Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines
Abstract
1. Introduction
2. Experimental Program
2.1. Material
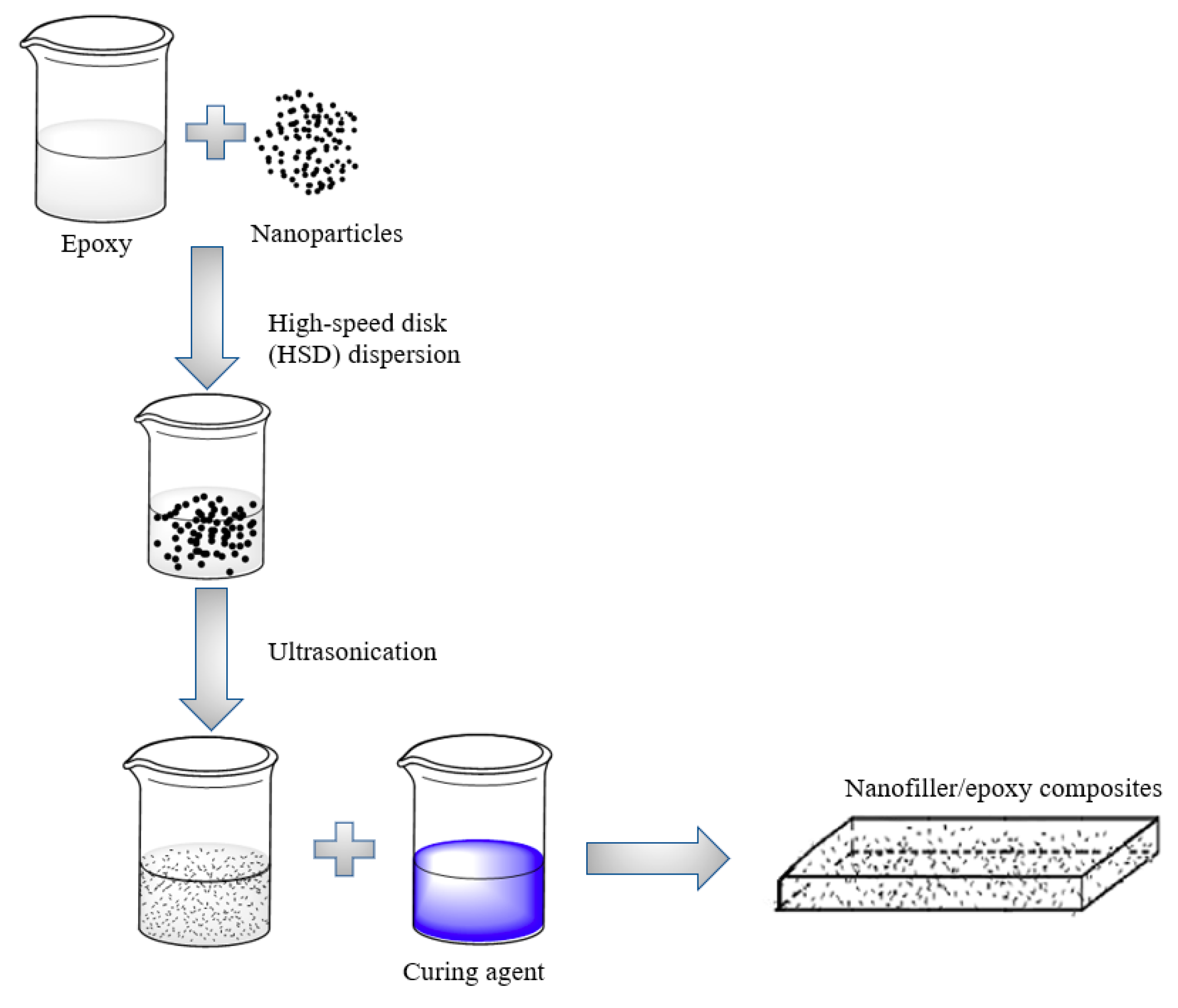
2.2. Fabrication of Nanofiller-reinforced Epoxy Composites and Test Sample Preparation
2.3. Characterization Methods
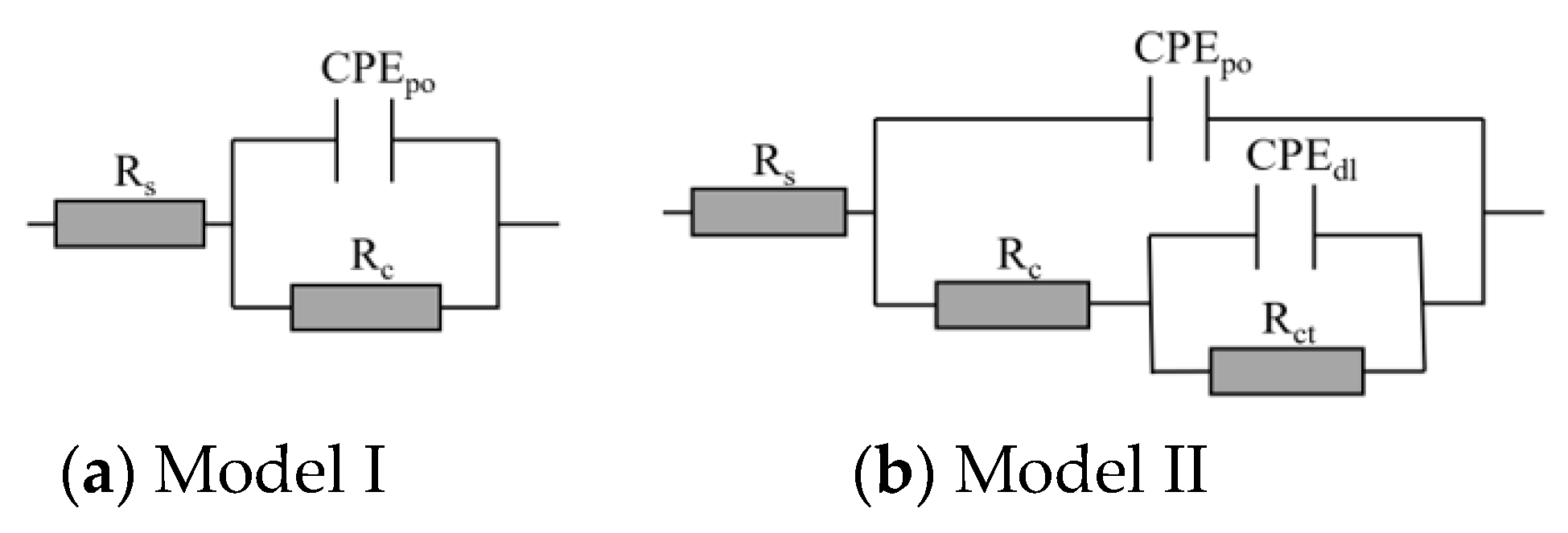
2.3.1. Corrosion Resistance of the Composite Coating Using EIS
2.3.2. Abrasion Resistance of the Composite Coating Using the Taber Abraser Method
2.3.3. Adhesion of the Composite Coating Using Tensile Button Testing
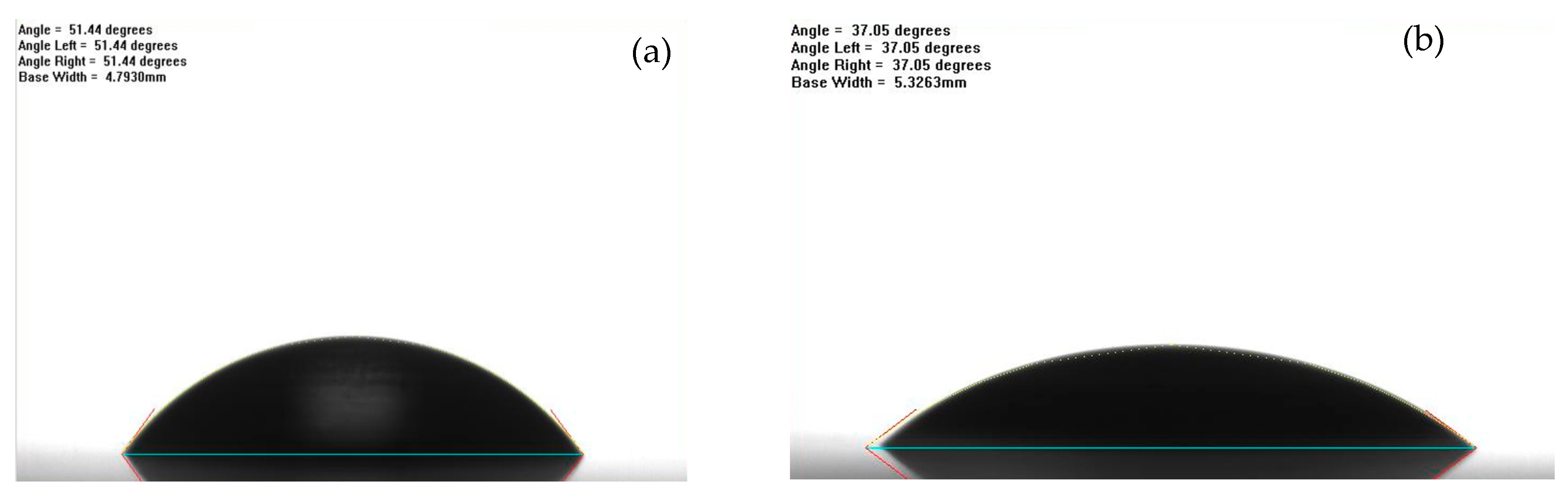
2.3.4. Contact Angle Testing of the Composite Coating
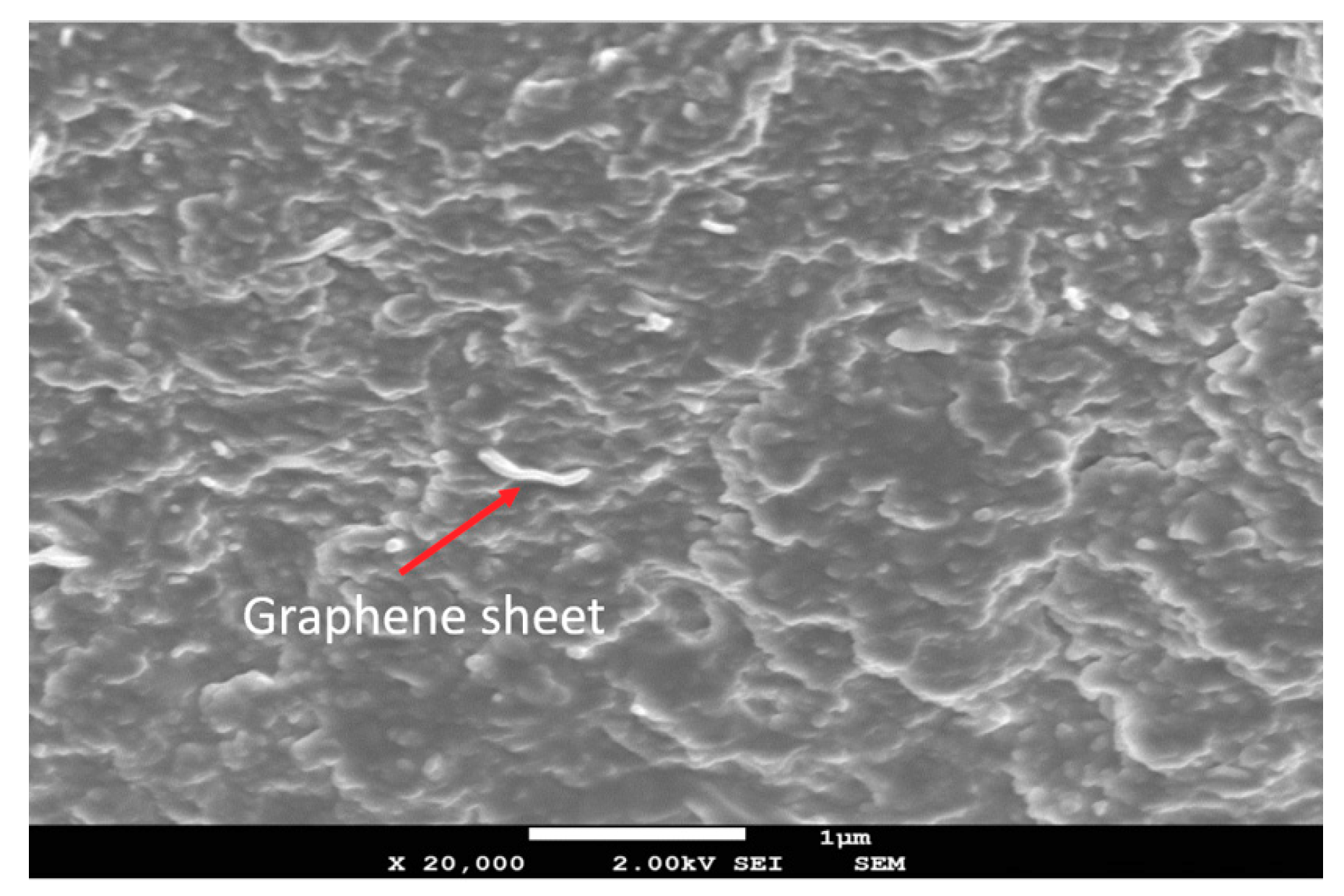
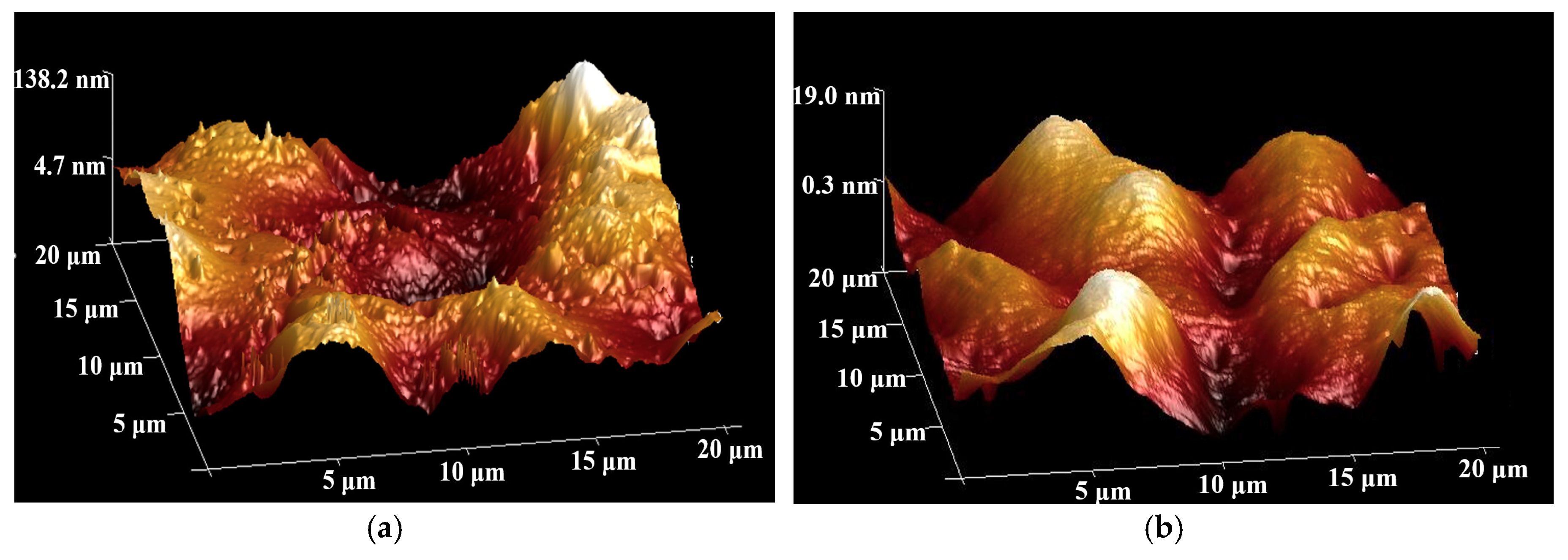
2.3.5. Microstructures Using Scanning Electron Microscopy and Atomic Force Microscopy
3. Results and Discussions
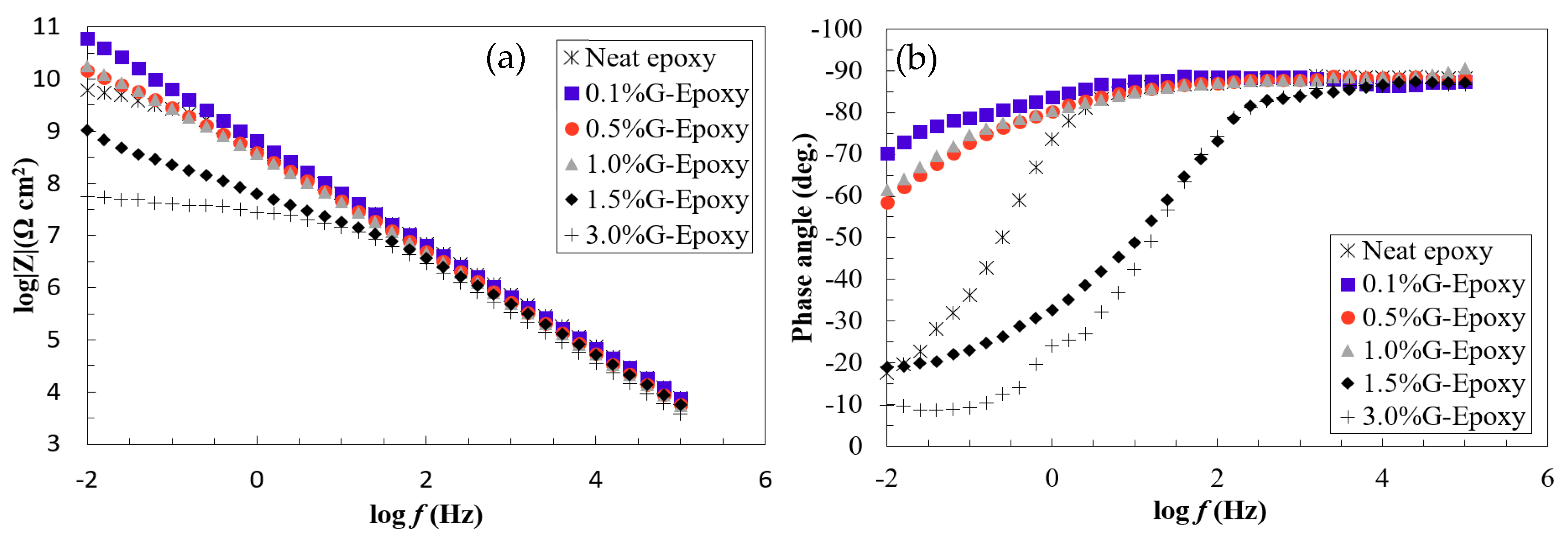
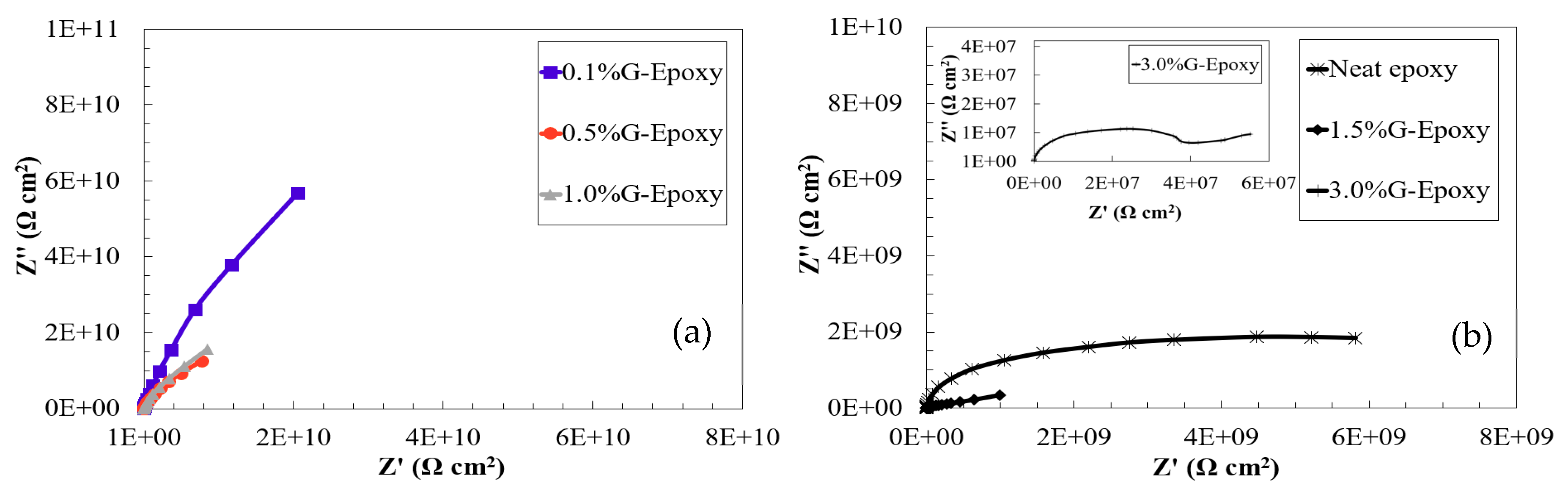
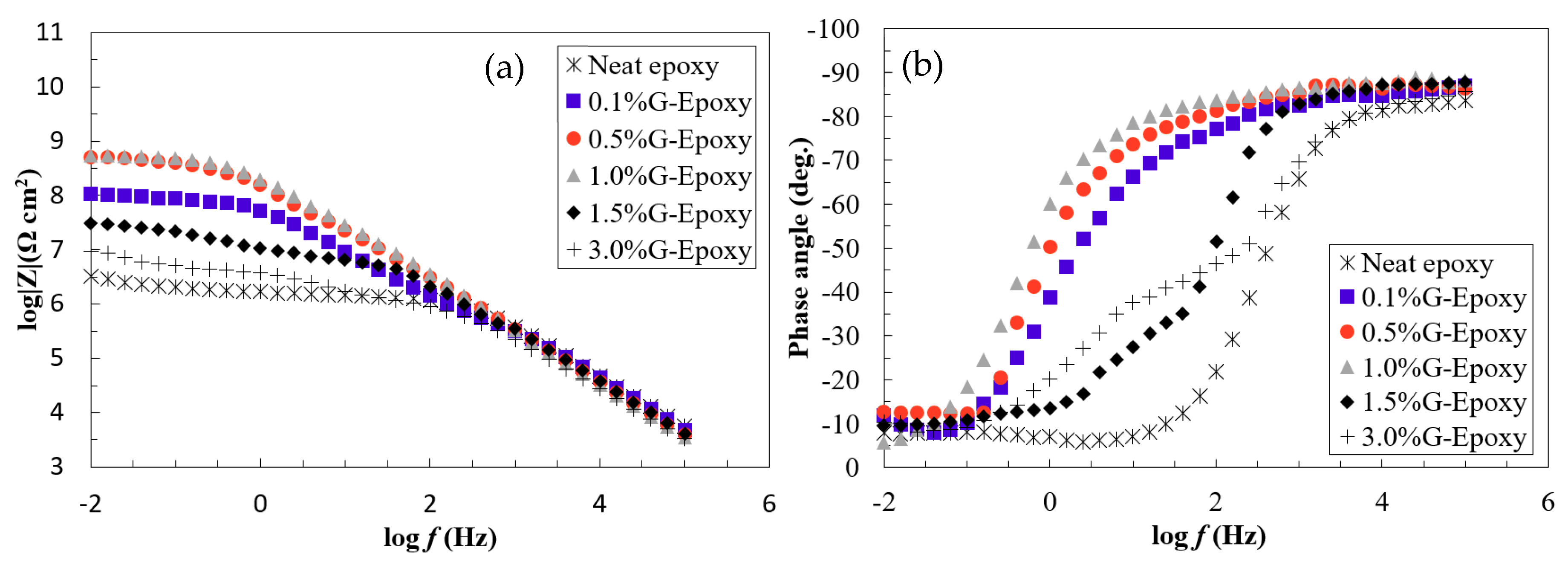
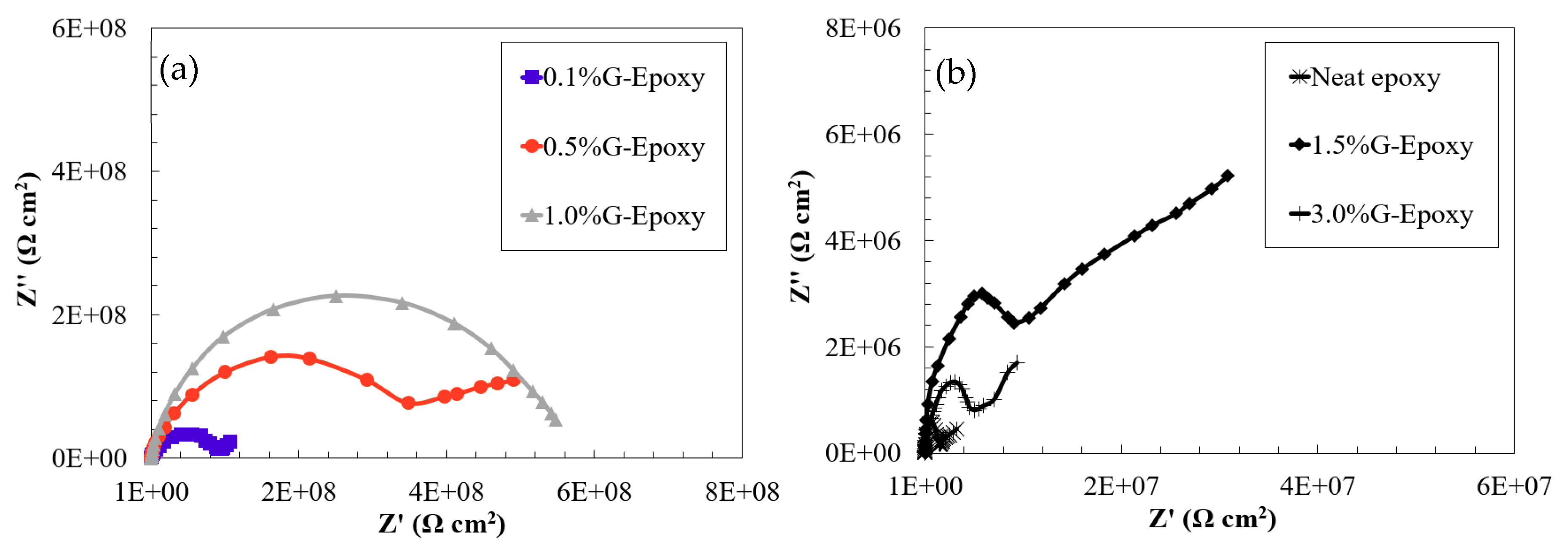
3.1. Corrosion Resistance of the New Nanocomposites in the Short Term
3.2. Corrosion Resistance of the New Nanocomposites in the Long Run
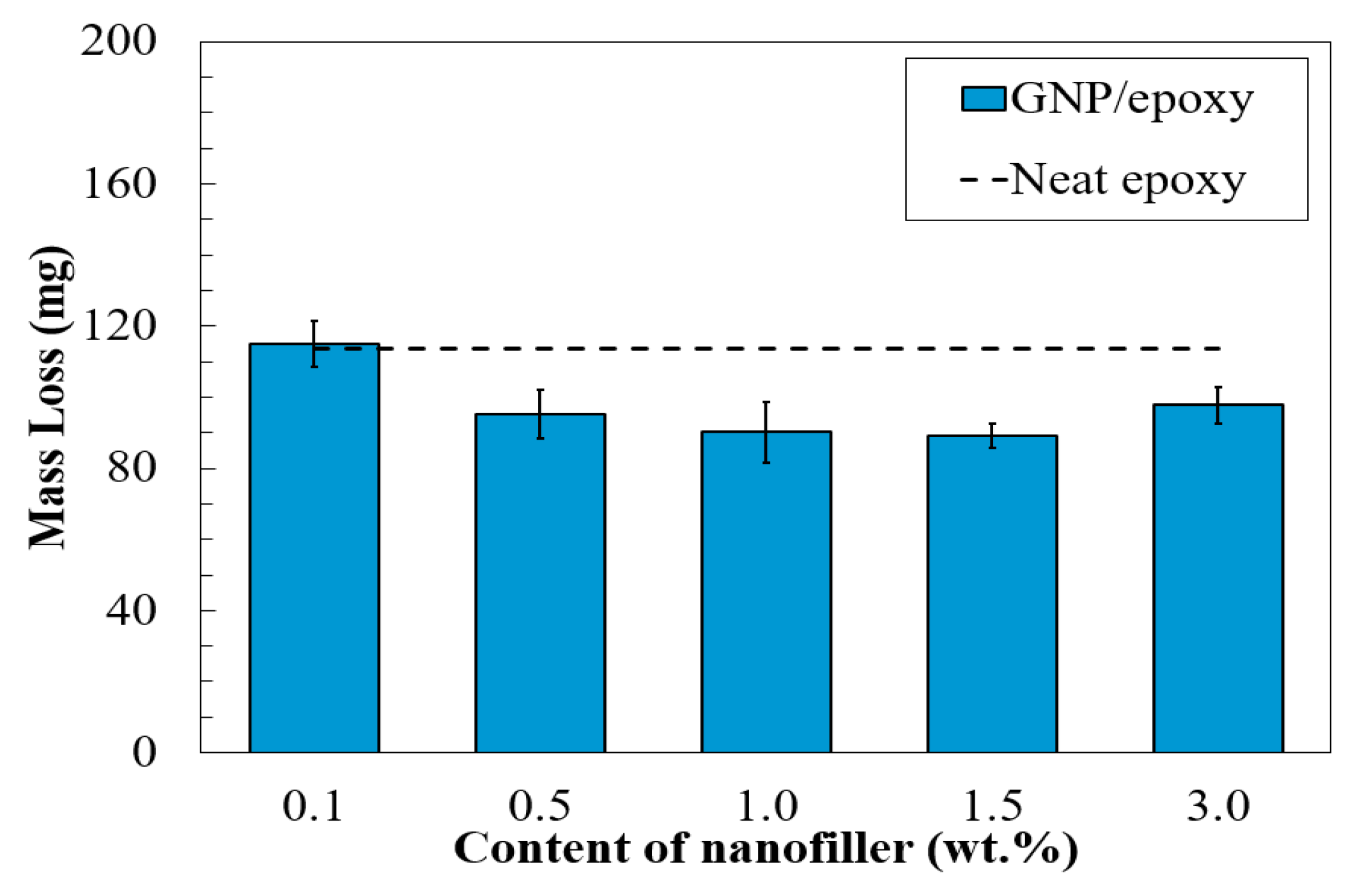
3.3. Abrasion Resistance of Nano-Reinforced Composites
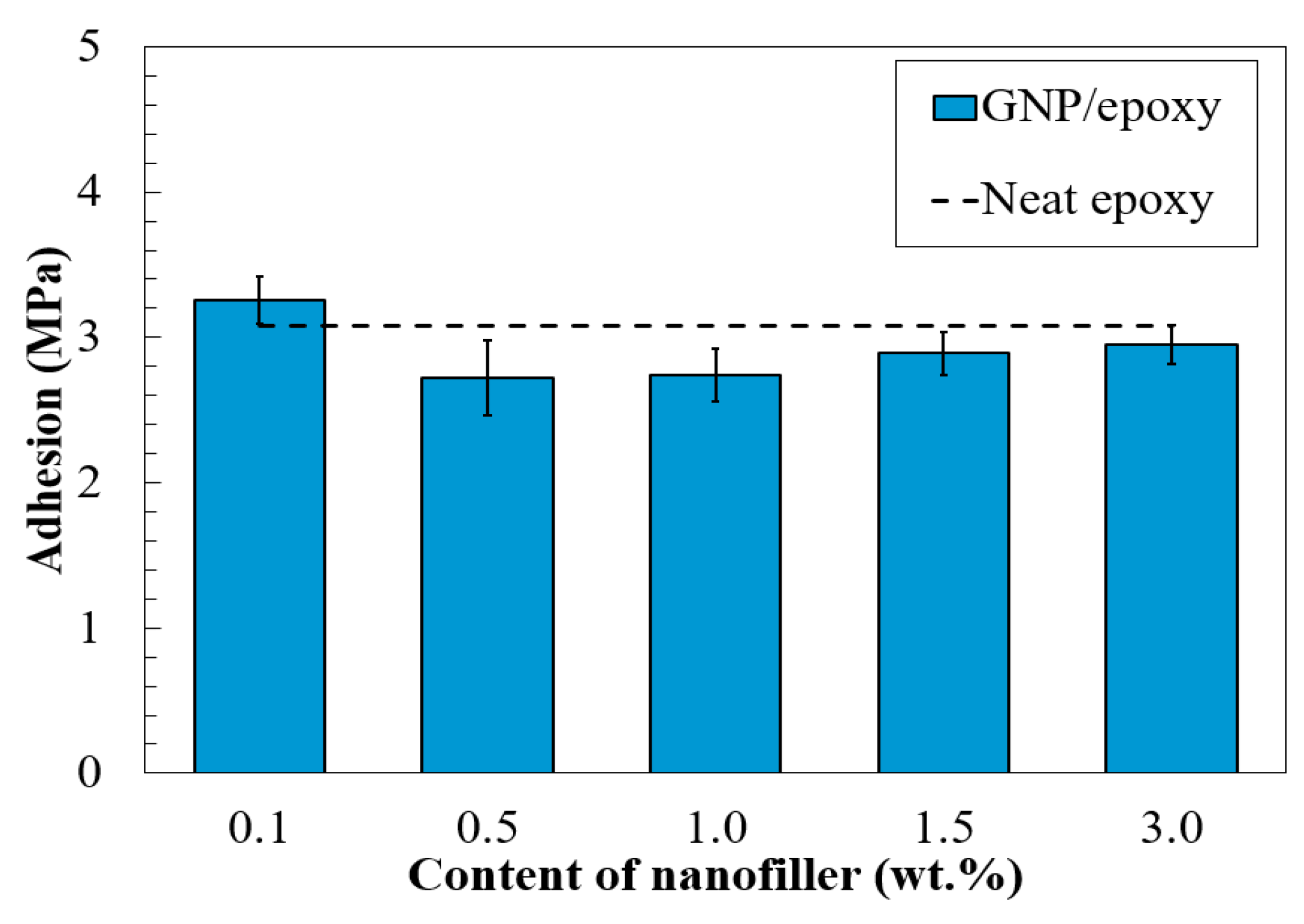
3.4. Adhesive Bonding Strength of Nano-Reinforced Composites to the Substrate
3.5. Surface Roughness of the Composite Coating
4. Conclusions
- (a)
- Graphene-based composite coatings exhibited enhanced mechanical and electrochemical properties that enabled the accommodation of the needs in wide structural applications, while maintaining high adhesion strength to the substrate.
- (b)
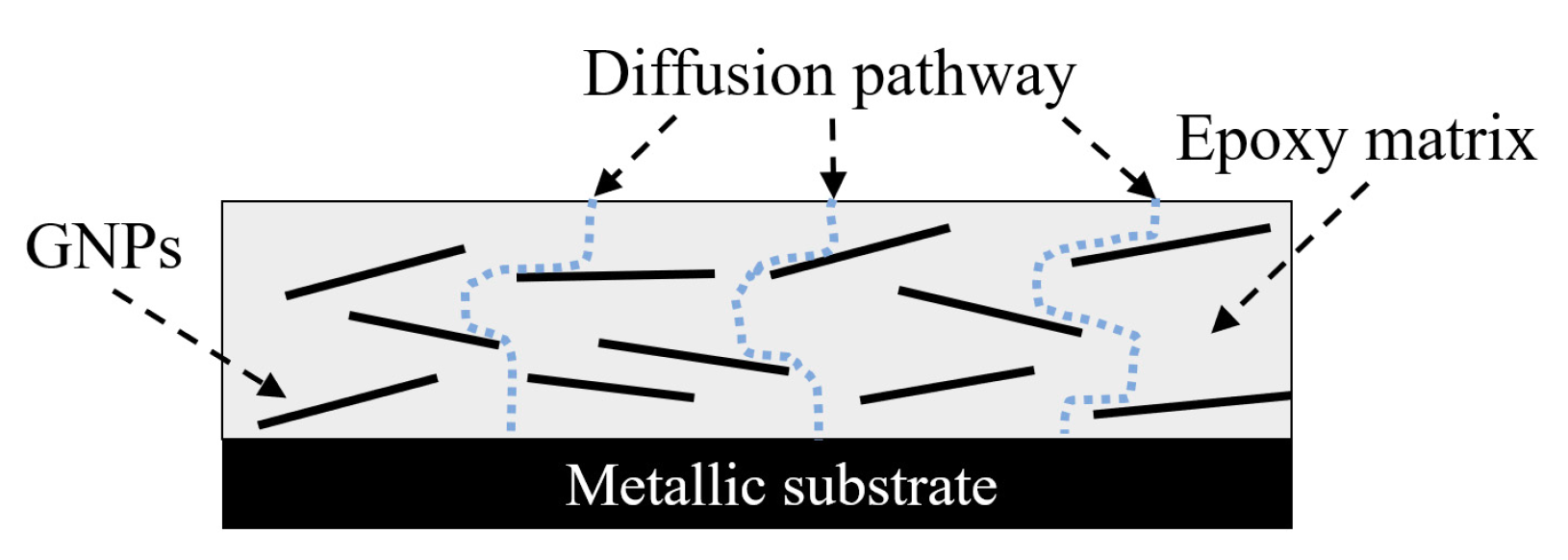
- Electrochemical behaviors of the test samples revealed that the nanocomposites with 0.1 to 1.0 wt.% of GNPs offered effective barrier properties for corrosion mitigation in both short- and long-term performances. Due to the agglomeration when increasing the high content of nanoparticles, the samples with 1.5 or 3.0 wt.% of GNPs exhibited some levels of reduction in corrosion resistance.
- (c)
- A similar trend was observed in the abrasion resistance. The results demonstrated no significant influence on the water contact angle and adhesion test by incorporating graphene in the epoxy resin. The graphene-based composite coatings with 0.5 to 1.5 wt.% of GNPs provided an over 20% increase in abrasion resistance in terms of low mass loss, as compared to the neat epoxy.
- (d)
- Results of the adhesion strength of the composites to the substrate showed that incorporation of the nanoparticles has minor effects on their interfacial bonding, showing a reduction within 10%. Moreover, the contact angle tests and surface roughness of the composite coatings both supported that the graphene particles smoothened the surface texture, as compared to the neat epoxy samples.
- (e)
- The composite coatings that were reinforced by graphene nanofillers display promising results in terms of enhanced mechanical and electrical properties, thereby offering the potential for widespread real-world applications, including oil and gas pipelines and bridges. Note that conclusions were mainly drawn from the findings in a short-term manner. Although the accelerated durability tests by 200 h exposure of salt spray confirmed the enhanced performance of the graphene-loaded composite coatings over conventional neat epoxy, it still necessitates the further investigation of the long-term durability tests in future studies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Kaseasbeh, Q.; Lin, Z.; Wang, Y.; Azarmi, F.; Qi, X. Electrochemical Characterization of Steel Bridge Welds under Simulated Durability Test. J. Bridge Eng. 2018, 23, 04018068. [Google Scholar] [CrossRef]
- Wang, X.; Qi, X.; Lin, Z.; Wang, J.; Gong, N. Electrochemical Characterization of the Soils Surrounding Buried or Embedded Steel Elements. In Proceedings of the ASCE 2016 Pipelines, Kansas City, MO, USA, 17–20 July 2016. [Google Scholar]
- Fessler, R.R. Pipeline Corrosion; US Department of Transportation Pipeline and Hazardous Materials Safety Administration: Baker, Evanston, IL, USA, 2008.
- Chiong, S.; Goh, P.; Ismail, A. Novel hydrophobic PVDF/APTES-GO nanocomposite for natural gas pipelines coating. J. Nat. Gas Sci. Eng. 2017, 42, 190–202. [Google Scholar] [CrossRef]
- Samimi, A. Use of Polyurethane Coating to Prevent Corrosion in Oil and Gas Pipelines Transfer. Int. J. Innov. Appl. Stud. 2012, 1, 186–193. [Google Scholar]
- Pan, H.; Gui, G.; Lin, Z.; Yan, C. Deep BBN Learning for Health Assessment toward Decision-Making on Structures under Uncertainties. KSCE J. Civ. Eng. 2018, 22, 928–940. [Google Scholar] [CrossRef]
- Lin, Z.; Pan, H.; Wang, X.; Li, M. Data-driven structural diagnosis and conditional assessment: From shallow to deep learning. In Sensors and Smart Structures Technologies for Civil, Mechanical, and Aerospace Systems 2018; International Society for Optics and Photonics: Denver, CO, USA, 2018; Volume 10598, p. 1059814. [Google Scholar]
- Gui, G.; Pan, H.; Lin, Z.; Li, Y.; Yuan, Z. Data-driven support vector machine with optimization techniques for structural health monitoring and damage detection. KSCE J. Civ. Eng. 2017, 21, 523–534. [Google Scholar] [CrossRef]
- Lin, Z.B.; Pan, H.; Ge, R.; Wang, X.; Wang, J.; Gong, N. Embedded Wireless Passive Sensor Networks for Health Monitoring of Welded Joints in Onshore Metallic Pipelines. In Proceedings of the ASCE 2017 Pipelines, Phoenix, AZ, USA, 6–9 August 2017. [Google Scholar]
- Dittanet, P.; Pearson, R.A.; Kongkachuichay, P. Thermo-mechanical behaviors and moisture absorption of silica nanoparticle reinforcement in epoxy resins. Int. J. Adhes. Adhes. 2017, 78, 74–82. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Ma, I.; Farah, Z.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–polymer composites. Diam. Relat. Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mat. Sci. Eng. R 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Shadlou, S.; Alishahi, E.; Ayatollahi, M. Fracture behavior of epoxy nanocomposites reinforced with different carbon nano-reinforcements. Compos. Struct. 2013, 95, 577–581. [Google Scholar] [CrossRef]
- Miller, S.G.; Bauer, J.L.; Maryanski, M.J.; Heimann, P.J.; Barlow, J.P.; Gosau, J.-M.; Allred, R.E. Characterization of epoxy functionalized graphite nanoparticles and the physical properties of epoxy matrix nanocomposites. Compos. Sci. Technol. 2010, 70, 1120–1125. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mat. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef]
- Singh, B.P.; Jena, B.K.; Bhattacharjee, S.; Besra, L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf. Coat. Technol. 2013, 232, 475–481. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Singh, B.P.; Nayak, S.; Nanda, K.K.; Jena, B.K.; Bhattacharjee, S.; Besra, L. The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 2013, 61, 47–56. [Google Scholar] [CrossRef]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/epoxy composites. Mater. Des. (1980–2015) 2015, 66, 142–149. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Ayatollahi, M.; Alishahi, E.; Doagou-R, S.; Shadlou, S. Tribological and mechanical properties of low content nanodiamond/epoxy nanocomposites. Compos. Part B-Eng. 2012, 43, 3425–3430. [Google Scholar] [CrossRef]
- Dideikin, A.T. Applications of Detonation Nanodiamonds. Detonat. Nano. Sci. Appl. 2014, 239. [Google Scholar]
- Tsai, J.-L.; Lu, T.-C. Investigating the load transfer efficiency in carbon nanotubes reinforced nanocomposites. Compos. Struct. 2009, 90, 172–179. [Google Scholar] [CrossRef]
- Zhang, G.; Karger-Kocsis, J.; Zou, J. Synergetic effect of carbon nanofibers and short carbon fibers on the mechanical and fracture properties of epoxy resin. Carbon 2010, 48, 4289–4300. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Song, L.; Hu, Y.; Xing, W.; Lu, H. Morphology, mechanical and thermal properties of graphene-reinforced poly (butylene succinate) nanocomposites. Compos. Sci. Technol. 2011, 72, 1–6. [Google Scholar] [CrossRef]
- Slonczewski, J.; Weiss, P. Band structure of graphite. Phys. Rev. 1958, 109, 272. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Spallas, J.P. Different contrast mechanisms in SEM imaging of graphene. Available online: http://www.toyo.co.jp/files/user/img/product/microscopy/pdf/5991-0782EN.pdf (accessed on 21 November 2018).
- Hiura, H.; Miyazaki, H.; Tsukagoshi, K. Determination of the number of graphene layers: Discrete distribution of the secondary electron intensity stemming from individual graphene layers. Appl. Phys. Express 2010, 3, 095101. [Google Scholar] [CrossRef]
- Kim, J.; Kim, F.; Huang, J. Seeing graphene-based sheets. Mater. Today 2010, 13, 28–38. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. A novel coating material that uses nano-sized SiO2 particles to intensify hydrophobicity and corrosion protection properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Zheng, W.; Li, W.; Zhao, H.; Wang, L. Long-term corrosion protection of mild steel by epoxy coating containing self-doped polyaniline nanofiber. Synth. Met. 2017, 229, 39–46. [Google Scholar] [CrossRef]
- Montazeri, A.; Chitsazzadeh, M. Effect of sonication parameters on the mechanical properties of multi-walled carbon nanotube/epoxy composites. Mater. Des. (1980–2015) 2014, 56, 500–508. [Google Scholar] [CrossRef]
- Prolongo, S.; Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]
- Moghadam, A.D.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B-Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- Shen, G.; Chen, Y.; Lin, L.; Lin, C.; Scantlebury, D. Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals. Electrochim. Acta 2005, 50, 5083–5089. [Google Scholar] [CrossRef]
- Avella, M.; Errico, M.E.; Martuscelli, E. Novel PMMA/CaCO3 nanocomposites abrasion resistant prepared by an in situ polymerization process. Nano Lett. 2001, 1, 213–217. [Google Scholar] [CrossRef]

| Items | Group | |
|---|---|---|
| Neat Epoxy | GNP (Graphene Nanoplatelets)/Epoxy | |
| Surface Roughness (Ra, nm) | 29 ± 5 | 4 ± 2 |
| Contact Angle (degrees) | 55 ± 10 | 41 ± 7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Qi, X.; Lin, Z.; Battocchi, D. Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines. Nanomaterials 2018, 8, 1005. https://doi.org/10.3390/nano8121005
Wang X, Qi X, Lin Z, Battocchi D. Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines. Nanomaterials. 2018; 8(12):1005. https://doi.org/10.3390/nano8121005
Chicago/Turabian StyleWang, Xingyu, Xiaoning Qi, Zhibin Lin, and Dante Battocchi. 2018. "Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines" Nanomaterials 8, no. 12: 1005. https://doi.org/10.3390/nano8121005
APA StyleWang, X., Qi, X., Lin, Z., & Battocchi, D. (2018). Graphene Reinforced Composites as Protective Coatings for Oil and Gas Pipelines. Nanomaterials, 8(12), 1005. https://doi.org/10.3390/nano8121005







