Time-Resolved Study of Nanomorphology and Nanomechanic Change of Early-Stage Mineralized Electrospun Poly(lactic acid) Fiber by Scanning Electron Microscopy, Raman Spectroscopy and Atomic Force Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Electrospinning Solutions
2.3. Electrospinning of PLA Nanofibers
2.4. Preparation of Simulated Body Fluid (SBF) and Mineralization in SBF
2.5. Characterization of Mineralized PLA Nanofibers
2.5.1. Scanning Electron Microscopy (SEM) Analysis of Mineralized PLA Nanofibers
2.5.2. Raman Analysis of Mineralized PLA Nanofibers
2.5.3. Atomic Force Microscopy (AFM) Imaging and Data Analysis
3. Results and Discussion
Characterization of Mineralized PLA Nanofibers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Naito, Y.; Shinoka, T.; Duncan, D.; Hibino, N.; Solomon, D.; Cleary, M.; Rathore, A.; Fein, C.; Church, S.; Breuer, C. Vascular tissue engineering: Towards the next generation vascular grafts. Adv. Drug Deliv. Rev. 2011, 63, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibers via Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Edirisinghe, M. Forming of polymer nanofibers by a pressurised gyration process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Fu, S.J. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C 2016, 58, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.P. Madurantakam Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Castano, O.; Kim, H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Jaiswala, A.K.; Kadamb, S.S.; Sonia, V.P.; Bellareb, J.R. Improved functionalization of electrospun PLLA/gelatin scaffold by alternate soaking method for bone tissue engineering. Appl. Surf. Sci. 2013, 268, 477–488. [Google Scholar] [CrossRef]
- Liverani, L.; Boccaccini, A. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Lópezd, D.C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.L.; Galotto, M.J. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef]
- Shehata, N.; Gaballah, S.; Samir, E.; Hamed, A.; Saad, M. Fluorescent Nanocomposite of Embedded Ceria Nanoparticles in Crosslinked PVA Electrospun Nanofibers. Nanomaterials 2016, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.R.; Jacobs, D.K.; Meredith, J.O.; Barker, G.; Clark, A.J.; Kirwan, K.; Stanger, J.; Tucker, N. A Design of Experiments (DoE) Approach to Material Properties Optimization of Electrospun Nanofibers. J. Appl. Polym. Sci. 2010, 117, 2251–2257. [Google Scholar] [CrossRef]
- Chang, W.M.; Wang, C.C.; Chen, C.Y. The combination of electrospinning and forcespinning: Effects on a viscoelastic jet and a single nanofiber. Chem. Eng. J. 2014, 244, 540–551. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of Nanofibers for Energy Applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Biomineralization: From Nature to Application. Met. Ions Life Sci. 2008, 4, 413–456. [Google Scholar] [CrossRef]
- Wu, X.; Mahalingam, S.; Vanoosten, S.K.; Wisdom, C.; Tamerler, C.; Edirisinghe, M. New Generation of Tunable Bioactive Shape Memory Mats Integrated with Genetically Engineered Proteins. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, M. Biomimetics: Materials fabrication through biology. Proc. Natl. Acad. Sci. USA 1999, 96, 14183–14185. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Three-dimensional mineralization of dense nanofibrillar collagen-bioglass hybrid scaffolds. Biomacromolecules 2010, 11, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Guo, P.; Keeney, M.; Yang, F.; Hsu, T.M.; Fuller, G.G.; Martin, C.R.; Zare, R.N. Preparation of mineralized nanofibers: Collagen fibrils containing calcium phosphate. Nano Lett. 2011, 11, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lu, F.; Zhu, W.; Wang, D.; Zhu, X.; Tan, G.; Wang, X.; Zhang, Y.; Li, L.; Ning, C. Bio-inspired citrate functionalized apatite coating on rapid prototyped titanium scaffold. Appl. Surf. Sci. 2014, 313, 947–953. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Yu, Z.; He, Z.; Yang, S.; Jiang, H. Nucleation and characterization of hydroxyapatite on thioglycolic acid-capped reduced graphene oxide/silver nanoparticles in simplified simulated body fluid. Appl. Surf. Sci. 2014, 289, 89–96. [Google Scholar] [CrossRef]
- Rhilassi, A.E.; Mourabet, M.; Boujaady, H.E.; Bennani-Ziatni, M.; Hamri, R.E.; Taitai, A. Adsorption and release of amino acids mixture onto apatitic calcium phosphates analogous to bone mineral. Appl. Surf. Sci. 2012, 259, 376–384. [Google Scholar] [CrossRef]
- Palazzo, B.; Walsh, D.; Iafisco, M.; Foresti, E.; Bertinetti, L.; Martra, G.; Bianchi, C.L.; Cappelletti, G.; Roveri, N. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals. Acta Biomater. 2009, 5, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Picker, A.; Kellermeier, M.; Seto, J.; Gebauer, D.; Cölfen, H. The multiple effects of amino acids on the early stages of calcium carbonate crystallization. Z. Kristallogr. Cryst. Mater. 2012, 227, 744–757. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; kim, H.J.; Park, C.H.; Kim, C.S. Three-dimensional cellulose sponge: Fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, T.H.; Jin, G.Z.; Park, J.H.; Yun, Y.R.; Jang, J.H.; Kim, H.W. Mineralized poly(lactic acid) scaffolds loading vascular endothelial growth factor and the in vivo performance in rat subcutaneous model. Soc. Biomater. 2013, 101, 1447–1455. [Google Scholar] [CrossRef]
- Chen, J.; Chu, B.; Hsiao, B.S. Mineralization of hydroxyapatite in electrospun nanofibrous poly(L-lactic acid) scaffolds. J. Biomed. Mater. Res. A 2006, 79, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.G.; Lan, J.L.; Zhang, C.J.; Cao, M.; Cai, Q.; Yang, X.P. Mineralization on polylactide/gelatin composite nanofibers using simulated body fluid containing amino acid. Appl. Surf. Sci. 2015, 349, 538–548. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Herrera, K.V.; Misiun, A.; Vogt, C. Preparation and characterization of poly (lactic acid)/poly (methyl methacrylate) blend tablets for application in quantitative analysis by micro Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 273–279. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA-PHB blends: Structural, thermal, mechanical and disintegration properties. Euro. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Cai, Q.; Xu, Q.; Feng, Q.; Cao, X.; Yang, X.; Deng, X. Biomineralization of electrospun poly(l-lactic acid)/gelatin composite fibrous scaffold by using a supersaturated simulated body fluid with continuous CO2 bubbling. Appl. Surf. Sci. 2011, 257, 10109–10118. [Google Scholar] [CrossRef]
- Paiva, A.O.; Costa, N.; Cachinho, S.C.P.; Fernandes, M.H.V. Evaluation of the influence of albumin on the mineralization of a glass by Atomic Force Microscopy. J. Mater. Sci. Mater. Med. 2007, 18, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rim, N.G.; Jung, H.S.; Shin, H. Control of Osteogenic Differentiation and Mineralization of Human Mesenchymal Stem Cells on Composite Nanofibers Containing Poly[lactic-co-(glycolic acid)] and Hydroxyapatite. Macromol. Biosci. 2010, 10, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, J.; Zhou, C. Effect of ammonia plasma treatment on the properties and cytocompatibility of a poly(l-lactic acid) film surface. J. Biomater. Sci. Polym. Ed. 2012, 23, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Sarapirom, S.; Yu, L.D.; Boonyawan, D.; Chaiwong, C. Effect of surface modification of poly(lactic acid) by low-pressure ammonia plasma on adsorption of human serum albumin. Appl. Surf. Sci. 2014, 310, 42–50. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tănase, E.E.; Mitelut, A.; Ailieseia, G.L.; Vasilea, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Massy, Z.A.; Drüeke, T.B. Vascular calcification. Curr. Opin. Nephrol. Hypertens. 2013, 22, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Marulanda, J.; Alqarni, S.; Murshed, M. Mechanisms of vascular calcification and associated diseases. Curr. Pharm. Des. 2014, 20, 5801–5810. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bomans, P.H.; Müller, F.A.; Will, J.; Frederik, P.M.; Sommerdijk, N.A. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. Biomineralization: A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.; de With, G.; Sommerdijk, N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed]



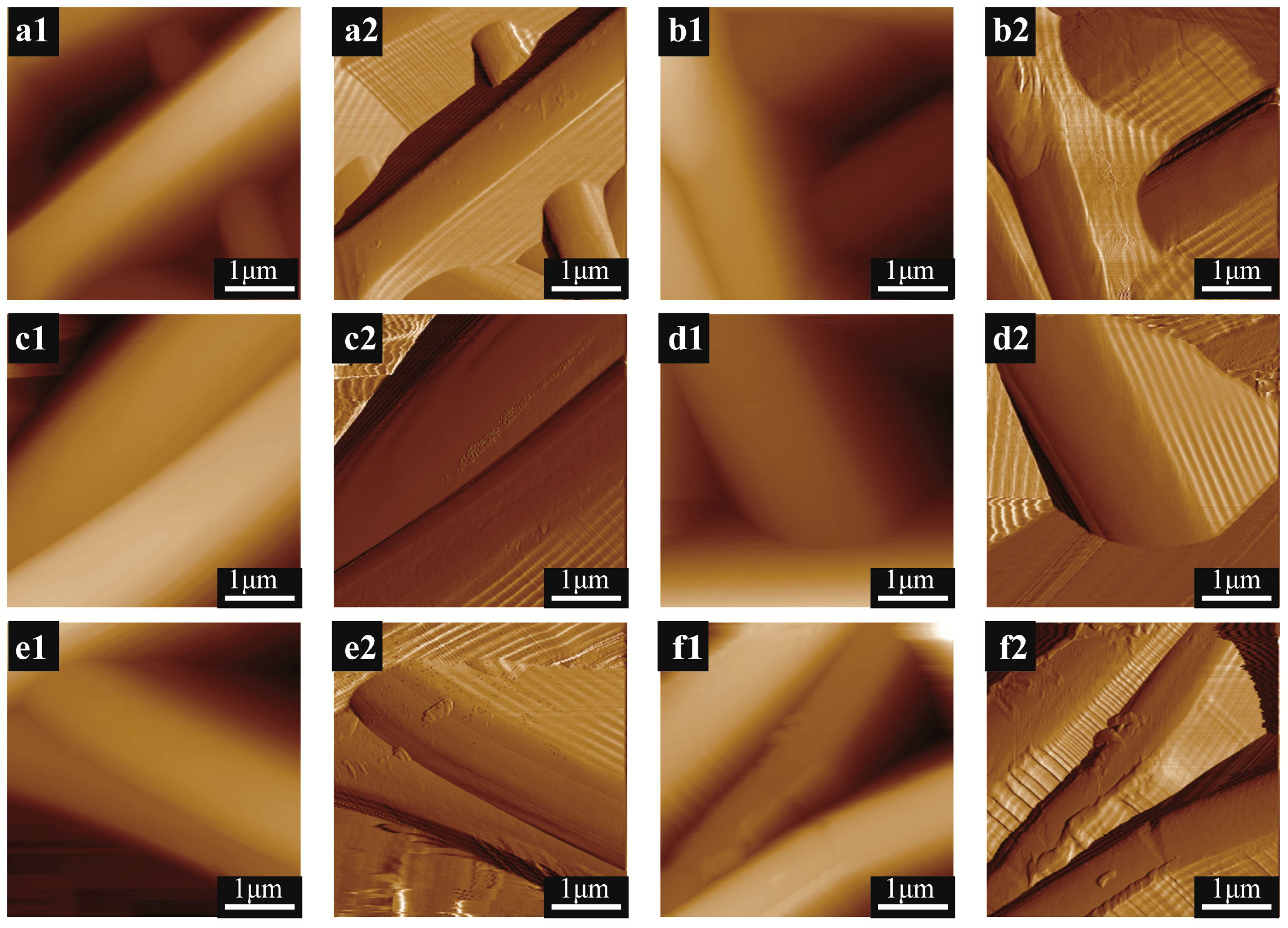


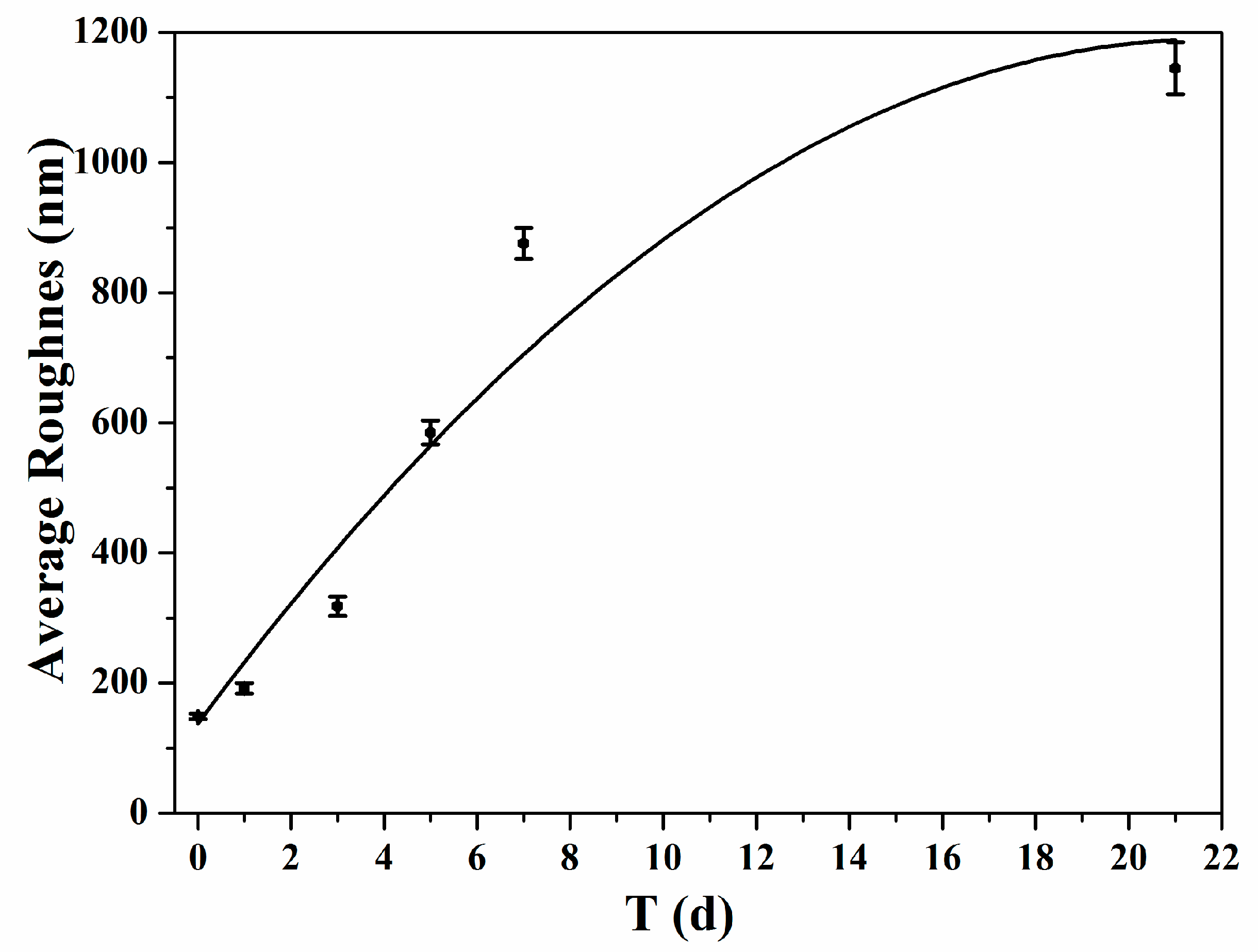
| Physical Matrix | Mineralization Days | Young’s Modulus (MPa) | Average Roughness (nm) | Surface Area (μm2) |
|---|---|---|---|---|
| Mineralized Condition | SBF (0-day) | 128 ± 5 | 149 ± 4 | 1.04 ± 0.14 |
| SBF (1-day) | 175 ± 8 | 192 ± 8 | 1.21 ± 0.17 | |
| SBF (3-day) | 355 ± 13 | 318 ± 15 | 1.99 ± 0.21 | |
| SBF (5-day) | 417 ± 18 | 585 ± 18 | 2.01 ± 0.23 | |
| SBF (7-day) | 625 ± 21 | 876 ± 24 | 2.14 ± 0.25 | |
| SBF (21-day) | 823 ± 30 | 1145 ± 40 | 2.28 ± 0.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Cai, Y.; Zhao, B.; Zhu, P. Time-Resolved Study of Nanomorphology and Nanomechanic Change of Early-Stage Mineralized Electrospun Poly(lactic acid) Fiber by Scanning Electron Microscopy, Raman Spectroscopy and Atomic Force Microscopy. Nanomaterials 2017, 7, 223. https://doi.org/10.3390/nano7080223
Wang M, Cai Y, Zhao B, Zhu P. Time-Resolved Study of Nanomorphology and Nanomechanic Change of Early-Stage Mineralized Electrospun Poly(lactic acid) Fiber by Scanning Electron Microscopy, Raman Spectroscopy and Atomic Force Microscopy. Nanomaterials. 2017; 7(8):223. https://doi.org/10.3390/nano7080223
Chicago/Turabian StyleWang, Mengmeng, Yin Cai, Bo Zhao, and Peizhi Zhu. 2017. "Time-Resolved Study of Nanomorphology and Nanomechanic Change of Early-Stage Mineralized Electrospun Poly(lactic acid) Fiber by Scanning Electron Microscopy, Raman Spectroscopy and Atomic Force Microscopy" Nanomaterials 7, no. 8: 223. https://doi.org/10.3390/nano7080223
APA StyleWang, M., Cai, Y., Zhao, B., & Zhu, P. (2017). Time-Resolved Study of Nanomorphology and Nanomechanic Change of Early-Stage Mineralized Electrospun Poly(lactic acid) Fiber by Scanning Electron Microscopy, Raman Spectroscopy and Atomic Force Microscopy. Nanomaterials, 7(8), 223. https://doi.org/10.3390/nano7080223





