Charge Transfer Tuned by the Surrounding Dielectrics in TiO2-Ag Composite Arrays
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation and Characterization
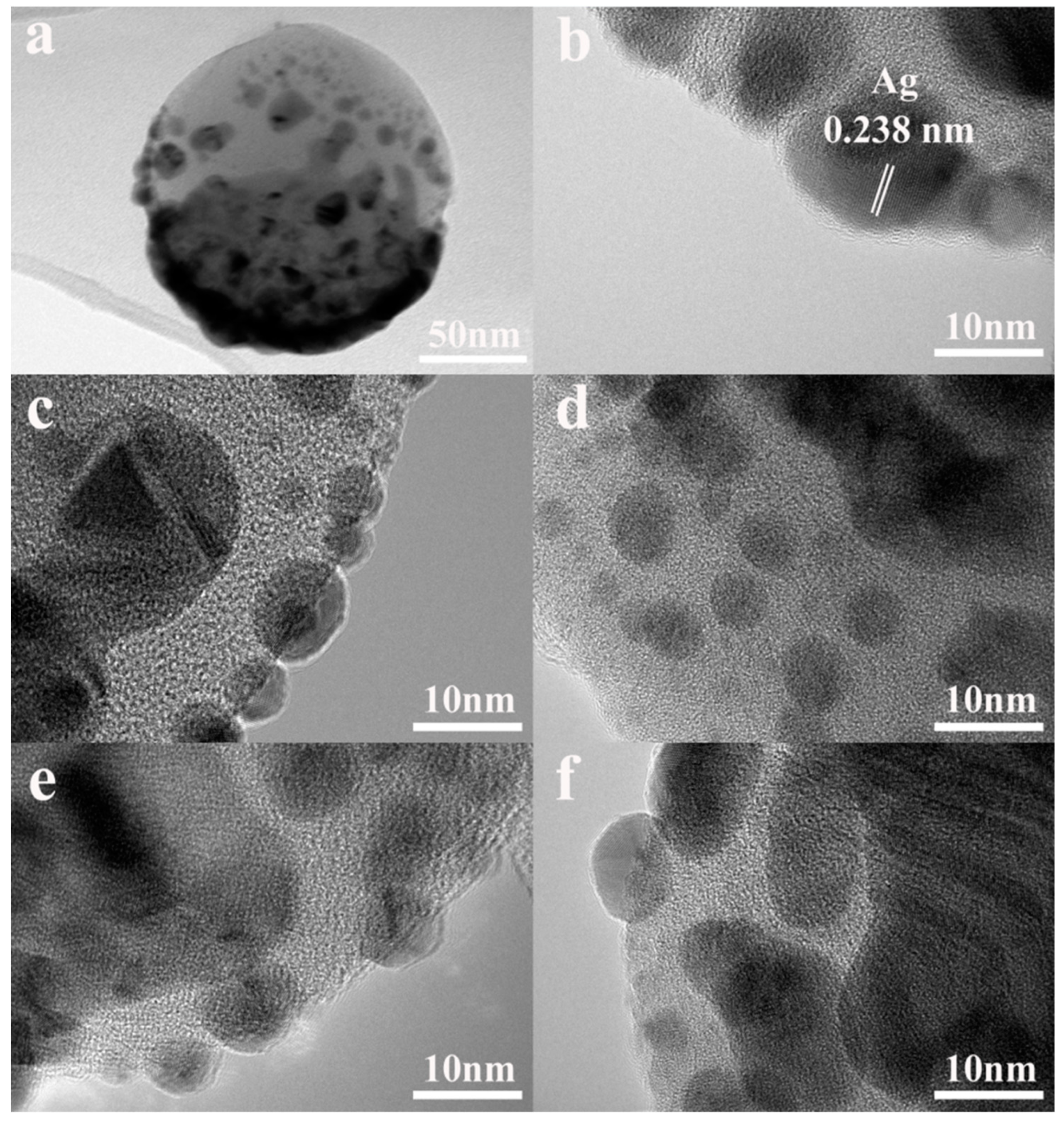
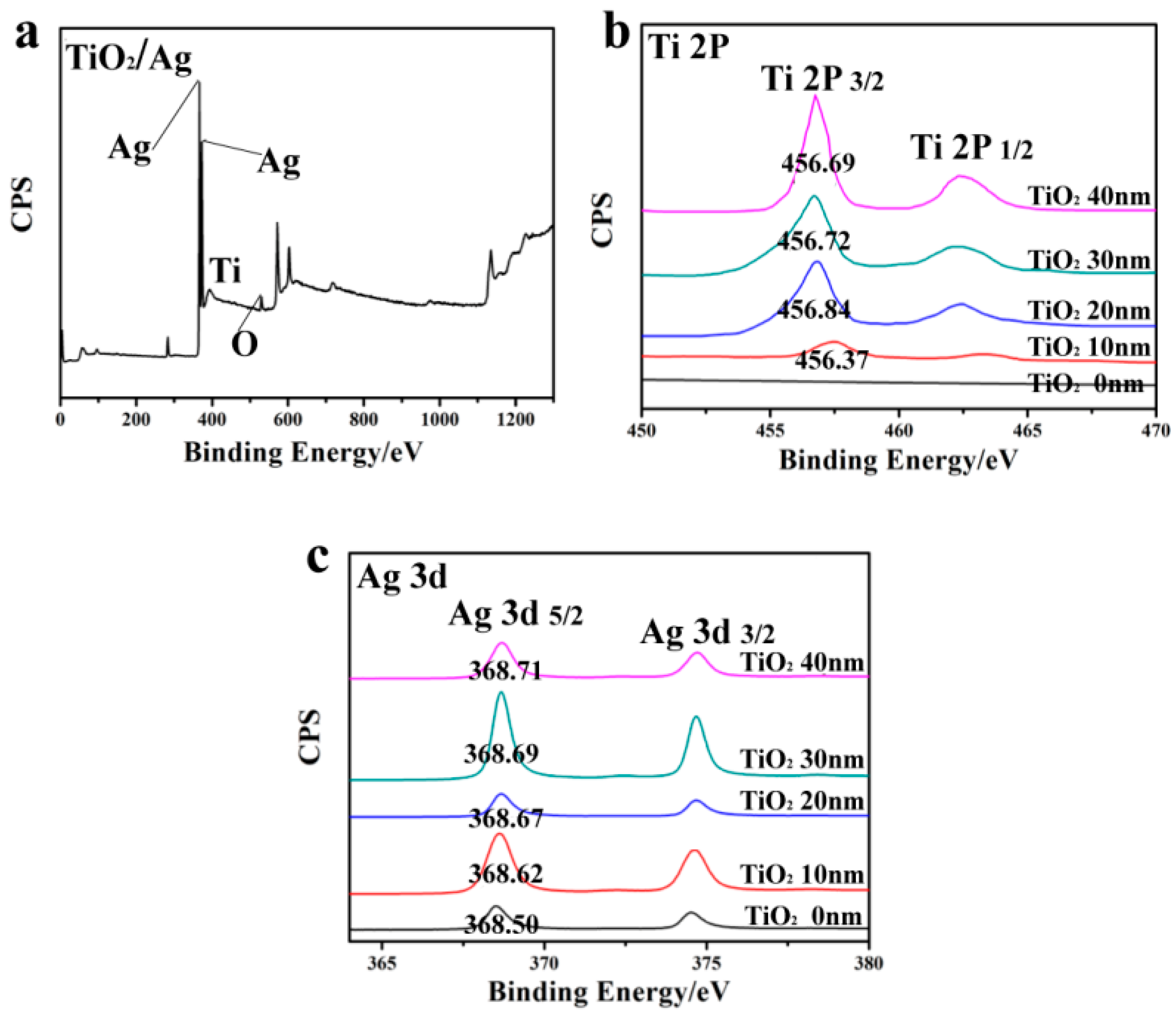
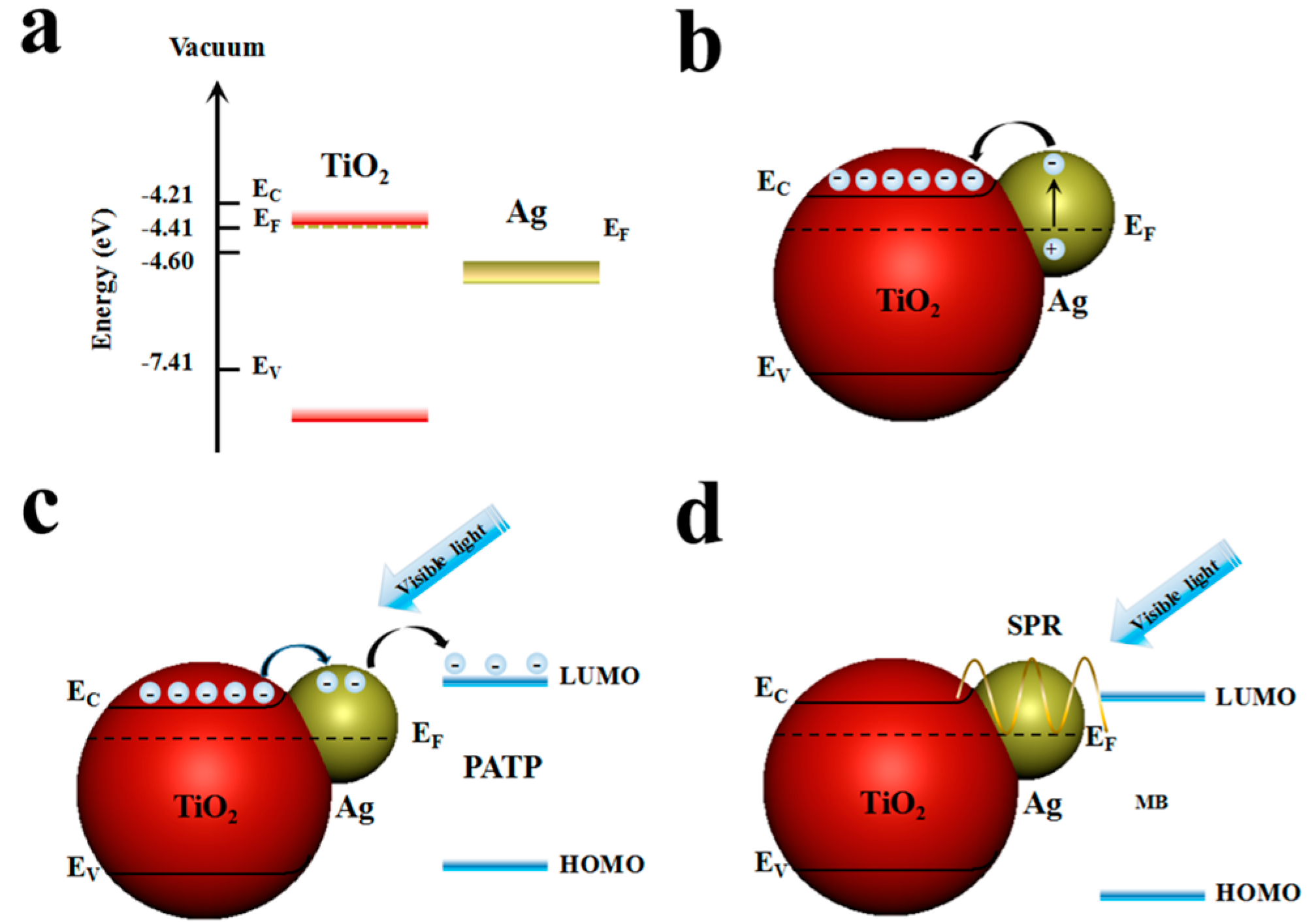
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, N.; Zou, Q.G.; Booksh, K.S. Characterization of electrografted 4-aminophenylalanine layers for lownon-specific binding of proteins. New J. Chem. 2012, 36, 963–970. [Google Scholar] [CrossRef]
- Zou, Q.G.; Kegel, L.L.; Booksh, K.S. Electrografted Diazonium Salt Layers for Antifouling on the Surface of Surface Plasmon Resonance Biosensors. Anal. Chem. 2014, 87, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Dina, N.E.; Gherman, A.M.R.; Chis, V.; Sârbu, C.; Wieser, A.; Bauer, D.; Haisch, C. Characterization of clinically relevant fungi via SERS fingerprinting assisted by novel chemometric models. Anal. Chem. 2018, 90, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 154–164. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, X.; Ren, W.; Wang, W.; Wang, E. Effect of Silver Nanoplates on Raman Spectra of p-Aminothiophenol Assembled on Smooth Macroscopic Gold and Silver Surface. J. Phys. Chem. C 2007, 111, 3259–3265. [Google Scholar] [CrossRef]
- Zou, Q.G.; Menegazzo, N.; Booksh, K.S. Development and Investigation of a Dual-Pad In-Channel Referencing Surface Plasmon Resonance Sensor. Anal. Chem. 2012, 84, 7891–7898. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Lin, X.F.; Yang, Z.L.; Liu, G.K.; Aroca, R.F.; Mao, B.W.; Tian, Z.Q. Surface-Enhanced Raman Scattering in the Ultraviolet Spectral Region: UV-SERS on Rhodium and Ruthenium Electrodes. J. Am. Chem. Soc. 2003, 125, 9598–9599. [Google Scholar] [CrossRef]
- Zhu, S.; Fan, C.; Wang, J.; He, J.; Liang, E.; Chao, M. Surface enhanced Raman scattering of 4-aminothiophenol sandwiched between Ag nanocubes and smooth Pt substrate: The effect of the thickness of Pt film. J. Appl. Phys. 2014, 116, 163–166. [Google Scholar] [CrossRef]
- Jiang, J.; Bosnick, K.; Maillard, M.; Brus, L. Single Molecule Raman Spectroscopy at the Junctions of Large Ag Nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhao, X.Y.; Chen, L.; Chen, S.; Wei, M.; Gao, M.; Wang, C.; Qu, X.; Zhang, Y.; Yang, J. Ordered Nanocap Array Composed of SiO2-Isolated Ag Islands as SERS Platform. Langmuir 2014, 30, 15285–15291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, P.; Yang, X.; Liang, W.; Sun, M. Surface plasmon-driven photocatalysis in ambient, aqueous and high-vacuum monitored by SERS and TERS. J. Photochem. Photobiol. C 2016, 27, 100–112. [Google Scholar] [CrossRef]
- Wang, T.T.; Raghunath, P.; Lin, Y.G.; Lin, M.C. A Synergistic Effect of Hydrogenation and Thiocyanate Treatments on Ag-loaded TiO2 Nanoparticles for Solar-to-Hydrogen Conversion. J. Phys. Chem. C 2017, 121, 9681–9690. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.X.; Zhao, B. Surface-Enhanced Raman Scattering of 4-Mercaptopyridine on the Surface of TiO2 Nanofibers Coated with Ag Nanoparticles. J. Phys. Chem. C 2007, 111, 12786–12791. [Google Scholar]
- Dai, Z.; Wang, G.; Xiao, X.; Wu, W.; Li, W.; Ying, J.; Zheng, J.; Mei, F.; Fu, L.; Wang, J. Obviously Angular, Cuboid-Shaped TiO2 Nanowire Arrays Decorated With Ag Nanoparticle as Ultrasensitive 3D Surface-Enhanced Raman Scattering Substrates. J. Phys. Chem. C 2014, 118, 22711–22718. [Google Scholar] [CrossRef]
- Karawdeniya, B.I.; Bandara, Y.N.D.; Whelan, J.C.; Dwyer, J.R. A General Strategy to Make an On-Demand Library of Structurally and Functionally Diverse SERS Substrates. Appl. Nano Mater. 2018, 1, 960–968. [Google Scholar] [CrossRef]
- Bu, Y.; Zhu, G.; Li, S.; Qi, R.; Bhave, G.; Zhang, D.; Han, R.; Sun, D.; Liu, X.; Hu, Z.; et al. Silver Nanoparticles Embedded Porous Silicon Disks enabled SERS Signal Amplification for Selective Glutathione Detection. Appl. Nano Mater. 2018, 1, 410–417. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, D.; Ni, H.; Wang, D.; Jiang, L.; Fu, D.; Han, X.; Zhang, C.; Chen, H.; Gu, Z.; et al. Multiplex Analysis on a Single Porous Hydrogel Bead with Encoded SERS Nanotags. Appl. Mater. Interfaces 2018, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Yan, C.; Chen, L.; Zhang, Y.J.; Yang, J.H. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Destouches, N.; Vitrant, G.; Lefkir, Y.; Epicier, T.; Vocanson, F.; Bakhti, S.; Fang, Y.; Bandyopadhyay, B. Ahmed, Understanding the Growth Mechanisms of Ag Nanoparticles Controlled by Plasmon-Induced Charge Transfers in Ag-TiO2 Films. J. Phys. Chem. C 2015, 119, 9496–9505. [Google Scholar] [CrossRef]
- Birke, R.L.; Lombardi, J.R. TDDFT Study of Charge-Transfer Raman Spectra of 4-Mercaptopyridine on Various ZnSe Nanoclusters as a Model for the SERS of 4-Mpy on Semiconductors. J. Phys. Chem. C 2018, 122, 4908–4927. [Google Scholar] [CrossRef]
- Zhang, X.; Mainka, M.; Paneff, F.; Hachmeister, H.; Beyer, A.; Gölzhäuser, A.; Huser, T. Surface-enhanced Raman spectroscopy of carbon nanomembranes from aromatic self-assembled monolayers. Langmuir 2018, 34, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Kavitha, R. A review on plasmonic metal-TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogeneratedelectrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zhou, Y.; Wang, Y. SERS activity of self-cleaning silver/titania nanoarray. Appl. Surf. Sci. 2014, 313, 549–557. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, W.; Mao, D.; Chen, C.; Shi, Y.; Lv, X.; Xie, J. Silver-loaded nitrogen-doped yolk-shell mesoporous TiO2 hollow microspheres with enhanced visible light photocatalytic activity. Nanoscale 2014, 7, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, L.; Li, S.; Liu, K.; Wu, S.; Peng, W. Controlled Preparation of Uniform TiO2-Catalyzed Silver Nanoparticle Films for Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2013, 117, 6861–6871. [Google Scholar] [CrossRef]
- Tanaka, A.; Nishino, Y.; Sakaguchi, S.; Yoshikawa, T.; Imamura, K.; Hashimoto, K.; Kominami, H. Functionalization of a plasmonic Au/TiO2 photocatalyst with an Ag co-catalyst for quantitative reduction of nitrobenzene to aniline in 2-propanol suspensions under irradiation of visible light. Chem. Commun. 2013, 49, 2551–2553. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Pu, Y.C.; Chang, K.D.; Liang, Y.F.; Liu, C.M.; Yeh, J.W.; Shih, H.C.; Hsu, Y.J. Ag-Nanoparticle-Decorated SiO2 Nanospheres Exhibiting Remarkable Plasmon-Mediated Photocatalytic Properties. J. Phys. Chem. C 2012, 116, 19039–19045. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, C.; Chen, L.; Liu, Y.; Li, J.; Zhang, Y.; Yang, J. Pillar-cap shaped arrays of Ag/SiO2 multilayers after annealing treatment as a SERS-active substrate. Colloids Surf. A 2016, 506, 96–103. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Dai, W.L.; Fan, K. Dependence of Ag Deposition Methods on the Photocatalytic Activity and Surface State of TiO2 with Twistlike Helix Structure. J. Phys. Chem. C 2009, 113, 8343–8349. [Google Scholar] [CrossRef]

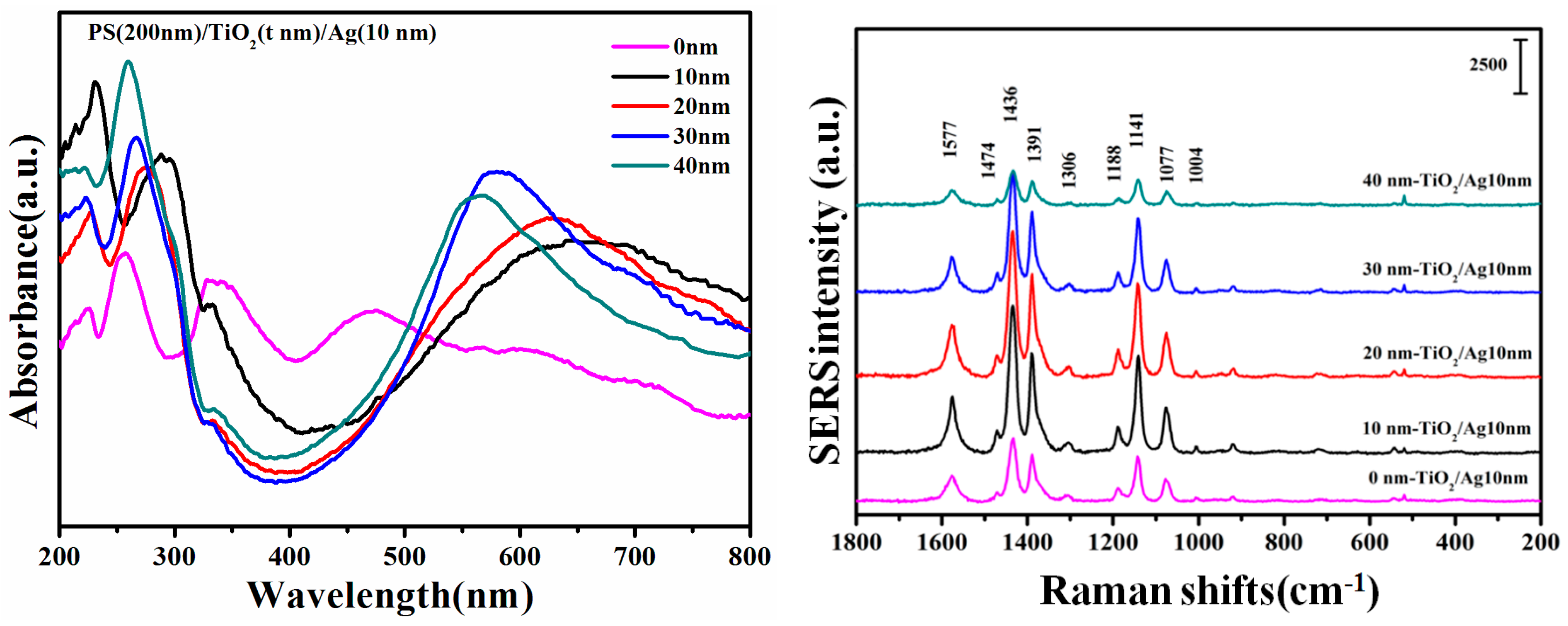
| Wavenumber (cm−1) PS/TiO2/Ag | Band Assignment |
|---|---|
| 1577m | νCC, 8b(b2) |
| 1474w | νCC, 19a(a1) |
| 1436νs | νCC + δCH, 19b(b2) |
| 1391s | δCH + νCC, 3(b2) |
| 1306w | νCC + δCH, 14(b2) |
| 1188w | δCH, 9a(a1) |
| 1141νs | δCH, 9b(b2) |
| 1077m | νCS, 7a(a1) |
| 1004w | γCC + γCC, 18a(a1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, C.; Li, C.; Lu, Z.; Ma, C.; Yan, Y.; Zhang, Y. Charge Transfer Tuned by the Surrounding Dielectrics in TiO2-Ag Composite Arrays. Nanomaterials 2018, 8, 1019. https://doi.org/10.3390/nano8121019
Wang Y, Yan C, Li C, Lu Z, Ma C, Yan Y, Zhang Y. Charge Transfer Tuned by the Surrounding Dielectrics in TiO2-Ag Composite Arrays. Nanomaterials. 2018; 8(12):1019. https://doi.org/10.3390/nano8121019
Chicago/Turabian StyleWang, Yaxin, Chao Yan, Chunxiang Li, Ziyang Lu, Changchang Ma, Yongsheng Yan, and Yongjun Zhang. 2018. "Charge Transfer Tuned by the Surrounding Dielectrics in TiO2-Ag Composite Arrays" Nanomaterials 8, no. 12: 1019. https://doi.org/10.3390/nano8121019
APA StyleWang, Y., Yan, C., Li, C., Lu, Z., Ma, C., Yan, Y., & Zhang, Y. (2018). Charge Transfer Tuned by the Surrounding Dielectrics in TiO2-Ag Composite Arrays. Nanomaterials, 8(12), 1019. https://doi.org/10.3390/nano8121019




