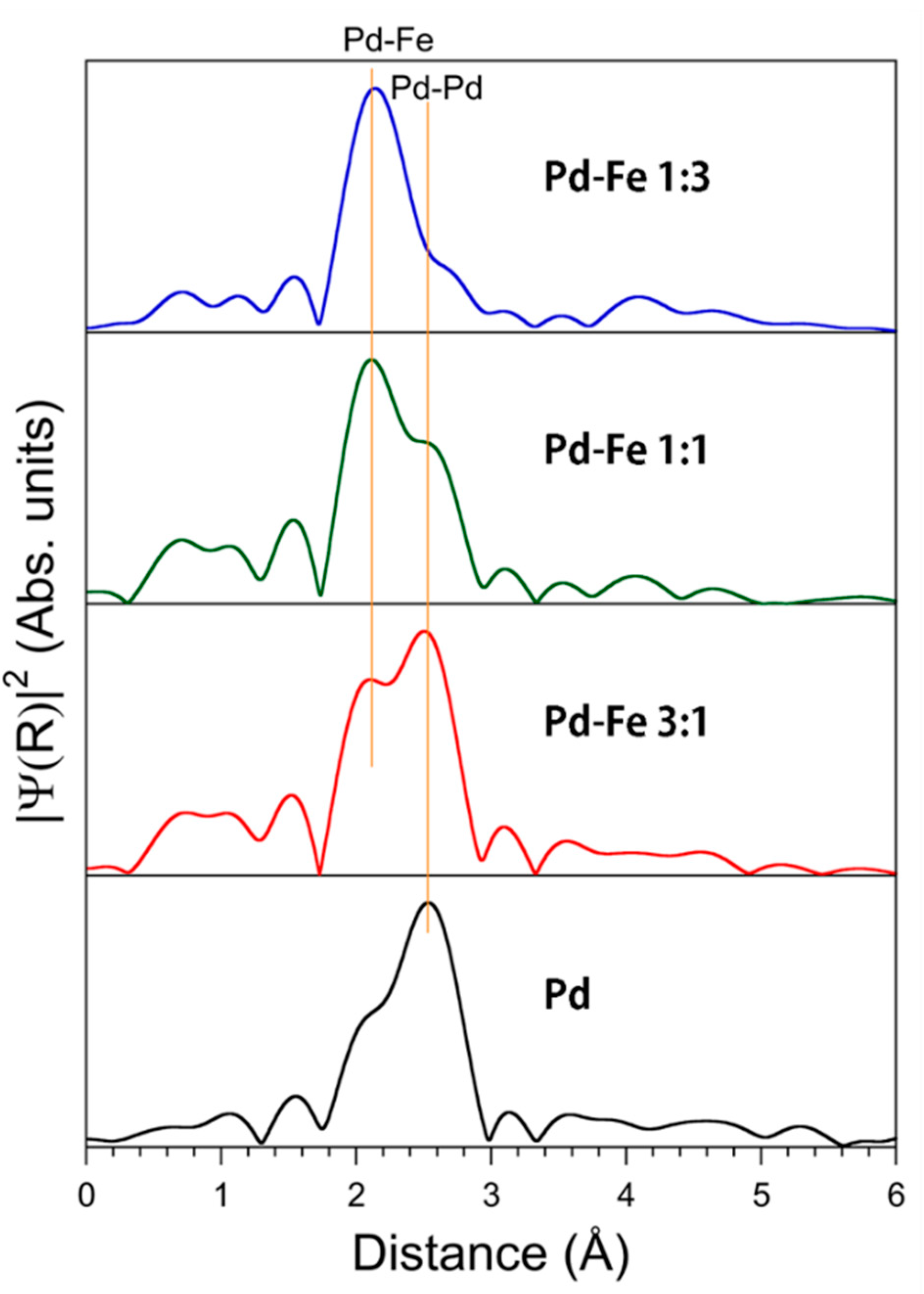
3.3. X-ray Absorption Near Edge Structure
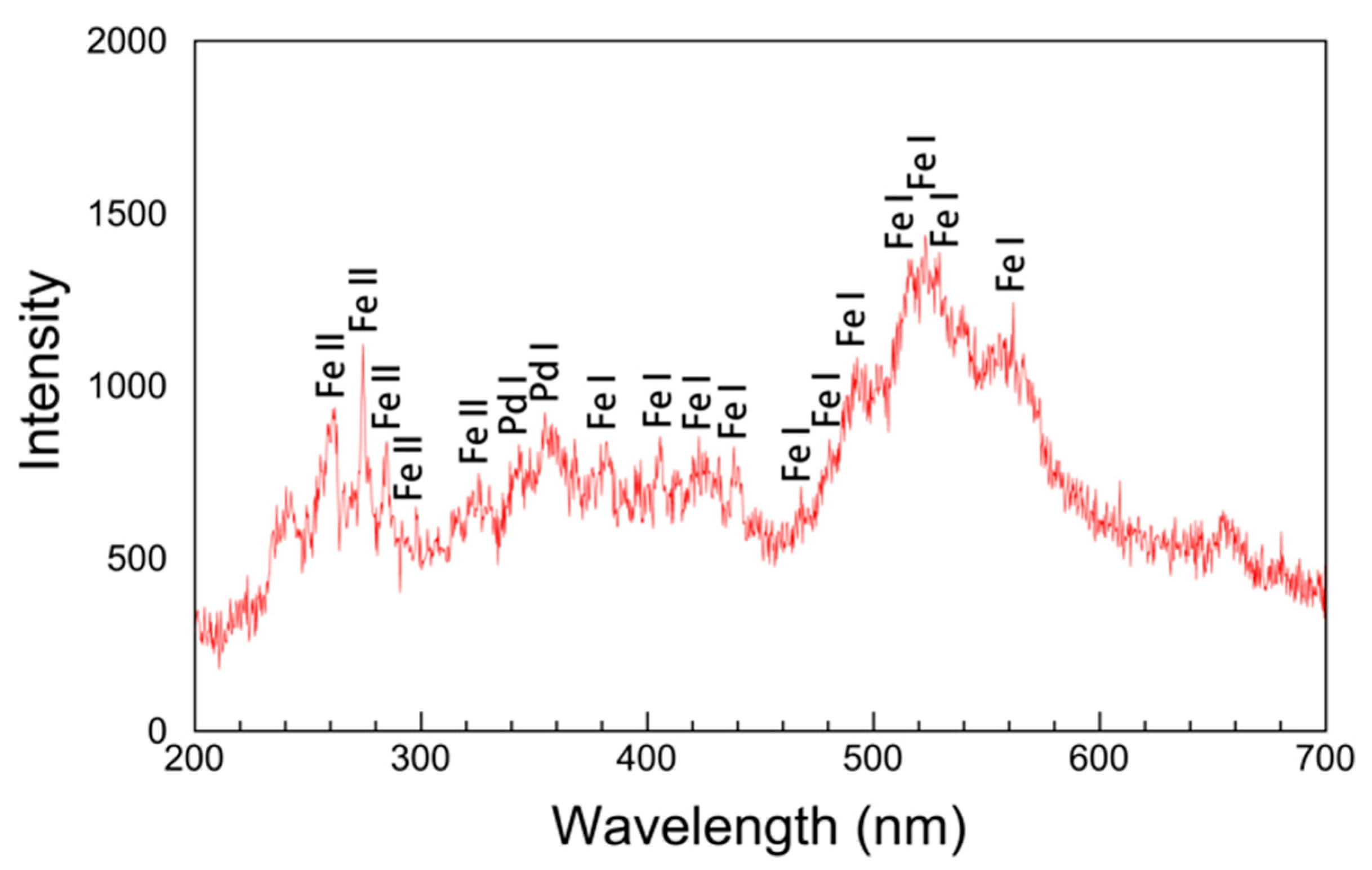
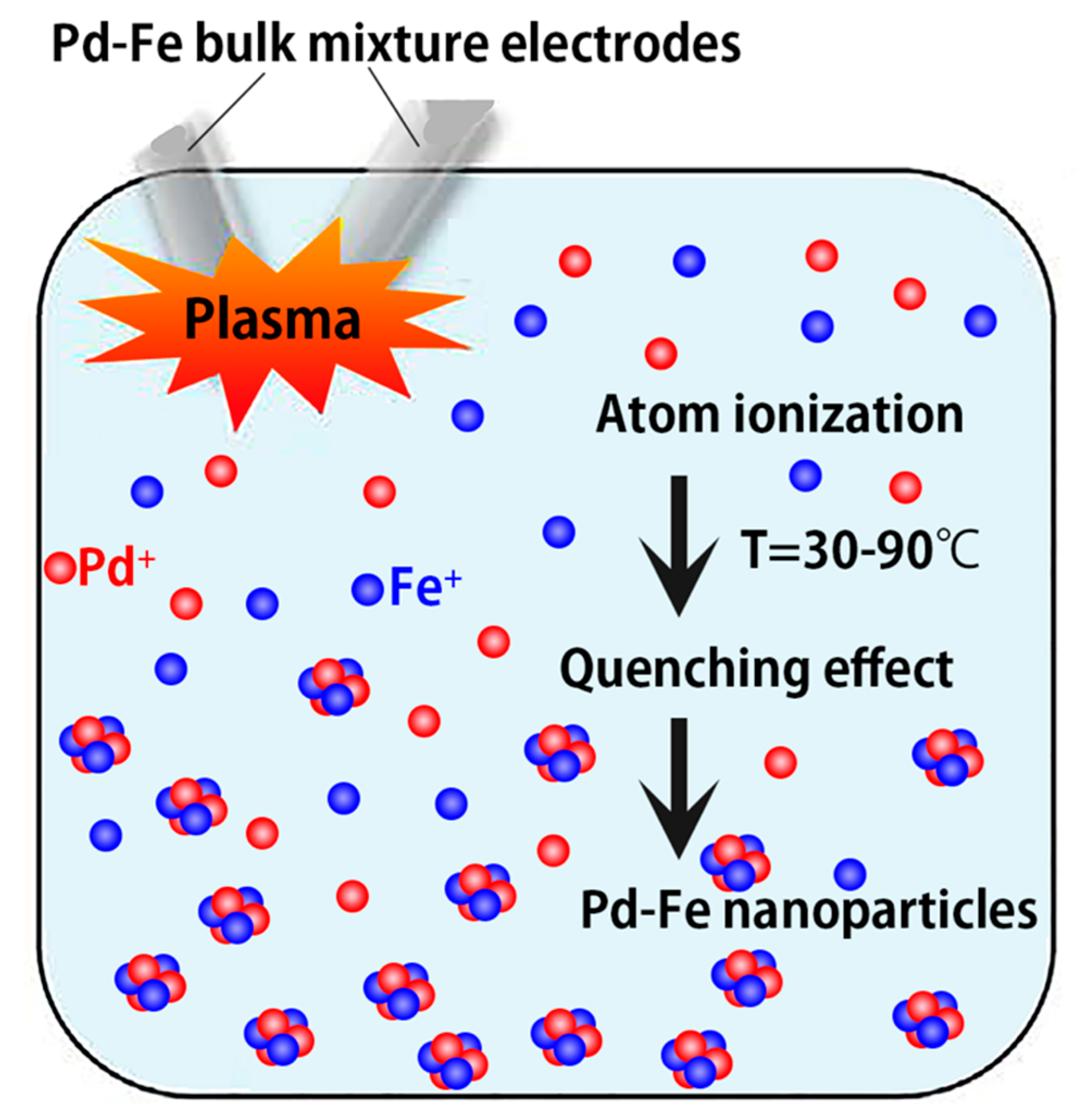
3.4. Atomic Emission Spectrum and Nanoparticle Formation Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gelatt, C.D.; Ehrenreich, H.; Weiss, J.A. Transition-metal hydrides: Electronic structure and the heats of formation. Phys. Rev. B 1978, 17, 1940–1957. [Google Scholar] [CrossRef]
- Vuillemin, J.J.; Priestly, M.G. De Haas-Van Alphen effect and Fermi surface in palladium. Phys. Rev. Lett. 1965, 14, 307–309. [Google Scholar] [CrossRef]
- Perkas, N.; Teo, J.; Shen, S.; Wang, Z.; Highfield, J.; Zhong, Z.; Gedanken, A. Supported Ru catalysts prepared by two sonication-assisted methods for preferential oxidation of CO in H2. Phys. Chem. Chem. Phys. 2011, 13, 15690–15698. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Cui, G.-F.; Shen, P.-K. Stability of Pd-Fe Alloy Catalysts. Wuli Huaxue Xuebao Acta Phys. Chim. Sin. 2009, 25, 213–217. [Google Scholar]
- Shao, Mi.; Sasaki, K.; Adzic, R.R. Pd-Fe Nanoparticles as Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Lim, T.T. Catalytic Reduction of Chlorobenzenes with Pd/Fe Nanoparticles: Reactive Sites, Catalyst Stability, Particle Aging, and Regeneration. Environ. Sci. Technol. 2007, 41, 7523–7529. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Kelgenbaeva, Z.; Yamamoto, K.; Chen, L.; Mashimo, T. Preparation of FePt Nanoparticles by Pulsed Plasma in Liquid Method. Eng. Mater. 2017, 730, 248–252. [Google Scholar] [CrossRef]
- Omurzak, E.; Jasnakunov, J.; Mairykova, N.; Abdykerimova, A.; Maatkasymova, A.; Sulaimankulova, S.; Matsuda, M.; Nishida, M.; Ihara, H.; Mashimo, T. Synthesis Method of Nanomaterial by Pulsed Plasma in Liquid. J. Nanosci. Nanotechnol. 2007, 7, 3157–3159. [Google Scholar] [CrossRef] [PubMed]
- Kelgenbaeva, Z.; Omurzak, E.; Takebe, S.; Sulaimankulova, S.; Abdullaeva, Z.; Iwamoto, C.; Mashimo, T. Synthesis of pure iron nanoparticles at liquid-liquid interface using pulsed plasma. J. Nanopart. Res. 2014, 16, 2603. [Google Scholar] [CrossRef]
- Abdullaeva, Z.; Omurzak, E.; Iwamoto, C.; Chen, L.; Mashimo, T. Onion-like carbon-encapsulated Co, Ni, and Fe magnetic nanoparticles with low cytotoxicity synthesized by a pulsed plasma in a liquid. Carbon 2012, 550, 1776–1785. [Google Scholar] [CrossRef]
- Omurzak, E.; Abdullaeva, Z.; Iwamoto, C.; Ihara, H.; Sulaimankulova, S.; Mashimo, T. Synthesis of Hollow Carbon Nano-Onions Using the Pulsed Plasma in Liquid. J. Nanosci. Nanotechnol. 2015, 15, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Omurzak, E.; Mashimo, T.; Iwamoto, C.; Matsumoto, Y.; Sulaimankulova, S. Synthesis of Blue Amorphous TiO2 and TinO2n−1 by the Impulse Plasma in Liquid. J. Nanosci. Nanotechnol. 2009, 9, 6372–6375. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mashimo, T.; Omurzak, E.; Okudera, H.; Iwamoto, C.; Yoshiasa, A. Pure Tetragonal ZrO2 Nanopartiles Synthesized by Pulsed Plasma in Liquid. J. Phys. Chem. C 2011, 15, 9370–9375. [Google Scholar] [CrossRef]
- Omurzak, E.; Mashimo, T.; Sulaimankulova, S.; Takebe, S.; Chen, L.; Abdullaeva, Z.; Iwamoto, C.; Oishi, Y.; Ihara, H.; Okudera, H.; et al. Wurtzite-type ZnS nanoparticles by pulsed electric discharge. Nanotechnology 2011, 22, 365602. [Google Scholar] [CrossRef] [PubMed]
- Ralchenko, Y.; Kramida, A.E.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database; Version 5; National Institute of Standards and Technology, 2011. Available online: http://physics.nist.gov/asd (accessed on 20 August 2018).
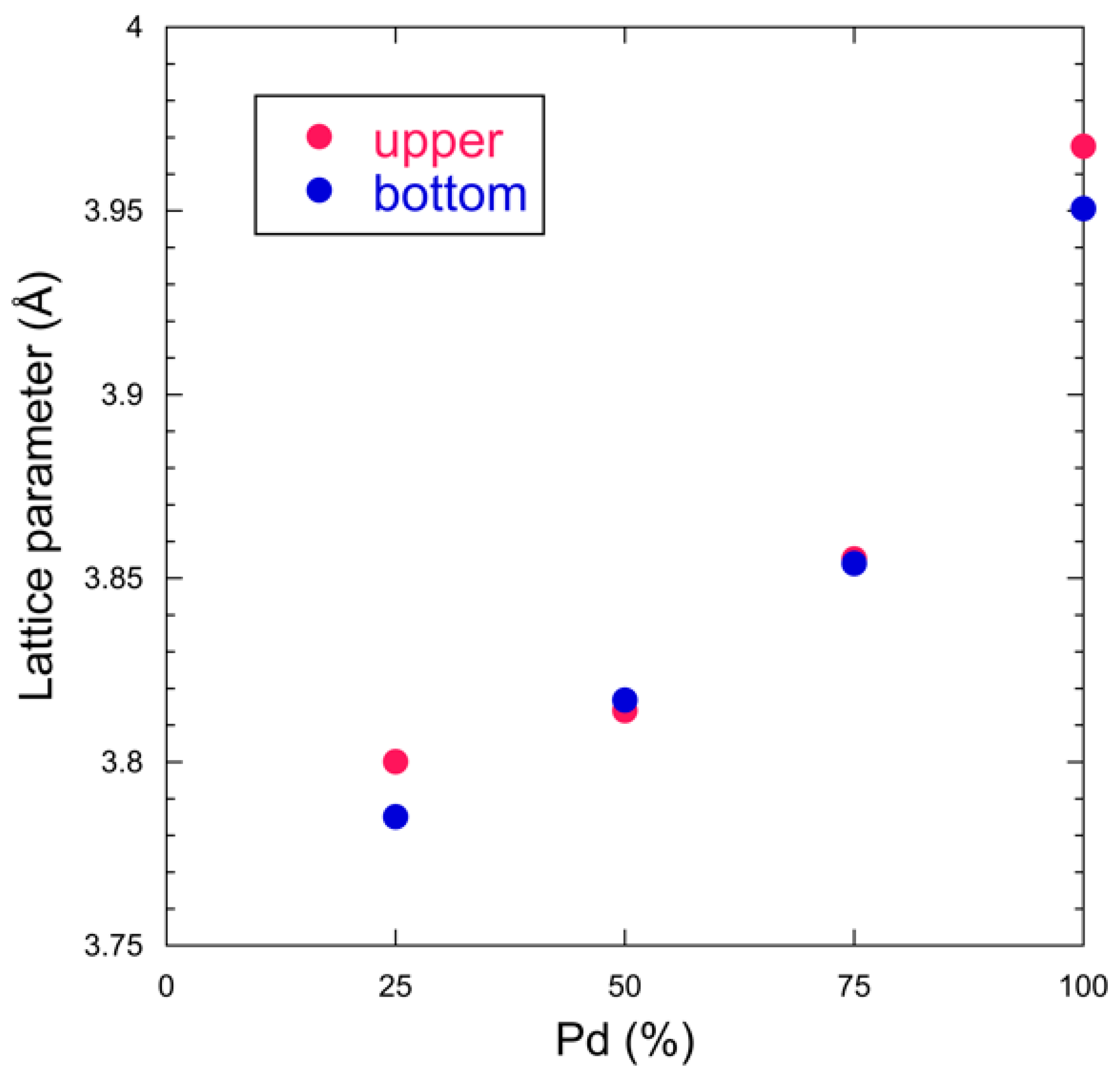
| Lattice Parameter (Å) | Upper | Bottom |
|---|---|---|
| Pd | 3.9676(39) | 3.9506(37) |
| Pd3Fe | 3.8553(102) | 3.8540(1) |
| PdFe | 3.8140(4) | 3.8168(13) |
| PdFe3 | 3.8001(57) | 3.7851(30) |
| Sample | Pd-Pd Distance (Å) | Pd-Fe Distance (Å) |
|---|---|---|
| Pd Upper | 2.76(1) | - |
| Pd3Fe Upper | 2.76(1) | 2.62(2) |
| PdFe Upper | 2.74(2) | 2.61(2) |
| PdFe3 Upper | 2.75(2) | 2.63(1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, S.; Mashimo, T.; Yamamoto, K.; Kelgenbaeva, Z.; Ma, W.; Kang, X.; Koinuma, M.; Isobe, H.; Yoshiasa, A. Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid. Nanomaterials 2018, 8, 1068. https://doi.org/10.3390/nano8121068
Tamura S, Mashimo T, Yamamoto K, Kelgenbaeva Z, Ma W, Kang X, Koinuma M, Isobe H, Yoshiasa A. Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid. Nanomaterials. 2018; 8(12):1068. https://doi.org/10.3390/nano8121068
Chicago/Turabian StyleTamura, Shota, Tsutomu Mashimo, Kenta Yamamoto, Zhazgul Kelgenbaeva, Weijan Ma, Xuesong Kang, Michio Koinuma, Hiroshi Isobe, and Akira Yoshiasa. 2018. "Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid" Nanomaterials 8, no. 12: 1068. https://doi.org/10.3390/nano8121068
APA StyleTamura, S., Mashimo, T., Yamamoto, K., Kelgenbaeva, Z., Ma, W., Kang, X., Koinuma, M., Isobe, H., & Yoshiasa, A. (2018). Synthesis of Pd-Fe System Alloy Nanoparticles by Pulsed Plasma in Liquid. Nanomaterials, 8(12), 1068. https://doi.org/10.3390/nano8121068