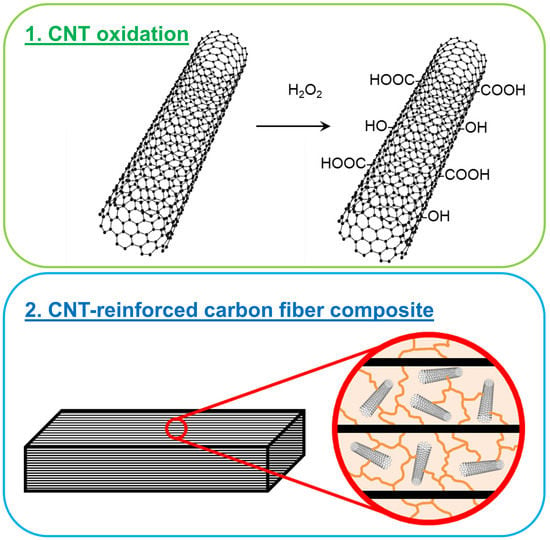
Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP
Abstract
1. Introduction
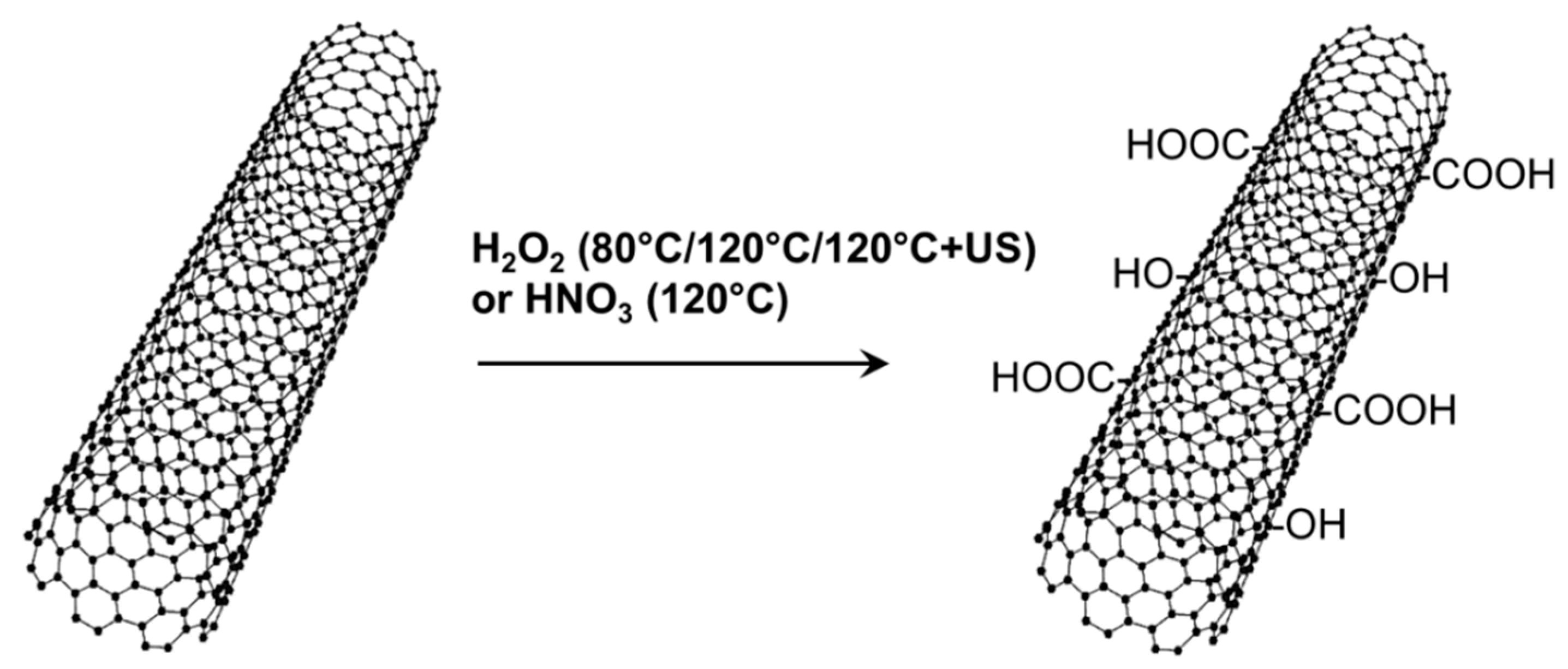
1.1. Oxidation of CNT
1.2. CNT-Reinforced Composites
2. Materials and Methods
2.1. Oxidation of CNT
2.2. X-ray Photoelectron Spectroscopy (XPS)
2.3. Raman Spectroscopy
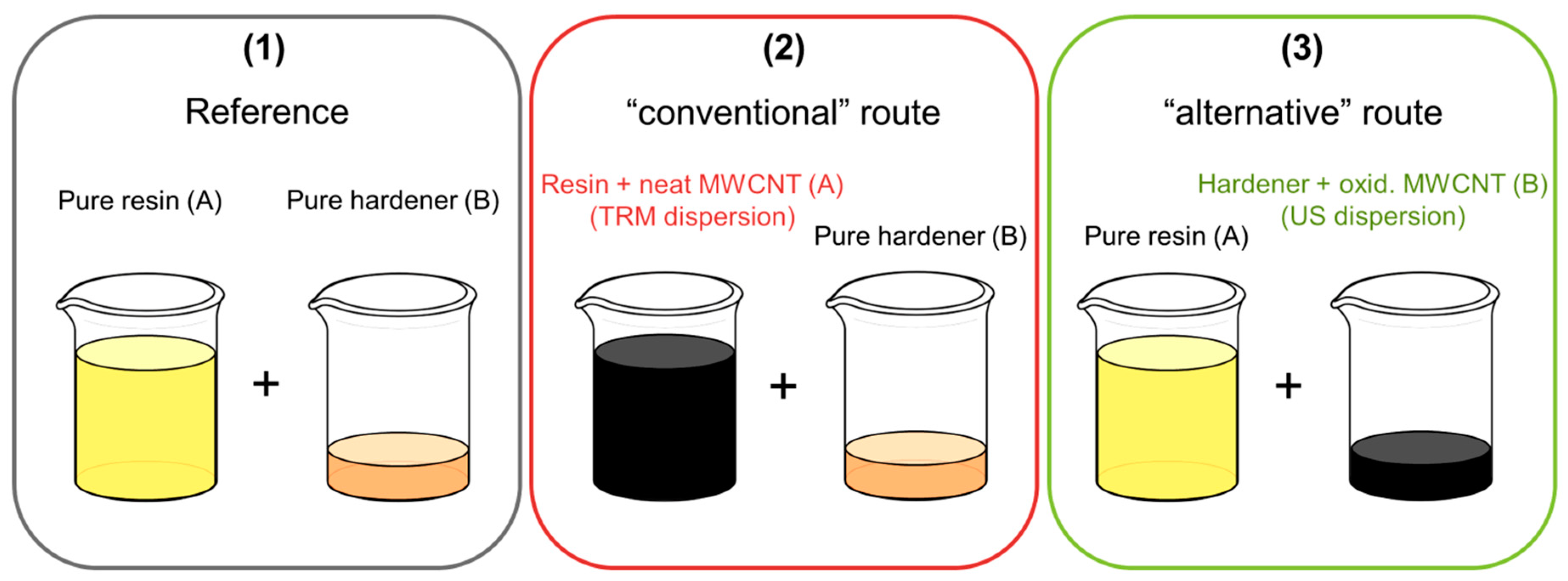
2.4. Dispersion
2.4.1. Three-Roll Mill
2.4.2. Ultrasonic
2.5. Preparation of CNT-Reinforced Composites
2.6. Mechanical Characterization of CNT-Reinforced Composites
3. Results and Discussion
3.1. XPS Measurements
3.2. Raman Spectroscopy
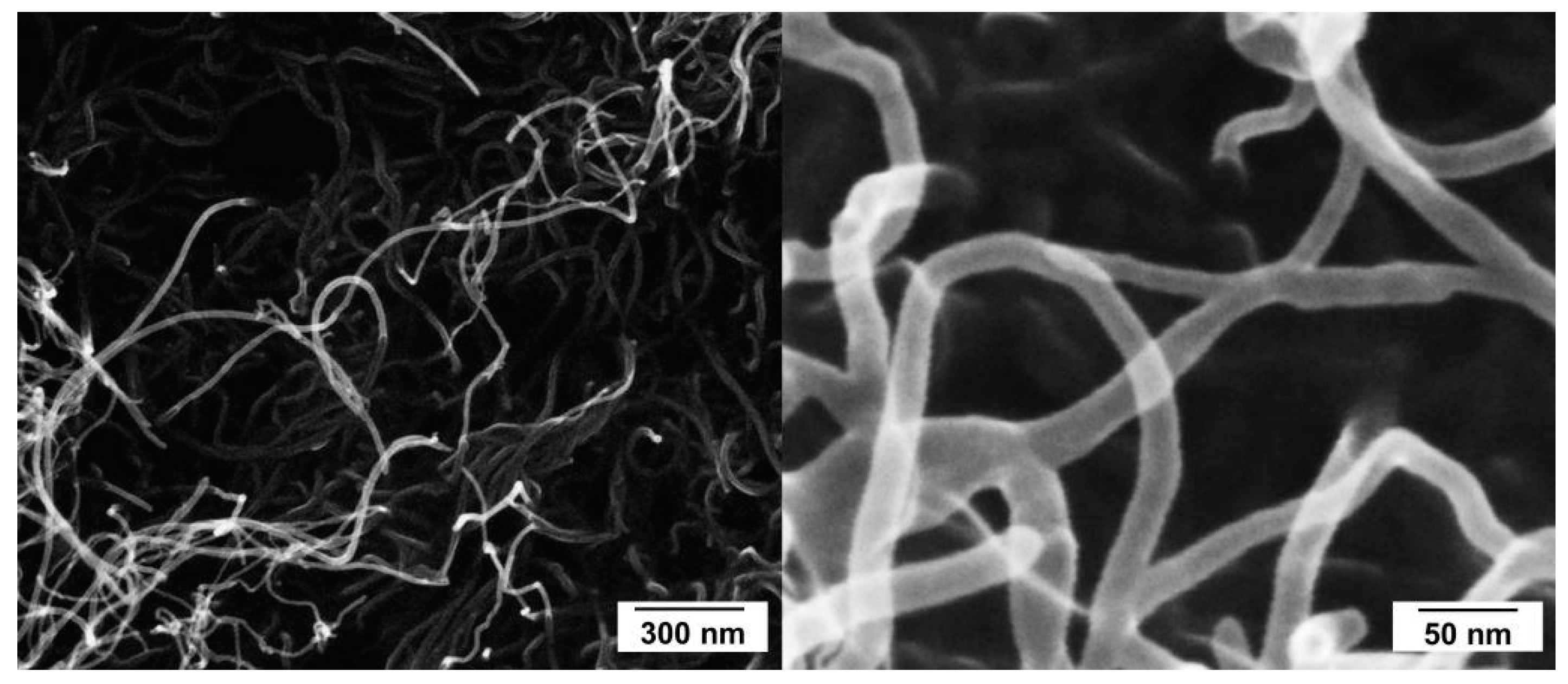
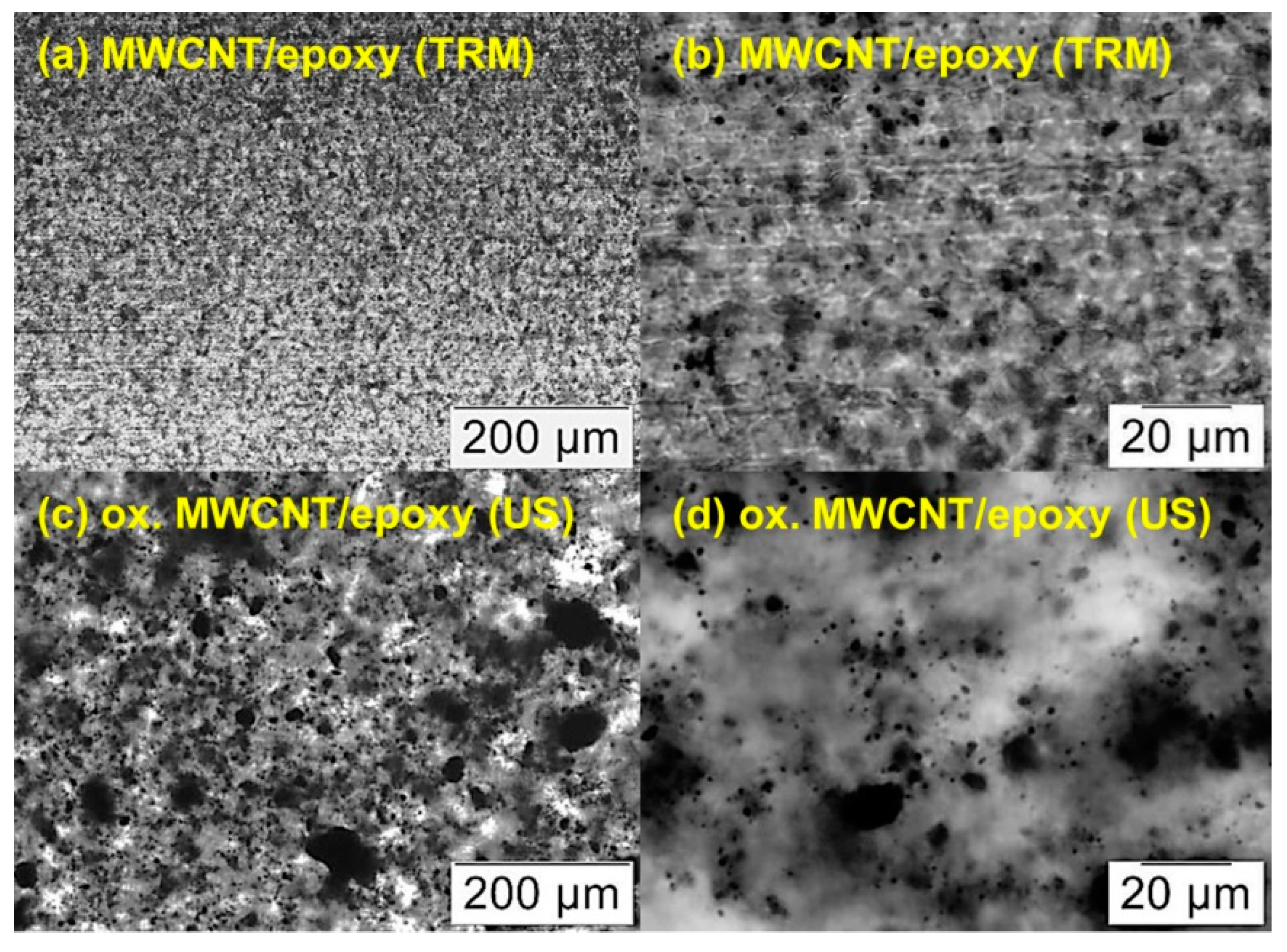
3.3. Dispersion of MWCNT
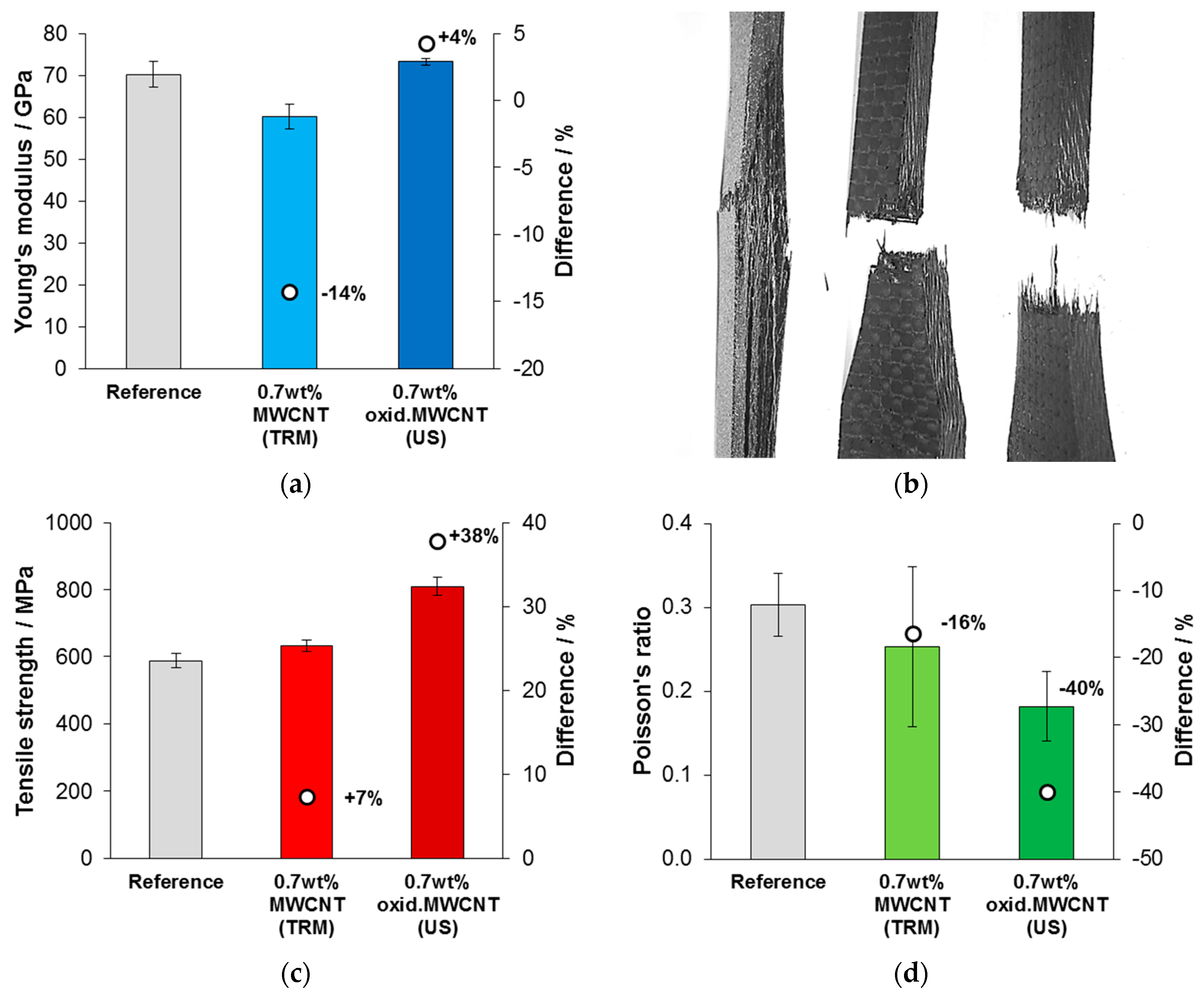
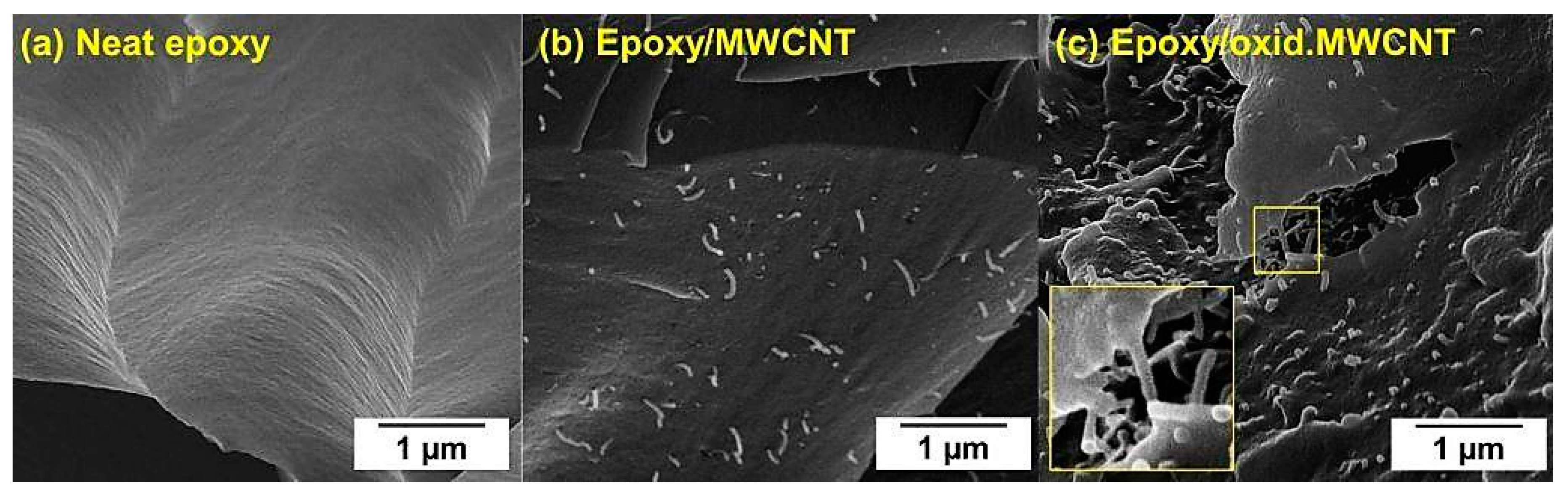
3.4. Mechanical Testing of CNT-Reinforced CFRP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.; Ruoff, R. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, R.; Wang, B.; Zhang, C. Load-transfer in functionalized carbon nanotubes/polymer composites. Chem. Phys. Lett. 2008, 457, 371–375. [Google Scholar] [CrossRef]
- Ma, P.C.; Zheng, Q.B.; Mäder, E.; Kim, J.K. Behavior of load transfer in functionalized carbon nanotube/epoxy nanocomposites. Polymer 2012, 53, 6081–6088. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xu, S.; Li, Y. Nanohelices from planar polymer self-assembled in carbon nanotubes. Sci. Rep. 2016, 6, 30310. [Google Scholar] [CrossRef] [PubMed]
- Stobinski, L.; Lesiak, B.; Kövér, L.; Tóth, J.; Biniak, S.; Trykowski, G.; Judek, J. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J. Alloys Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Zhou, W.; Sasaki, S.; Kawasaki, A. Effective control of nanodefects in multiwalled carbon nanotubes by acid treatment. Carbon 2014, 78, 121–129. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Nanyan, Z.; Jining, X.; Vijay, K.V. Functionalization of carbon nanotubes by potassium permanganate assisted with phase transfer catalyst. Smart. Mater. Struct. 2002, 11, 962. [Google Scholar]
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, H. Effects of oxidation by hydrogen peroxide on the structures of multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2006, 45, 6483–6488. [Google Scholar] [CrossRef]
- Rodríguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Lozano-Castello, D.; Lillo-Ródenas, M.; Cazorla-Amorós, D. Controlling Porosity to Improve Activated Carbon Applications; Springer: Berlin, Germany, 2007; pp. 97–106. [Google Scholar]
- Milowska, K.Z. Influence of Carboxylation on Structural and Mechanical Properties of Carbon Nanotubes: Composite Reinforcement and Toxicity Reduction Perspectives. J. Phys. Chem. C 2015, 119, 26734–26746. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Mater. Sci. Semicond. Process. 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Charlier, J.C. Defects in carbon nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Y.; Peng, J.; Zhang, X. Effects of Stone-Wales Defects on the Thermal Conductivity of Carbon Nanotubes. J. Heat. Transfer. 2012, 134, 092401. [Google Scholar]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening carbon nanotubes with oxygen and implications for filling. Nature 1993, 362, 522–525. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M.; Hiura, H.; Tanigaki, K. Purification of nanotubes. Nature 1994, 367, 519. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Naumenko, V.; Kuznetsova, A.; Yates, J.T.; Liu, J.; Smalley, R.E. Infrared Spectral Evidence for the Etching of Carbon Nanotubes: Ozone Oxidation at 298 K. J. Am. Chem. Soc. 2000, 122, 2383–2384. [Google Scholar] [CrossRef]
- Peng, K.; Liu, L.Q.; Li, H.; Meyer, H.; Zhang, Z. Room temperature functionalization of carbon nanotubes using an ozone/water vapor mixture. Carbon 2011, 49, 70–76. [Google Scholar] [CrossRef]
- Sham, M.L.; Kim, J.K. Surface functionalities of multi-wall carbon nanotubes after UV/Ozone and TETA treatments. Carbon 2006, 44, 768–777. [Google Scholar] [CrossRef]
- Ago, H.; Kugler, T.; Cacialli, F.; Salaneck, W.R.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Work Functions and Surface Functional Groups of Multiwall Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 8116–8121. [Google Scholar] [CrossRef]
- Guadagno, L.; De Vivo, B.; Di Bartolomeo, A.; Lamberti, P.; Sorrentino, A.; Tucci, V.; Vertuccio, L.; Vittoria, V. Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 2011, 49, 1919–1930. [Google Scholar] [CrossRef]
- Sharma, K.; Shukla, M. Three-Phase Carbon Fiber Amine Functionalized Carbon Nanotubes Epoxy Composite: Processing, Characterisation, and Multiscale Modeling. J. Nanomater. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Wu, J.; Guo, J.; Zhang, Q.; Gao, L.; Li, H.; Deng, H.; Jiang, W.; Sui, G.; Yang, X. Effect of different amino functionalized carbon nanotubes on curing behavior and mechanical properties of carbon fiber/epoxy composites. Polym. Compos. 2018, 39, E733–E744. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, S.; Mehta, R. Pristine and amino functionalized carbon nanotubes reinforced glass fiber epoxy composites. Compos. Part A Appl. Sci. Manuf. 2015, 76, 92–101. [Google Scholar] [CrossRef]
- Gojny, F.H.; Nastalczyk, J.; Roslaniec, Z.; Schulte, K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chem. Phys. Lett. 2003, 370, 820–824. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, D.; Mathur, R.B.; Dhami, T.L. Influence of Surface Modified MWCNTs on the Mechanical, Electrical and Thermal Properties of Polyimide Nanocomposites. Nanoscale Res. Lett. 2008, 3, 444–453. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Rafiee, R. A review of the mechanical properties of isolated carbon nanotubes and carbon nanotube composites. Mech. Compos. Mater. 2010, 46, 155–172. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Nanocyl SA. Technical Data Sheet Nanocyl NC7000. Available online: http://www.nanocyl.com/wp-content/uploads/2016/07/DM-TI-02-TDS-NC7000-V08.pdf (accessed on 13 August 2018).
- White, C.M.; Banks, R.; Hamerton, I.; Watts, J.F. Characterisation of commercially CVD grown multi-walled carbon nanotubes for paint applications. Prog. Org. Coat. 2016, 90, 44–53. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Phan, C.H.; Jaafar, M.; Koh, Y.H. Mild functionalization of carbon nanotubes filled epoxy composites: Effect on electromagnetic interferences shielding effectiveness. J. Appl. Polym. Sci. 2015, 132, 42557. [Google Scholar] [CrossRef]
- Špitalský, Z.; Krontiras, C.A.; Georga, S.N.; Galiotis, C. Effect of oxidation treatment of multiwalled carbon nanotubes on the mechanical and electrical properties of their epoxy composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 778–783. [Google Scholar] [CrossRef]
- Tchoul, M.N.; Ford, W.T.; Lolli, G.; Resasco, D.E.; Arepalli, S. Effect of Mild Nitric Acid Oxidation on Dispersability, Size, and Structure of Single-Walled Carbon Nanotubes. Chem. Mater. 2007, 19, 5765–5772. [Google Scholar] [CrossRef]
- Andrade, N.F.; Martinez, D.S.T.; Paula, A.J.; Silveira, J.V.; Alves, O.L.; Souza Filho, A.G. Temperature effects on the nitric acid oxidation of industrial grade multiwalled carbon nanotubes. J. Nanopart. Res. 2013, 15, 1761. [Google Scholar] [CrossRef]
- Avantage. XPS Software Database, version 5.981; Thermo Fisher Scientific Inc.: Basingstokecity, UK.
- Hantsche, H. High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]
- Zheng, M.; Diner, B.A. Solution Redox Chemistry of Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.G.O. Organikum: Organisch-chemisches Grundpraktikum; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Sundara, R. Hot peroxide bleaching. Can. Chem. News 1998, 50, 15–17. [Google Scholar]
- Tsuruoka, S.; Matsumoto, H.; Castranova, V.; Porter, D.W.; Yanagisawa, T.; Saito, N.; Kobayashi, S.; Endo, M. Differentiation of chemical reaction activity of various carbon nanotubes using redox potential: Classification by physical and chemical structures. Carbon 2015, 95, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, S.; Matsumoto, H.; Koyama, K.; Akiba, E.; Yanagisawa, T.; Cassee, F.R.; Saito, N.; Usui, Y.; Kobayashi, S.; Porter, D.W.; et al. Radical scavenging reaction kinetics with multiwalled carbon nanotubes. Carbon 2015, 83, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Weydemeyer, E.J.; Sawdon, A.J.; Peng, C.A. Controlled cutting and hydroxyl functionalization of carbon nanotubes through autoclaving and sonication in hydrogen peroxide. Chem. Commun. 2015, 51, 5939–5942. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.L.; Lago, R.M.; Chen, Y.K.; Green, M.L.H.; Harris, P.J.F.; Tsang, S.C. Mechanical damage of carbon nanotubes by ultrasound. Carbon 1996, 34, 814–816. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Jorio, A.; Dresselhaus, M.S.; Dresselhaus, G. Observations of the D-band feature in the Raman spectra of carbon nanotubes. Phys. Rev. B 2001, 64, 073403. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Gardea, F.; Lagoudas, D.C. Characterization of electrical and thermal properties of carbon nanotube/epoxy composites. Compos. Part B Eng. 2014, 56, 611–620. [Google Scholar] [CrossRef]
- Khare, K.S.; Khare, R. Effect of Carbon Nanotube Dispersion on Glass Transition in Cross-Linked Epoxy–Carbon Nanotube Nanocomposites: Role of Interfacial Interactions. J. Phys. Chem. B 2013, 117, 7444–7454. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Islam, M.E.; Mahdi, T.H.; Hosur, M.V.; Jeelani, S. Characterization of Carbon Fiber Reinforced Epoxy Composites Modified with Nanoclay and Carbon Nanotubes. Procedia. Eng. 2015, 105, 821–828. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Park, S.J. Silane modification of carbon nanotubes and its effects on the material properties of carbon/CNT/epoxy three-phase composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 478–483. [Google Scholar] [CrossRef]
- Singer, G.; Rennhofer, H.; Sinn, G.; Unterlass, M.M.; Wendrinsky, J.; Windberger, U.; Lichtenegger, H.C. Processing of Carbon Nanotubes and Carbon Nanofibers towards High Performance Carbon Fiber Reinforced Polymers. Key Eng. Mater. 2017, 742, 31–37. [Google Scholar] [CrossRef]
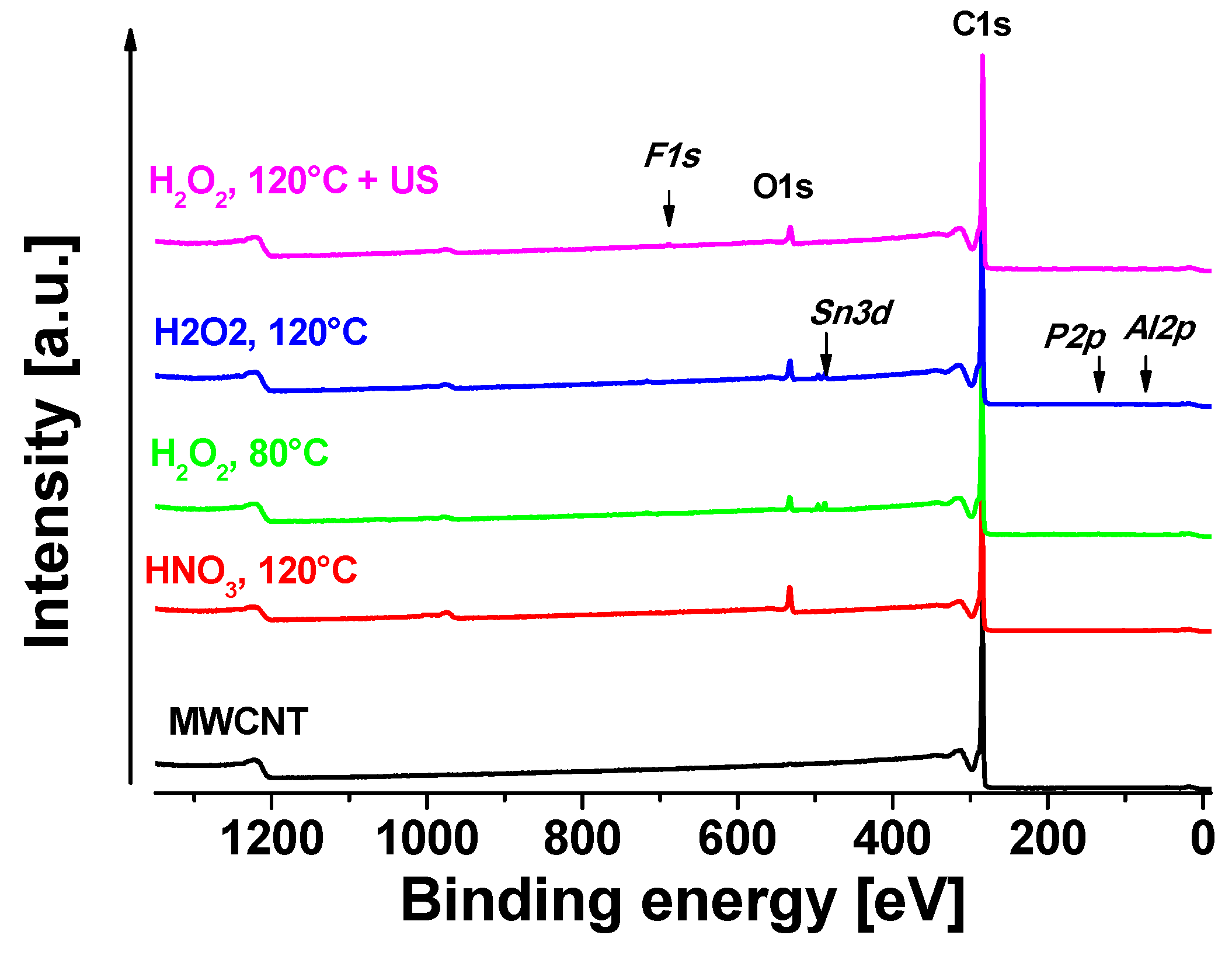
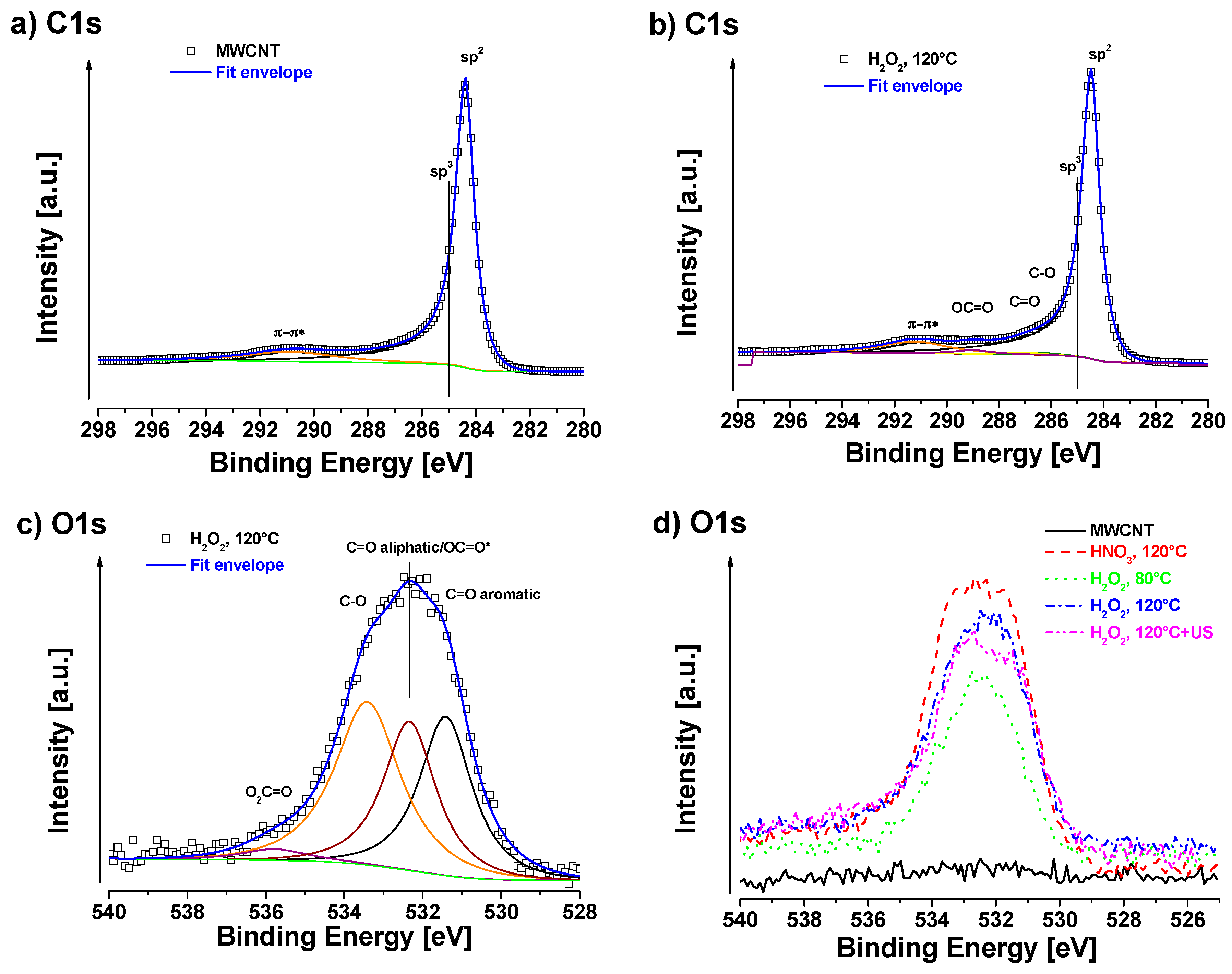
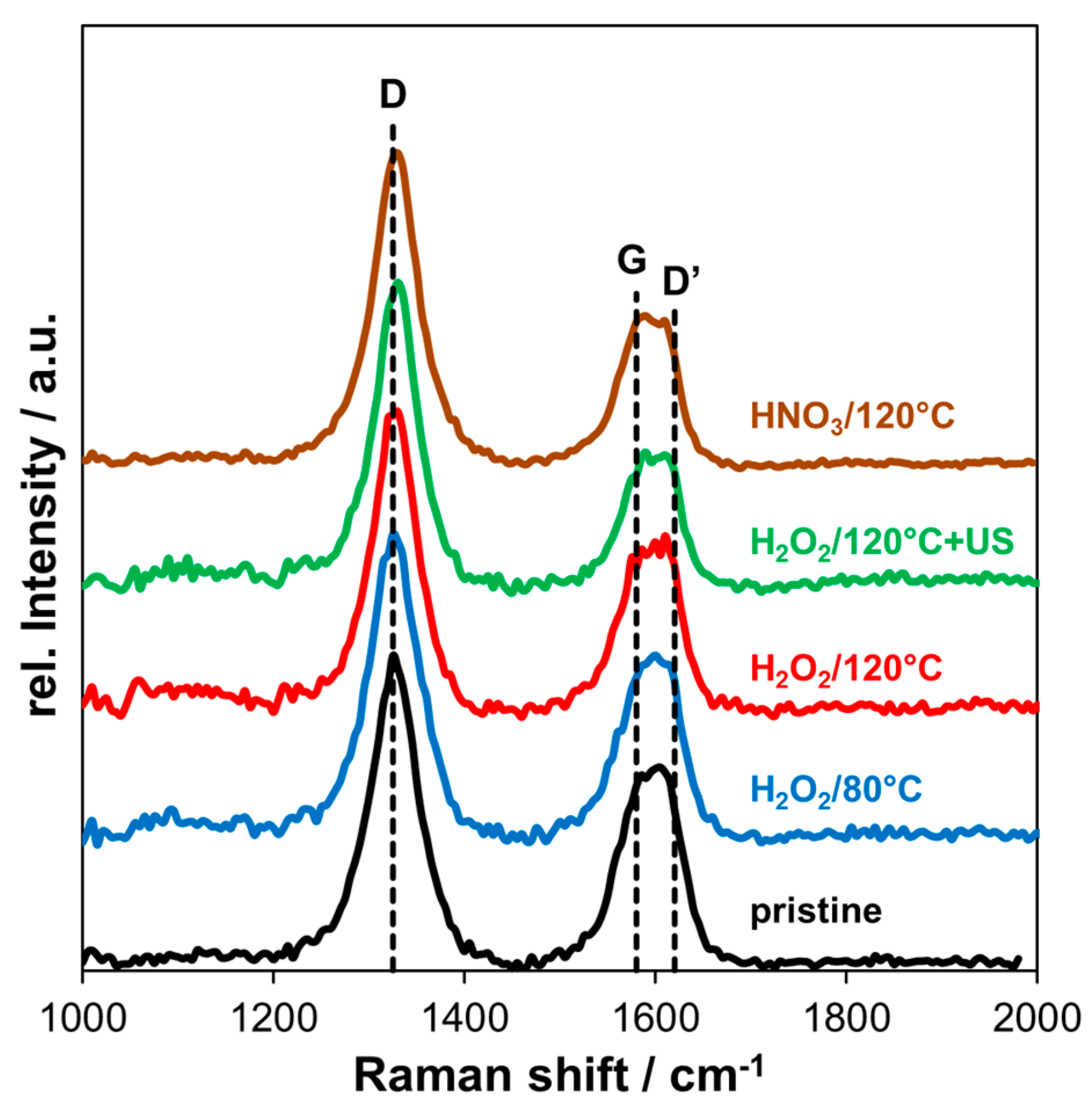
| C1s | O1s | F1s | Al2p | Sn3d | P2p |
|---|---|---|---|---|---|
| 1.000 | 2.881 | 4.118 | 0.560 | 37.257 | 1.353 |
| Sample | Surface Chemical Composition [at%] (Peak Positions in eV) | |||||
|---|---|---|---|---|---|---|
| C1s | O1s | Sn3d | P2p | Al2p | F1s | |
| sp2/sp3/C-O/C=O/OC=O/π-π* | C=Oar/OC=Ox/C-O/O2C=O | SnO2 | PO4 | Al2O3 | C-F | |
| (248.4/285/286.4/287.1/289.2/291.2) | (531.6/532.5/533.5/534.8) | (487.8) | (134.5) | (75.3) | (689.3) | |
| MWCNT | 99.9 | 0.1 | — | — | — | — |
| 100/0/0/0/0/0 | — | |||||
| HNO3, 120 °C | 91.5 | 8.5 | — | — | — | — |
| 82.1/3.7/2.0/0.7/3.6/7.8 | 33.5/25.3/38.1/3.1 | |||||
| H2O2, 80 °C | 96.4 | 3.2 | 0.2 | 0.2 | — | — |
| 87.9/0/0.7/0.9/1.0/9.5 | 29.7/36.0/31.6/2.7 | |||||
| H2O2, 120 °C | 95.0 | 4.7 | 0.1 | 0.1 | 0.2 | — |
| 86.9/0/1.0/0.9/1.7/9.6 | 30.2/28.3/38.8/2.7 | |||||
| H2O2, 120 °C + US | 95.3 | 4.3 | — | — | 0.2 | 0.3 |
| 87.9/0/1.5/0.2/1.6/8.9 | 29.4/17.3/46.9/6.4 | |||||
| Filler Type | Filler Content | Dispersion | Flexural Modulus [GPa] | Change [%] | Flexural Strength [MPa] | Change [%] |
|---|---|---|---|---|---|---|
| Reference | - | - | 40.2 (±1.8) | - | 520 (±20) | - |
| MWCNT | 0.7 wt% | TRM in resin | 44.1 (±2.7) | +10 | 580 (±45) | +12 |
| oxidized MWCNT | 0.7 wt% | US in hardener | 49.0 (±0.7) | +22 | 810 (±30) | +56 |
| Filler Type | Functionalization | Filler Content | Dispersion | Property | Change | Reference |
|---|---|---|---|---|---|---|
| oxidized MWCNT | H2O2 | 0.7 wt% | sonication in hardener | E | +4% | this study |
| σ | +38% | |||||
| Ef | +22% | |||||
| σf | +56% | |||||
| oxidized MWCNT | no information | 0.3 wt% | sonication and TRM in resin | Ef | +15% | [61] |
| σf | +19% | |||||
| oxidized MWCNT | H2SO4/HNO3 | 1.0 wt% | magnetic stirring in mixture of resin and hardener | E | +16% | [62] |
| σ | +7% | |||||
| silanized MWCNT | 3-aminopropyl-triethoxysilane | 1.0 wt% | E | +34% | ||
| σ | +16% | |||||
| MWCNT-NH2 | no information | 0.7 wt% | TRM in resin | Ef | +13% | [63] |
| σf | +18% | |||||
| MWCNT-NH2 | H2SO4/HNO3, SOCl2, Diethylamine | 1.0 wt% | sonication and ball milling in resin | E | +49% | [31] |
| σ | +52% | |||||
| σf | +38% | |||||
| MWCNT-NH2 | oxidation, SOCl2, 1,2-ethylene-diamine | 0.5 wt% | mechanical stirring and sonication in resin | Ef | +6% | [32] |
| σf | +23% | |||||
| MWCNT-NH2 | oxidation, SOCl2, diethyltoluene-diamine | 0.5 wt% | Ef | +18% | ||
| σf | +37% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, G.; Siedlaczek, P.; Sinn, G.; Rennhofer, H.; Mičušík, M.; Omastová, M.; Unterlass, M.M.; Wendrinsky, J.; Milotti, V.; Fedi, F.; et al. Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials 2018, 8, 912. https://doi.org/10.3390/nano8110912
Singer G, Siedlaczek P, Sinn G, Rennhofer H, Mičušík M, Omastová M, Unterlass MM, Wendrinsky J, Milotti V, Fedi F, et al. Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials. 2018; 8(11):912. https://doi.org/10.3390/nano8110912
Chicago/Turabian StyleSinger, Gerald, Philipp Siedlaczek, Gerhard Sinn, Harald Rennhofer, Matej Mičušík, Maria Omastová, Miriam M. Unterlass, Josef Wendrinsky, Valeria Milotti, Filippo Fedi, and et al. 2018. "Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP" Nanomaterials 8, no. 11: 912. https://doi.org/10.3390/nano8110912
APA StyleSinger, G., Siedlaczek, P., Sinn, G., Rennhofer, H., Mičušík, M., Omastová, M., Unterlass, M. M., Wendrinsky, J., Milotti, V., Fedi, F., Pichler, T., & Lichtenegger, H. C. (2018). Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials, 8(11), 912. https://doi.org/10.3390/nano8110912