Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc
Abstract
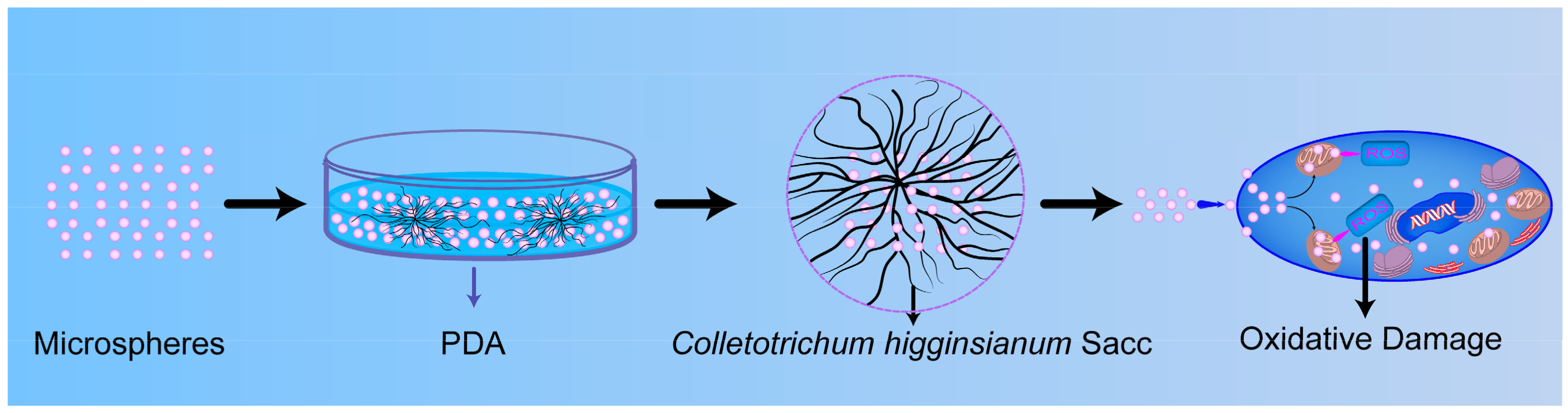
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
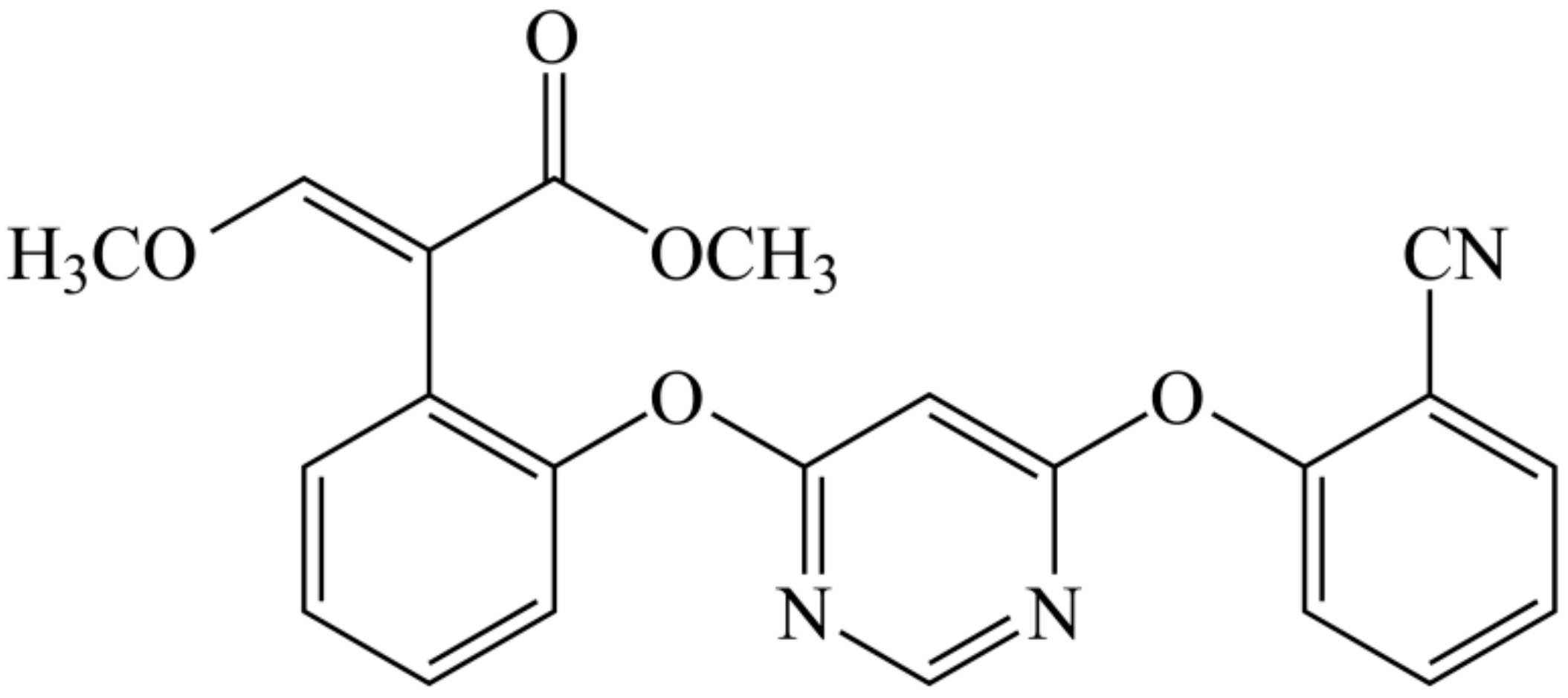
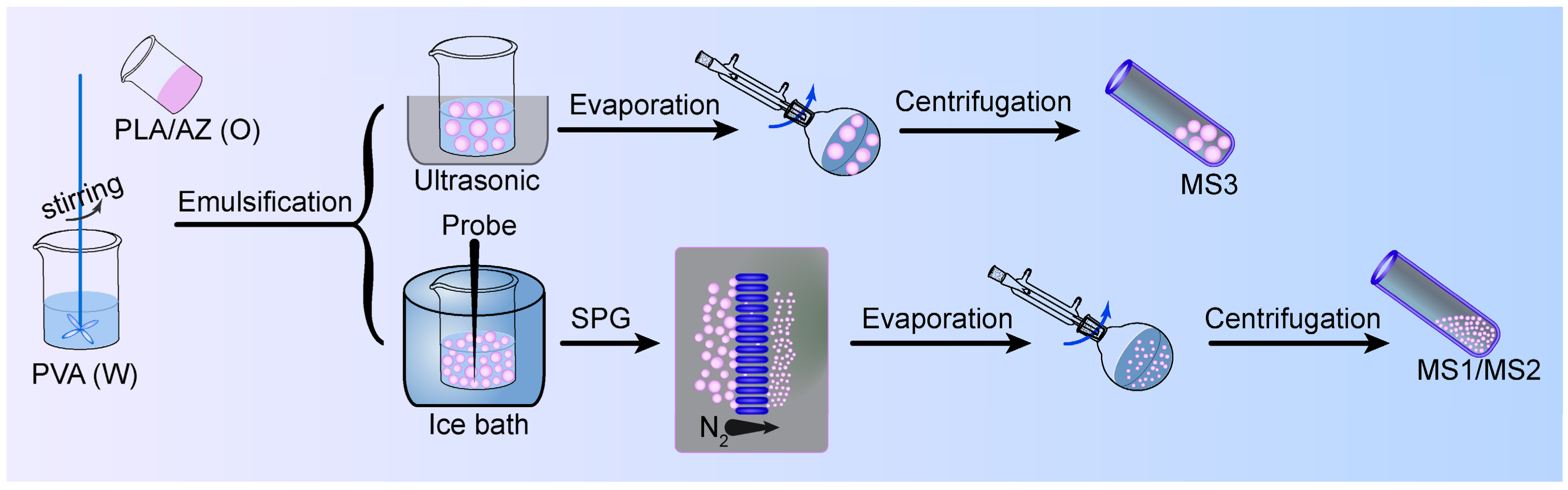
2.2.1. Preparation of Azoxystrobin Microspheres
2.2.2. Size and Morphological Characterizations of Microspheres
2.2.3. Drug Loading and Encapsulation Efficiency of Microspheres
2.2.4. In Vitro Release of Azoxystrobin from Microspheres
2.2.5. Stability Evaluation of MS1 Microspheres
2.2.6. Contact Angle Analysis of Microspheres
2.2.7. In Vitro Antagonistic Activity Assay
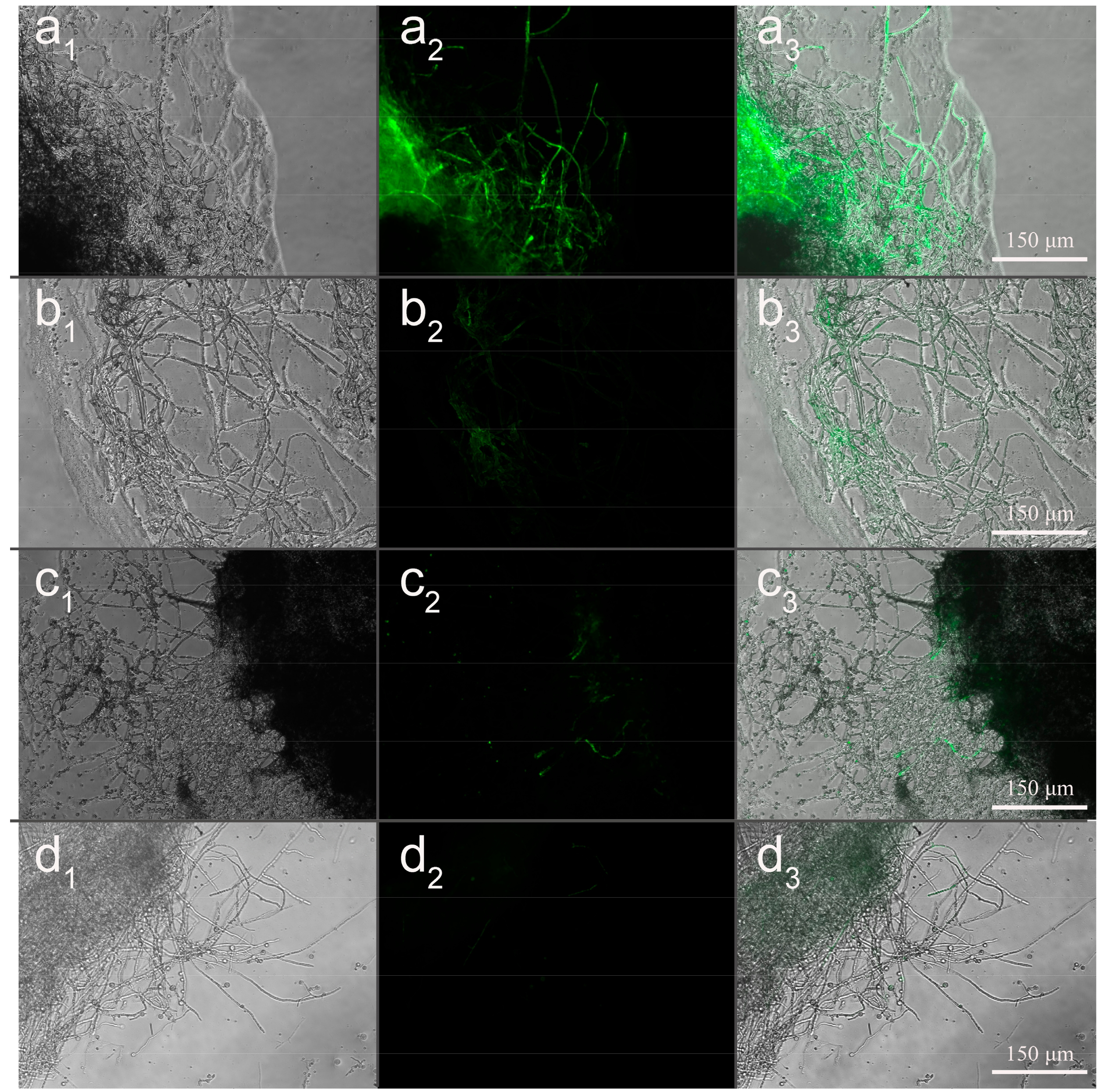
2.2.8. Mycelial Uptake Efficiency Assay
2.2.9. Reactive Oxygen Species Assay
2.2.10. Oxidative Stress Assay
2.2.11. Statistical Analysis
3. Results and Discussion
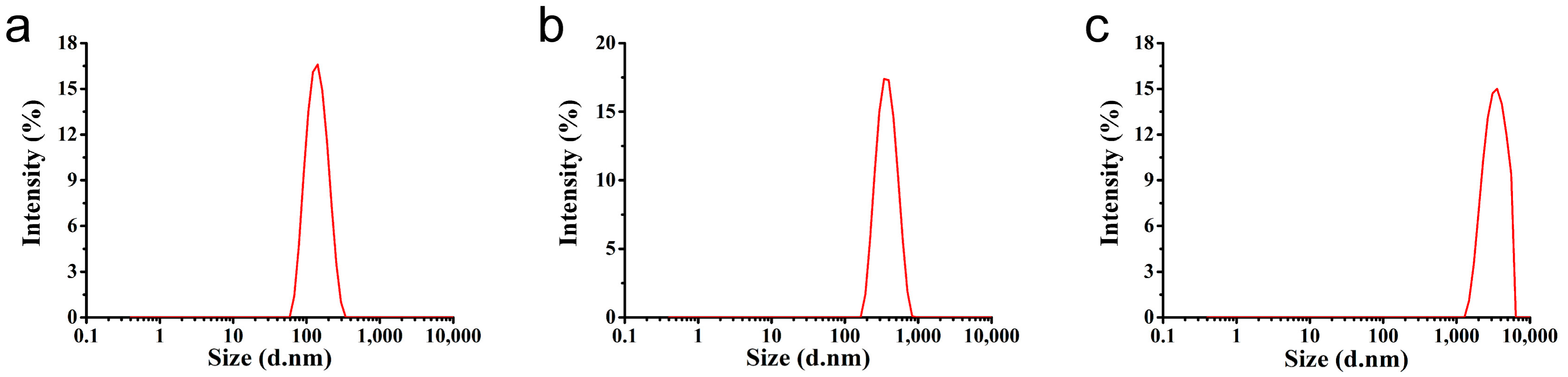
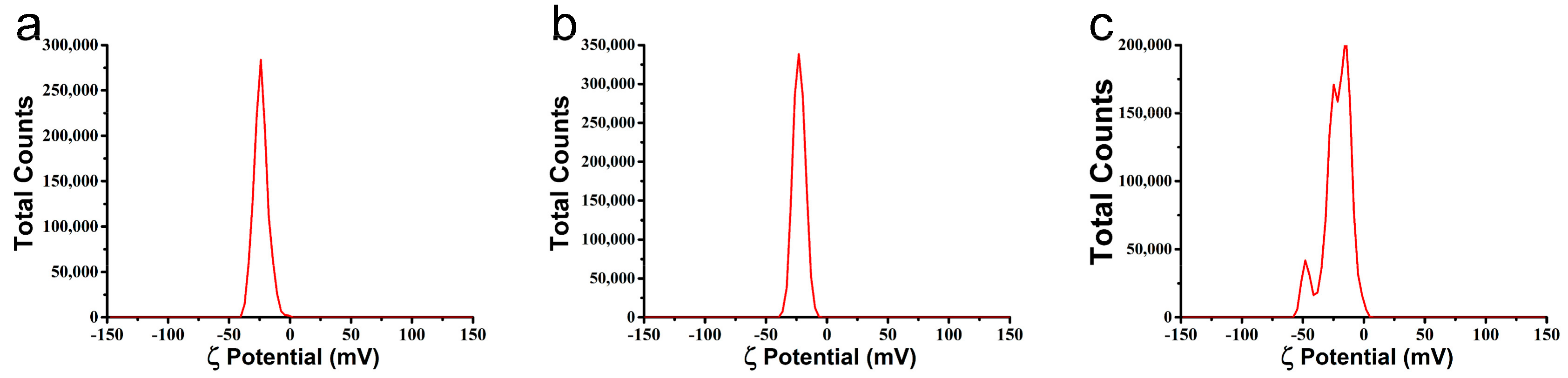
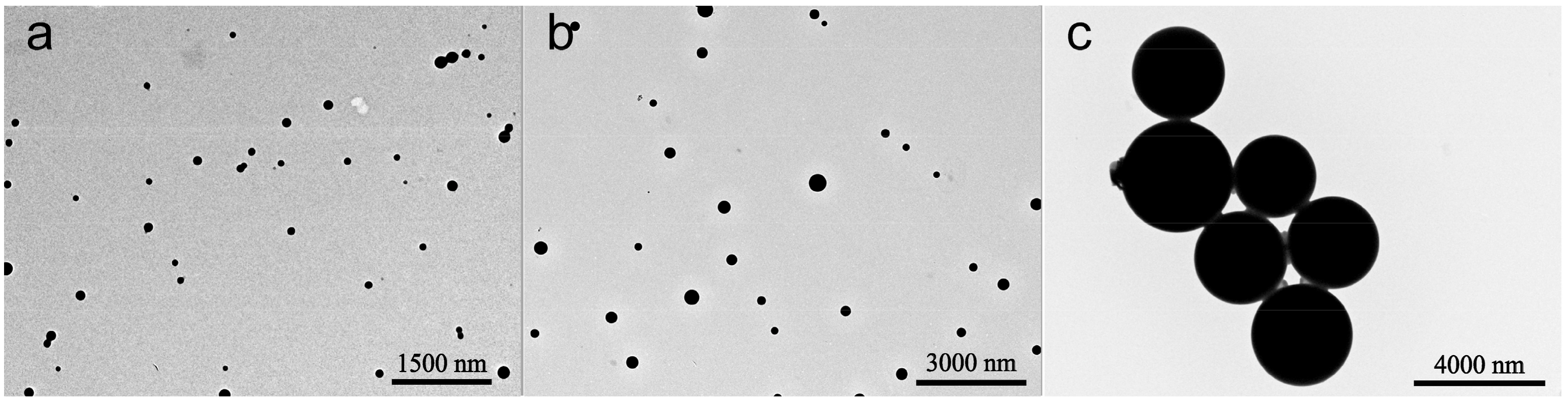
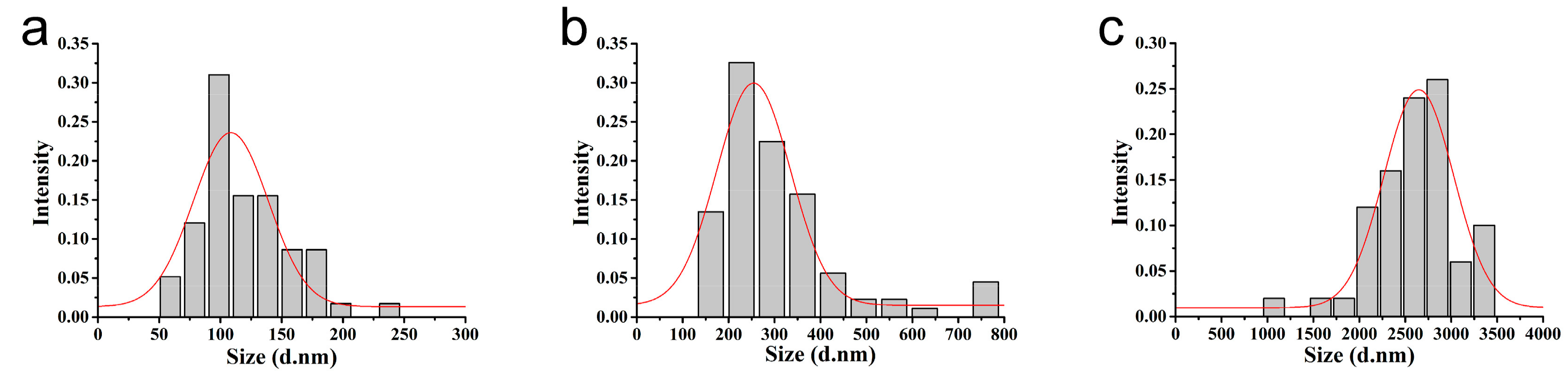
3.1. Characterization of the Microspheres
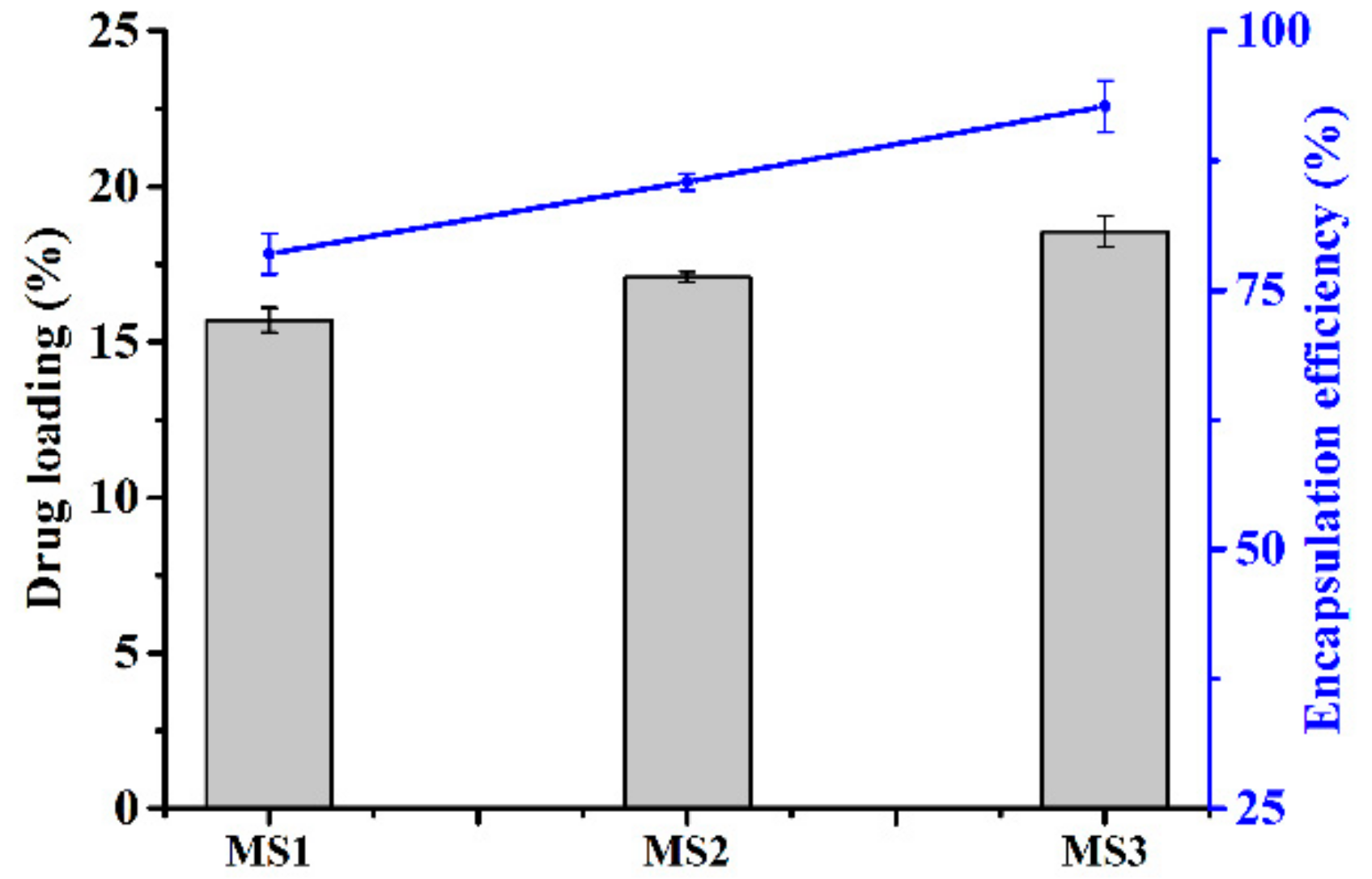
3.2. Azoxystrobin Loading and Encapsulation Efficiency
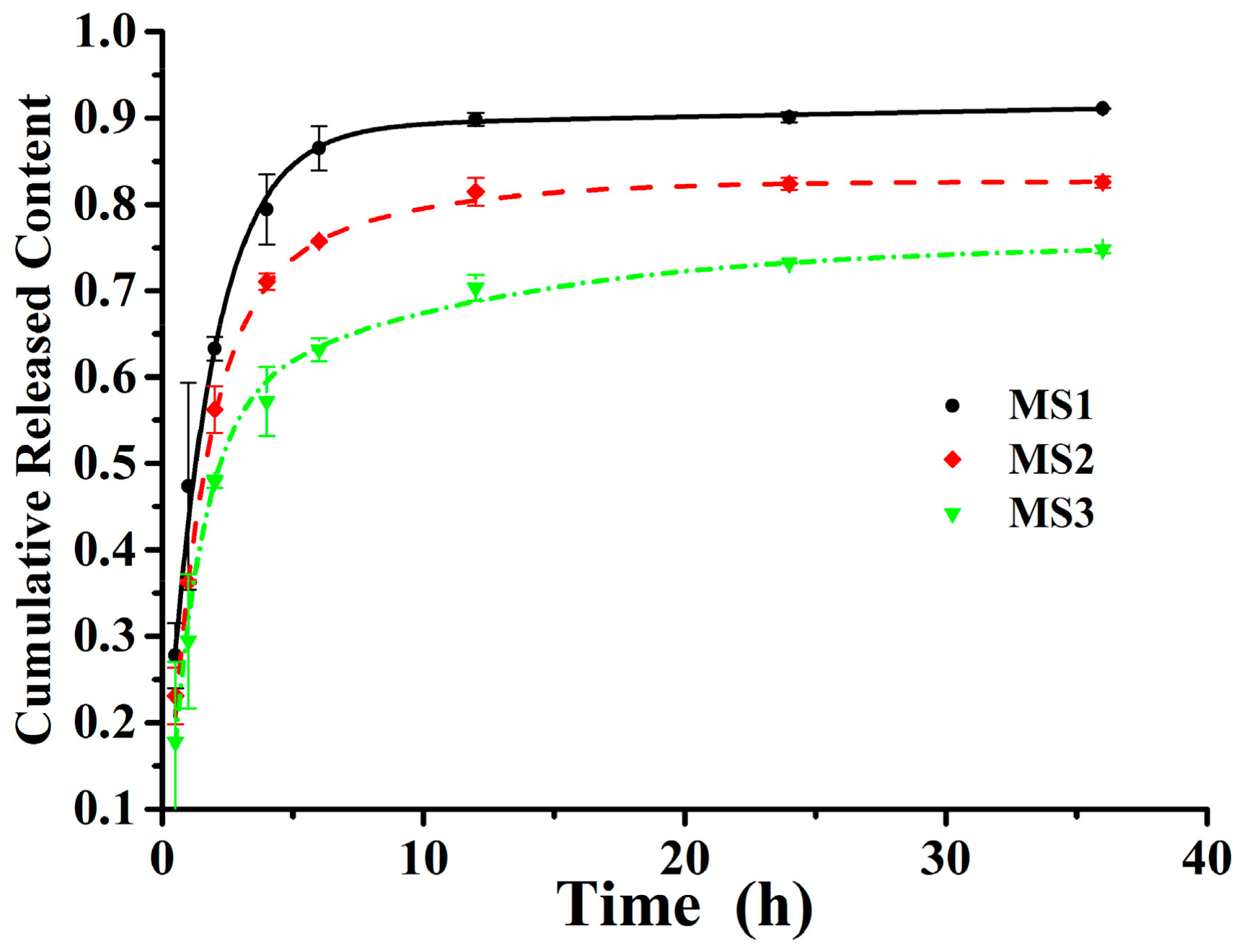
3.3. In Vitro Release Profiles of Microspheres
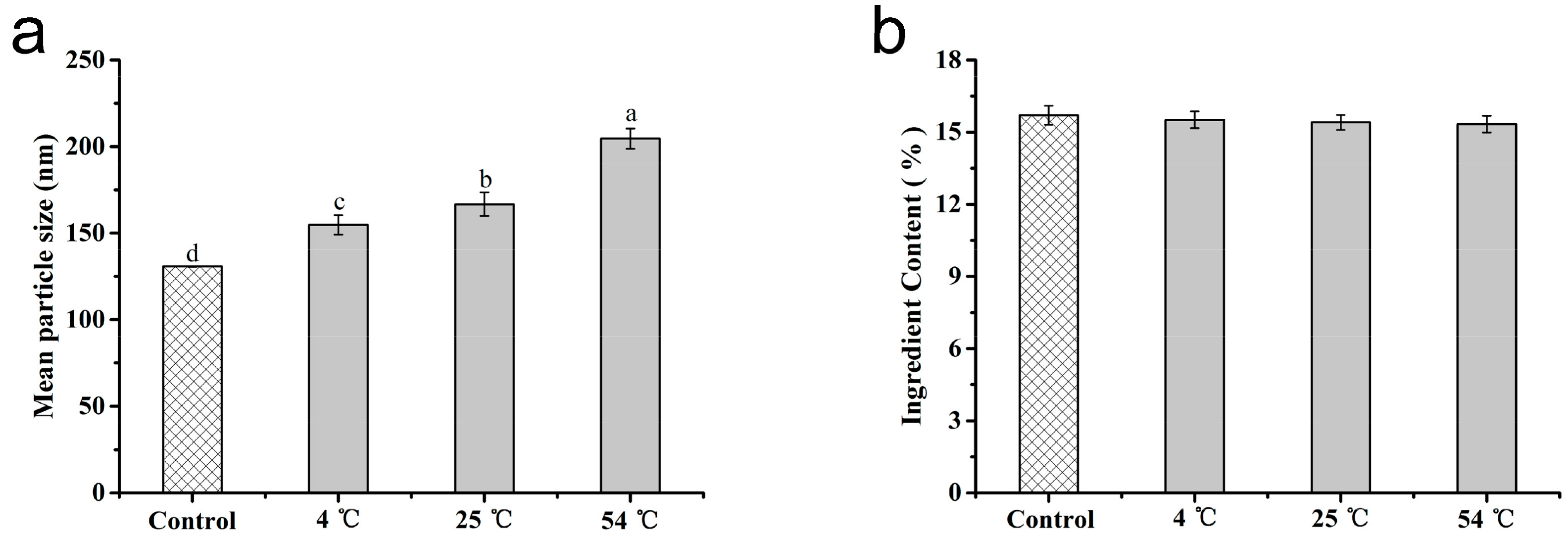
3.4. Stability of the Microspheres
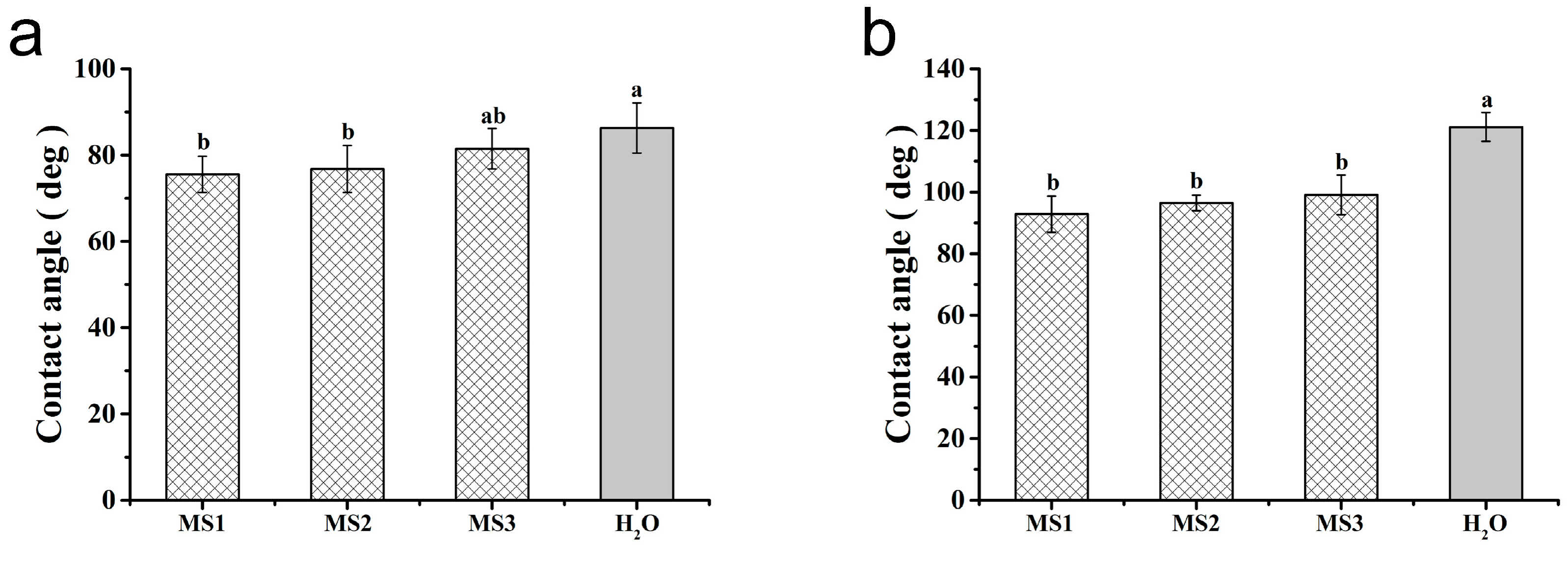
3.5. Effect of Microsphere Size on Contact Angle
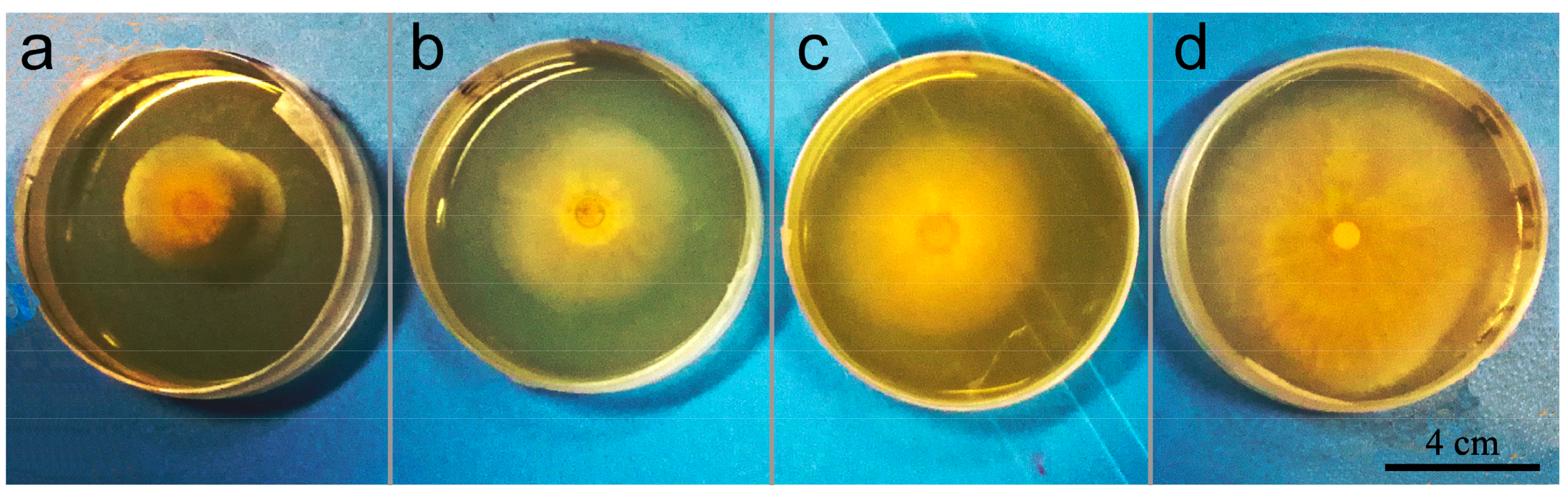
3.6. In Vitro Antagonistic Activity of Microspheres
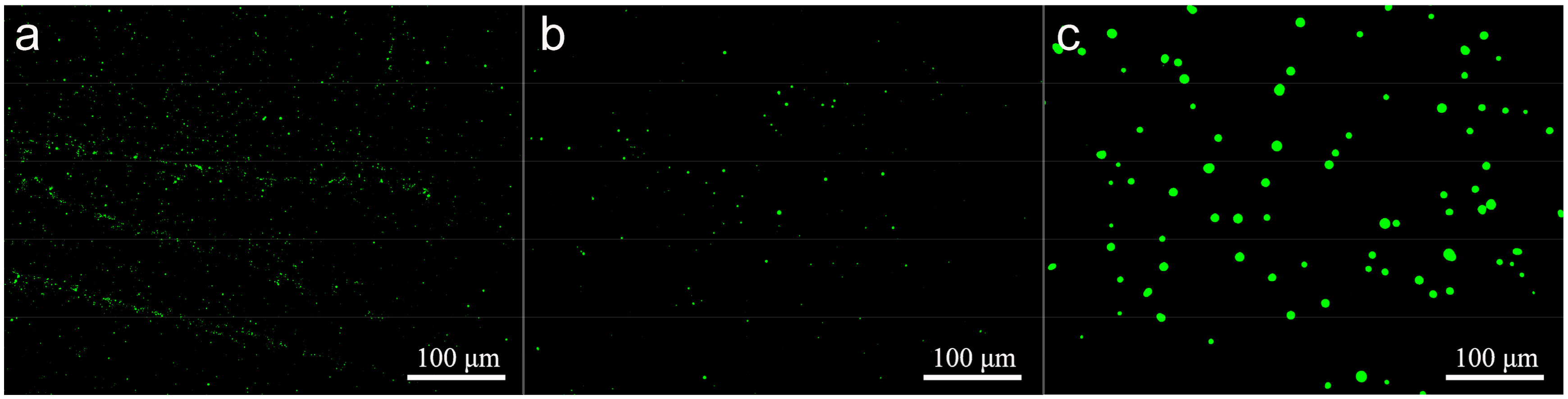
3.7. Effect of Microsphere Size on Intracellular Uptake
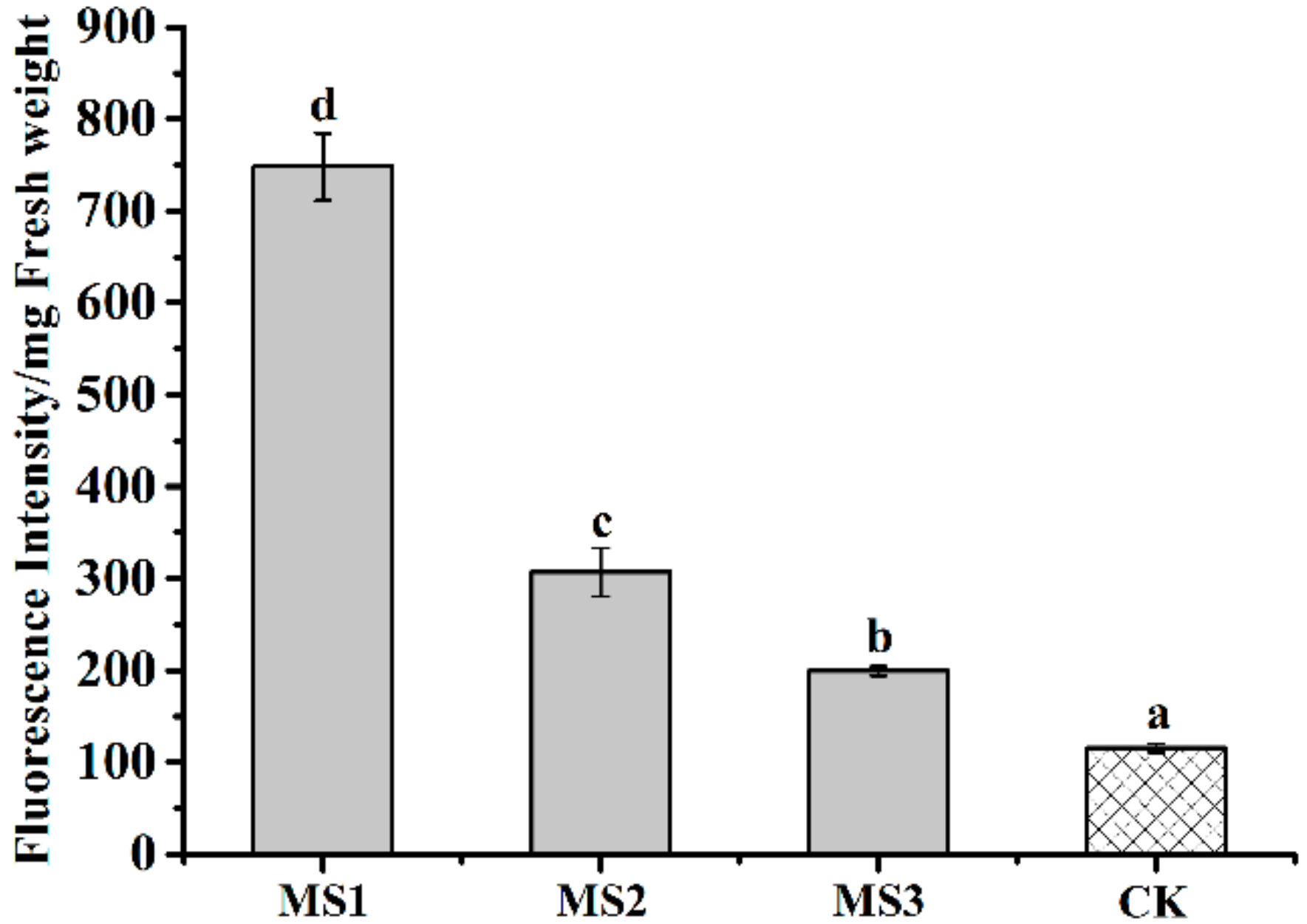
3.8. Effect of Microsphere Size on Intracellular ROS
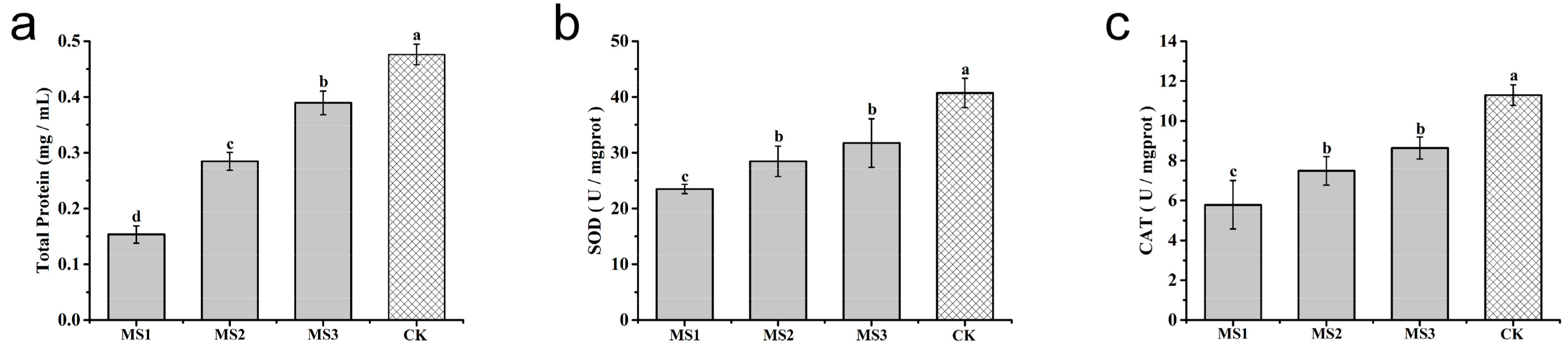
3.9. Effect of Microsphere Size on Antioxidase Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enserink, M.; Hines, P.J.; Vignieri, S.N.; Wigginton, N.S.; Yeston, J.S. The Pesticide Paradox. Science 2013, 341, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C. Applications of nanotechnology in agriculture and water quality management. Environ. Chem. Lett. 2017, 15, 591–605. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Laxmi, K.R.; Lijina, S.R.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Mena, P.; García-Viguera, C.; Moreno, D.A. Brassica Foods as a Dietary Source of Vitamin, C: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Artemyeva, A.M.; Solovyeva, A.E. Quality evaluation of some cultivar types of leafy Brassica rapa. Acta Hortic. 2006, 706, 121–128. [Google Scholar] [CrossRef]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; Van Themaat, E.V.L.; Van der Does, H.C.; Hacquard, S.; Stüber, K.; Will, I.; Schmalenbach, W.; et al. Sequential Delivery of Host-Induced Virulence Effectors by Appressoria and Intracellular Hyphae of the Phytopathogen Colletotrichum higginsianum. PLOS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a New Class of Active Substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health Part B 2007, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. The QoI Fungicides, the Rise and Fall of a Successful Class of Agricultural Fungicides; Carisse, O., Ed.; InTechOpen: London, UK, 2010; pp. 203–220. ISSN 978-953-307-266-1. [Google Scholar]
- Esser, L.; Quinn, B.; Li, Y.F.; Zhang, M.; Elberry, M.; Yu, L.; Yu, C.A.; Xia, D. Crystallographic studies of quinol oxidation site inhibitors: A modified classification of inhibitors for the cytochrome bc(1) complex. J. Mol. Biol. 2004, 341, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ortuno, D.; Tores, J.A.; De Vicente, A.; Perez-Garcia, A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 2008, 11, 1–9. [Google Scholar] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Musyanovych, A.; Schmitz-Wienke, J.; Mailänder, V.; Walther, P.; Landfester, K. Preparation of biodegradable polymer nanoparticles by miniemulsion technique and their cell interactions. Macromol. Biosci. 2008, 8, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdisciplinary Reviews. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Guo, W.; Yu, Q.; Guan, S.; Guo, L.; Shen, T.; Tang, H.; Gan, Z. Poly(d,l-lactic acid)-block-poly(N-(2-hydroxypropyl)methacrylamide) nanoparticles for overcoming accelerated blood clearance and achieving efficient anti-tumor therapy. Polym. Chem. 2016, 7, 5719–5729. [Google Scholar] [CrossRef]
- Qiao, J.-B.; Jang, Y.; Fan, Q.-Q.; Chang, S.-H.; Xing, L.; Cui, P.-F.; He, Y.-J.; Lee, S.; Hwang, S.; Cho, M.-H.; et al. Aerosol delivery of biocompatible dihydroergotamine-loaded PLGA-PSPE polymeric micelles for efficient lung cancer therapy. Polym. Chem. 2017, 8, 1540–1554. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thauvin, C.; Schwarz, B.; Delie, F.; Allémann, E. Functionalized PLA polymers to control loading and/or release properties of drug-loaded nanoparticles. Int. J. Pharm. 2017, 542, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Florian, P.E.; Roseanu, A.; Moisei, M.; Craciunescu, O.; Astete, C.E.; Sabliov, C.M. Cytotoxicity and intracellular fate of PLGA and chitosan-coated PLGA nanoparticles in Madin-Darby bovine kidney (MDBK) and human colorectal adenocarcinoma (Colo 205) cells. J. Biomed. Mater. Res. Part A 2015, 103, 3599–3611. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Junior, F.H.; Huang, N.; Vachon, J.-J.; Rehder, V.L.G.; Do Egito, E.S.T.; Vauthier, C. Match of solubility parameters between oil and surfactants as a rational approach for the formulation of microemulsion with a high dispersed volume of copaiba oil and low surfactant content. Pharm. Res. 2016, 33, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Ibrahim, H.; Dormoi, J.; Briolant, S.; Pradines, B.; Moreno, A.; Mazier, D.; Legrand, P.; Nepveu, F. Albumin-bound nanoparticles of practically water-insoluble antimalarial lead greatly enhance its efficacy. Int. J. Pharm. 2014, 464, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Albisa, A.; Piacentini, E.; Sebastian, V.; Arruebo, M.; Santamaria, J.; Giorno, L. Preparation of Drug-Loaded PLGA-PEG Nanoparticles by Membrane-Assisted Nanoprecipitation. Pharm. Res. 2017, 34, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, B.; Yang, F.; Wang, X.; Shen, H.; Wu, D. Preparation of uniform starch microcapsules by premix membrane emulsion for controlled release of avermectin. Carbohydr. Polym. 2016, 136, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 2012, 55, 7969–7980. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2015, 51, 15768–15771. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Z.; Wei, J.; El Ghzaoui, A.; Li, S. Micelles formed by self-assembling of polylactide/poly(ethylene glycol) block copolymers in aqueous solutions. J. Colloid Interface Sci. 2007, 314, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials 2009, 30, 5757–5766. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.T.; Li, S.; Deratani, A. Reverse micelles prepared from amphiphilic polylactide-b-poly(ethylene glycol) block copolymers for controlled release of hydrophilic drugs. Int. J. Pharm. 2015, 495, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, Y.; Zhang, F.; Yin, Z.; Hu, Q.; Xiao, X.; Zhou, Z.; Wu, Y.; Sheng, W.; Zeng, Y. Acidic pH-induced charge-reversal nanoparticles for accelerated endosomal escape and enhanced microRNA modulation in cancer cells. Chem. Commun. 2016, 52, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Liu, Y.; Cao, L.; Wu, Y.; Huang, Q. Size-Dependent Effect of Prochloraz-Loaded mPEG-PLGA Micro- and Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 6231–6237. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A. review. Food Hydrocolloids 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. J. Control. Release 2008, 127, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhu, A.; Song, X.; Ji, L. Novel surfactant for preparation of poly(l-lactic acid) nanoparticles with controllable release profile and cytocompatibility for drug delivery. Colloids Surf. B 2014, 115, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jinlai, O.U.; Luo, R.; Liang, W.; Ouyang, P.; Zeng, F.; Chen, Y.; Zhenxia, X.U.; Zhao, W.; Sha, L.I. Preparation and Release Behavior of Pectin Nanoparticles Loading Doxorubicin. J. Pharm. Biomed. Sci. 2015, 5, 385–393. [Google Scholar]
- Yao, J.; Zhang, S.; Li, W.; Du, Z.; Li, Y. In vitro drug controlled-release behavior of an electrospun modified poly(lactic acid)/bacitracin drug delivery system. RSC Adv. 2016, 6, 515–521. [Google Scholar] [CrossRef]
- Hong, J.S.; Srivastava, D.; Lee, I. Fabrication of poly(lactic acid) nano- and microparticles using a nanomixer via nanoprecipitation or emulsion diffusion. J. Appl. Polym. Sci. 2018, 135, 46199. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, H.; Putz, K.W.; Brinson, L.C. Effect of particle agglomeration and interphase on the glass transition temperature of polymer nanocomposites. J. Polym. Sci. Part B 2011, 49, 740–748. [Google Scholar] [CrossRef]
- Pandey, S.K.; Ghosh, S.; Maiti, P.; Haldar, C. Therapeutic efficacy and toxicity of tamoxifen loaded PLA nanoparticles for breast cancer. Int. J. Biol. Macromol. 2015, 72, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yao, J.; Liang, J.; Zeng, Z.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H. Development of functionalized abamectin poly(lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017, 7, 11271–11280. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Liang, J.; Yu, M.; Guo, L.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H.; Zeng, Z. Bioinspired Development of P(St–MAA)–Avermectin Nanoparticles with High Affinity for Foliage To Enhance Folia Retention. J. Agric. Food Chem. 2018, 66, 6578–6584. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Sheng, W.-B.; Li, W.; Tong, Y.-B.; Liu, Z.-Y.; Zhou, F. Adhesive Polydopamine Coated Avermectin Microcapsules for Prolonging Foliar Pesticide Retention. ACS Appl. Mater. Interfaces 2014, 6, 19552–19558. [Google Scholar] [CrossRef] [PubMed]
- Nairn, J.J.; Forster, W.A.; Van Leeuwen, R.M. Quantification of physical (roughness) and chemical (dielectric constant) leaf surface properties relevant to wettability and adhesion. Pest Manag. Sci. 2011, 67, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.M.; Singh, V.N.; Kumar, M.; Singh, J.P. Effect of nanoparticle size on sessile droplet contact angle. J. Appl. Phys. 2008, 103, 367. [Google Scholar] [CrossRef]
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.Y.; Quinn, J.F.; Whittaker, M.R.; Truong, N.P.; Davis, T.P. Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2018, 1800438. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Osborne, O.J.; Lin, S.; Chang, C.H.; Ji, Z.; Yu, X.; Wang, X.; Lin, S.; Xia, T.; Nel, A.E. Organ-Specific and Size-Dependent Ag Nanoparticle Toxicity in Gills and Intestines of Adult Zebrafish. ACS Nano 2015, 9, 9573–9584. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Damann, K.; Leonardi, C.; Sabliov, C.M. Size dependency of PLGA-nanoparticle uptake and antifungal activity against Aspergillus flavus. Nanomedicine 2011, 6, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food. Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hal, L.A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.M.; Hollomon, D.W. A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Qo site of Complex III. Pest Manag. Sci. 2003, 59, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.-H.; Fu, Y.-Y.; Zhang, K.-Z.; Zhang, M.; Jiang, H.-W.; Fan, L.-X.; Nan, F.-J.; Yuan, C.-G.; Li, J.; Zhou, Y.-B.; et al. Azoxystrobin, a mitochondrial complex III Qo site inhibitor, exerts beneficial metabolic effects in vivo and in vitro. Biochim. Biophys. Acta 2014, 1840, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chung, J.M.; Chung, K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008, 447, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Cui, B.; Zhao, X.; Wang, Y.; Zeng, Z.; Sun, C.; Yang, D.; Liu, G.; Gao, J.; Cui, H. Preparation, characterization, and evaluation of azoxystrobin nanosuspension produced by wet media milling. Appl. Nanosci. 2018, 8, 297–307. [Google Scholar] [CrossRef]
- Kaneko, I.; Ishii, H. Effect of azoxystrobin on activities of antioxidant enzymes and alternative oxidase in wheat head blight pathogens Fusarium graminearum and Microdochium nivale. J. Gen. Plant Pathol. 2009, 75, 388. [Google Scholar] [CrossRef]
- Asada, K. The Water-Water Cycle In Chloroplasts: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
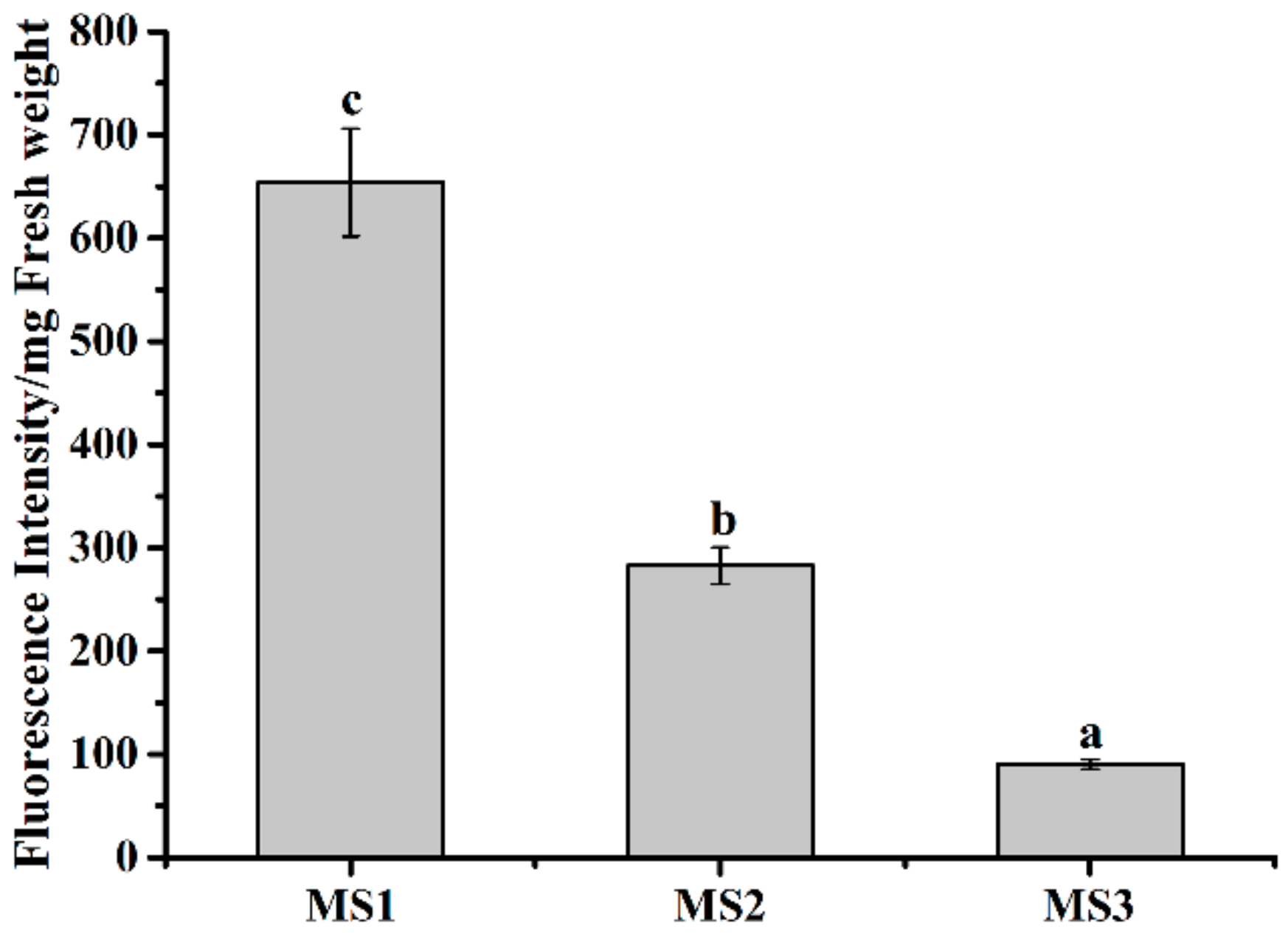
| Sample | Kinetic Equations | R2 |
|---|---|---|
| MS1 | y = 796.50 − 0.81e−0.57x − 795.61e−0.75x | 0.9983 |
| MS2 | y = 0.83 − 0.67e−0.74x − 0.17e−0.17x | 0.9999 |
| MS3 | y = 0.75 − 0.59e−0.85x − 0.20e−0.09x | 0.9971 |
| Sample | Toxicity Regressive Equation | R2 | LC50 (μg/mL) | Toxicity Index |
|---|---|---|---|---|
| MS1 | y = 0.4106 + 0.2961 x | 0.9995 | 2.0386 | 10.1137 |
| MS2 | y = 0.2445 + 0.2149 x | 0.9913 | 12.7246 | 1.6732 |
| MS3 | y = 0.1451 + 0.2118 x | 0.9620 | 21.2905 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Cui, B.; Zhao, X.; Zhi, H.; Zeng, Z.; Wang, Y.; Sun, C.; Liu, G.; Gao, J.; Cui, H. Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc. Nanomaterials 2018, 8, 857. https://doi.org/10.3390/nano8100857
Yao J, Cui B, Zhao X, Zhi H, Zeng Z, Wang Y, Sun C, Liu G, Gao J, Cui H. Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc. Nanomaterials. 2018; 8(10):857. https://doi.org/10.3390/nano8100857
Chicago/Turabian StyleYao, Junwei, Bo Cui, Xiang Zhao, Heng Zhi, Zhanghua Zeng, Yan Wang, Changjiao Sun, Guoqiang Liu, Jinming Gao, and Haixin Cui. 2018. "Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc" Nanomaterials 8, no. 10: 857. https://doi.org/10.3390/nano8100857
APA StyleYao, J., Cui, B., Zhao, X., Zhi, H., Zeng, Z., Wang, Y., Sun, C., Liu, G., Gao, J., & Cui, H. (2018). Antagonistic Effect of Azoxystrobin Poly (Lactic Acid) Microspheres with Controllable Particle Size on Colletotrichum higginsianum Sacc. Nanomaterials, 8(10), 857. https://doi.org/10.3390/nano8100857








