Electrochemical Enantiomer Recognition Based on sp3-to-sp2 Converted Regenerative Graphene/Diamond Electrode
Abstract
:1. Introduction
2. Materials and Experimental
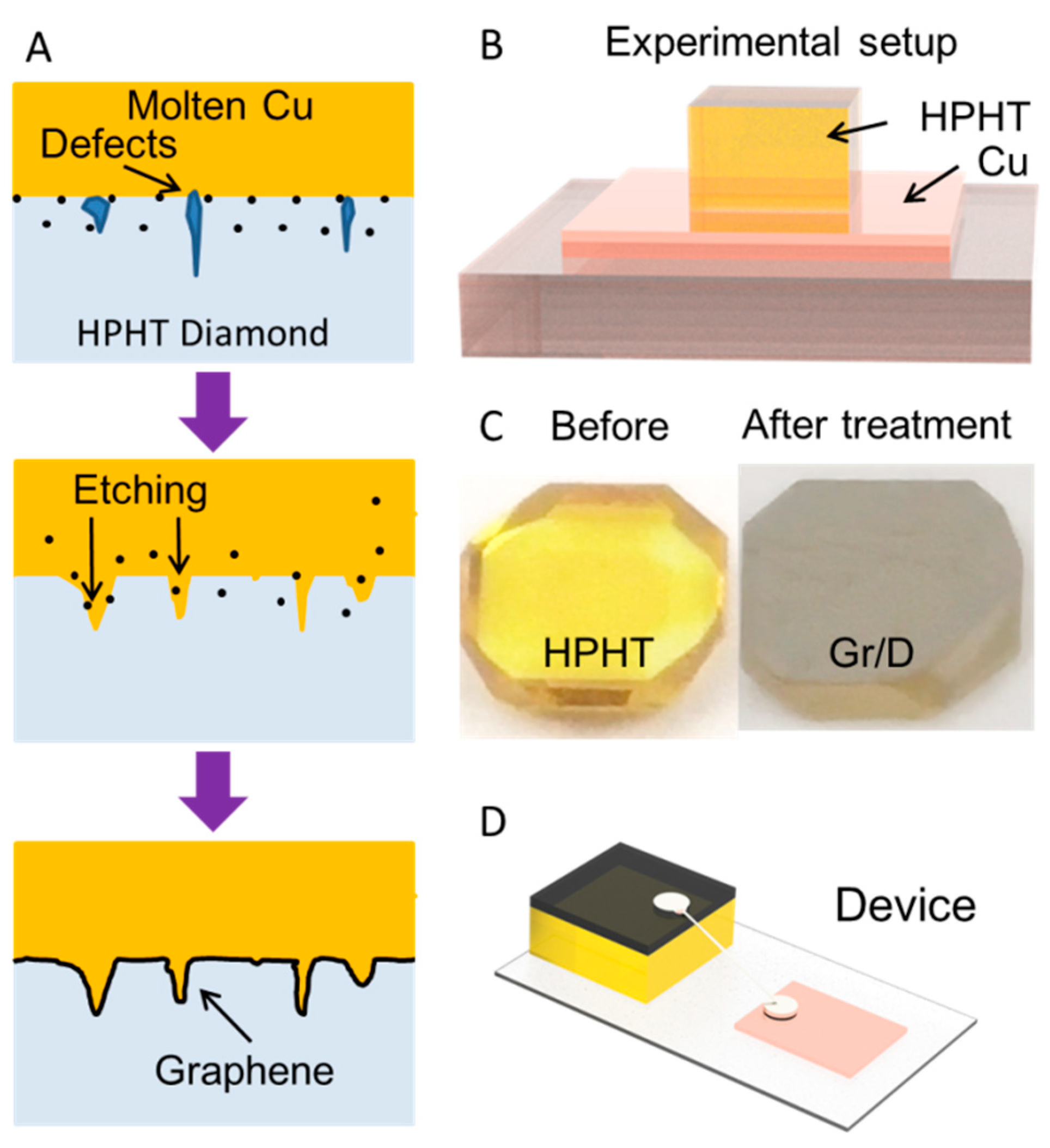
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, T.; Abrahams, I.; Dennis, T.J.S. Structural Identification of 19 Purified Isomers of the OPV Acceptor Material bisPCBM by 13C NMR and UV–Vis Absorption Spectroscopy and High-Performance Liquid Chromatography. J. Phys. Chem. A 2018, 122, 4138–4152. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Bhushan, R. Development of liquid chromatographic methods for enantioseparation and sensitive detection of β-adrenolytics/β2-agonists in human plasma using a single enantiomer reagent. J. Chromatogr. B 2017, 1061, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-H.; Pan, L.-S.; Jow, J.-J.; Chen, H.-R.; Ling, T.-R. Molecularly imprinted electrochemical sensor, formed on Ag screen-printed electrodes, for the enantioselective recognition of d and l phenylalanine. Biosens. Bioelectron. 2018, 105, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Rapini, R.; Piletsky, S. Recent advances in electrochemical sensors based on chiral and nano-sized imprinted polymers. Curr. Opin. Electrochem. 2018, 7, 146–152. [Google Scholar] [CrossRef]
- Batalla, P.; Martín, A.; López, M.A.N.; González, M.A.C.; Escarpa, A. Enzyme-based microfluidic chip coupled to graphene electrodes for the detection of D-amino acid enantiomer-biomarkers. Anal. Chem. 2015, 87, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.S.; Kalambate, P.K.; Srivastava, A.K. Enantioselective analysis of Moxifloxacin hydrochloride enantiomers with graphene-β-Cyclodextrin-nanocomposite modified carbon paste electrode using adsorptive stripping differential pulse Voltammetry. Electrochim. Acta 2017, 248, 258–269. [Google Scholar] [CrossRef]
- Dong, S.; Bi, Q.; Qiao, C.; Sun, Y.; Zhang, X.; Lu, X.; Zhao, L. Electrochemical sensor for discrimination tyrosine enantiomers using graphene quantum dots and β-cyclodextrins composites. Talanta 2017, 173, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; González-Campo, A.; Riba-Moliner, M.; Baeza, M.; Mas-Torrent, M. Chiral magnetic-nanobiofluids for rapid electrochemical screening of enantiomers at a magneto nanocomposite graphene-paste electrode. Biosens. Bioelectron. 2018, 105, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Doepel, L.; Hewage, I.; Lapierre, H. Milk protein yield and mammary metabolism are affected by phenylalanine deficiency but not by threonine or tryptophan deficiency. J. Dairy Sci. 2016, 99, 3144–3156. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Rai, R.K.; Arora, A.; Sinha, N.; Thakur, A.K. Therapeutic implication of L-phenylalanine aggregation mechanism and its modulation by D-phenylalanine in phenylketonuria. Sci. Rep. 2014, 4, 3875. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Massalski, T. The Au-C (gold-carbon) system. J. Phase Equilib. 1984, 5, 378–379. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Ye, C.; Sun, H.; Dai, D.; Wei, Q.; Lai, G.; Wu, T.; Yu, A.; Fu, L.; et al. Highly stable and regenerative graphene–diamond hybrid electrochemical biosensor for fouling target dopamine detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, H.; Hao, H.; Chen, Y.; Dong, C.; Wang, G. β-Cyclodextrin functionalized Mn-doped ZnS quantum dots for the chiral sensing of tryptophan enantiomers. Polym. Chem. 2015, 6, 591–598. [Google Scholar] [CrossRef]
- Freeman, R.; Finder, T.; Bahshi, L.; Willner, I. β-cyclodextrin-modified CdSe/ZnS quantum dots for sensing and chiroselective analysis. Nano Lett. 2009, 9, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Facile and efficient electrochemical enantiomer recognition of phenylalanine using β-Cyclodextrin immobilized on reduced graphene oxide. Biosens. Bioelectron. 2017, 94, 714–718. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhang, H.; Ye, C.; Yuan, Q.; Chee, K.W.A.; Su, W.; Yu, A.; Yu, J.; Lin, C.-T.; Dai, D.; et al. Electrochemical Enantiomer Recognition Based on sp3-to-sp2 Converted Regenerative Graphene/Diamond Electrode. Nanomaterials 2018, 8, 1050. https://doi.org/10.3390/nano8121050
Gao J, Zhang H, Ye C, Yuan Q, Chee KWA, Su W, Yu A, Yu J, Lin C-T, Dai D, et al. Electrochemical Enantiomer Recognition Based on sp3-to-sp2 Converted Regenerative Graphene/Diamond Electrode. Nanomaterials. 2018; 8(12):1050. https://doi.org/10.3390/nano8121050
Chicago/Turabian StyleGao, Jingyao, Haoyang Zhang, Chen Ye, Qilong Yuan, Kuan W. A. Chee, Weitao Su, Aimin Yu, Jinhong Yu, Cheng-Te Lin, Dan Dai, and et al. 2018. "Electrochemical Enantiomer Recognition Based on sp3-to-sp2 Converted Regenerative Graphene/Diamond Electrode" Nanomaterials 8, no. 12: 1050. https://doi.org/10.3390/nano8121050
APA StyleGao, J., Zhang, H., Ye, C., Yuan, Q., Chee, K. W. A., Su, W., Yu, A., Yu, J., Lin, C.-T., Dai, D., & Fu, L. (2018). Electrochemical Enantiomer Recognition Based on sp3-to-sp2 Converted Regenerative Graphene/Diamond Electrode. Nanomaterials, 8(12), 1050. https://doi.org/10.3390/nano8121050






