Optimizing the Removal of Rhodamine B in Aqueous Solutions by Reduced Graphene Oxide-Supported Nanoscale Zerovalent Iron (nZVI/rGO) Using an Artificial Neural Network-Genetic Algorithm (ANN-GA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of nZVI and nZVI/rGO
2.3. Characterization of nZVI and nZVI/rGO
2.4. Batch Experiments
2.5. Box-Behnken Design
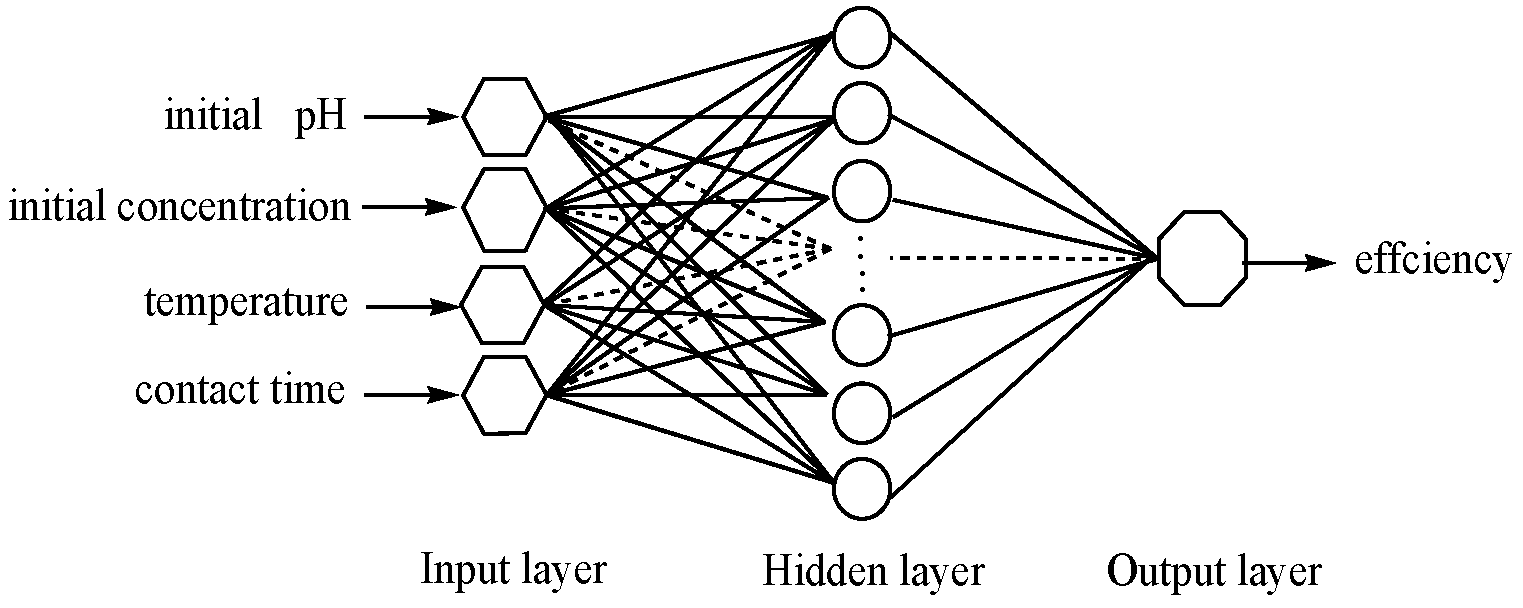
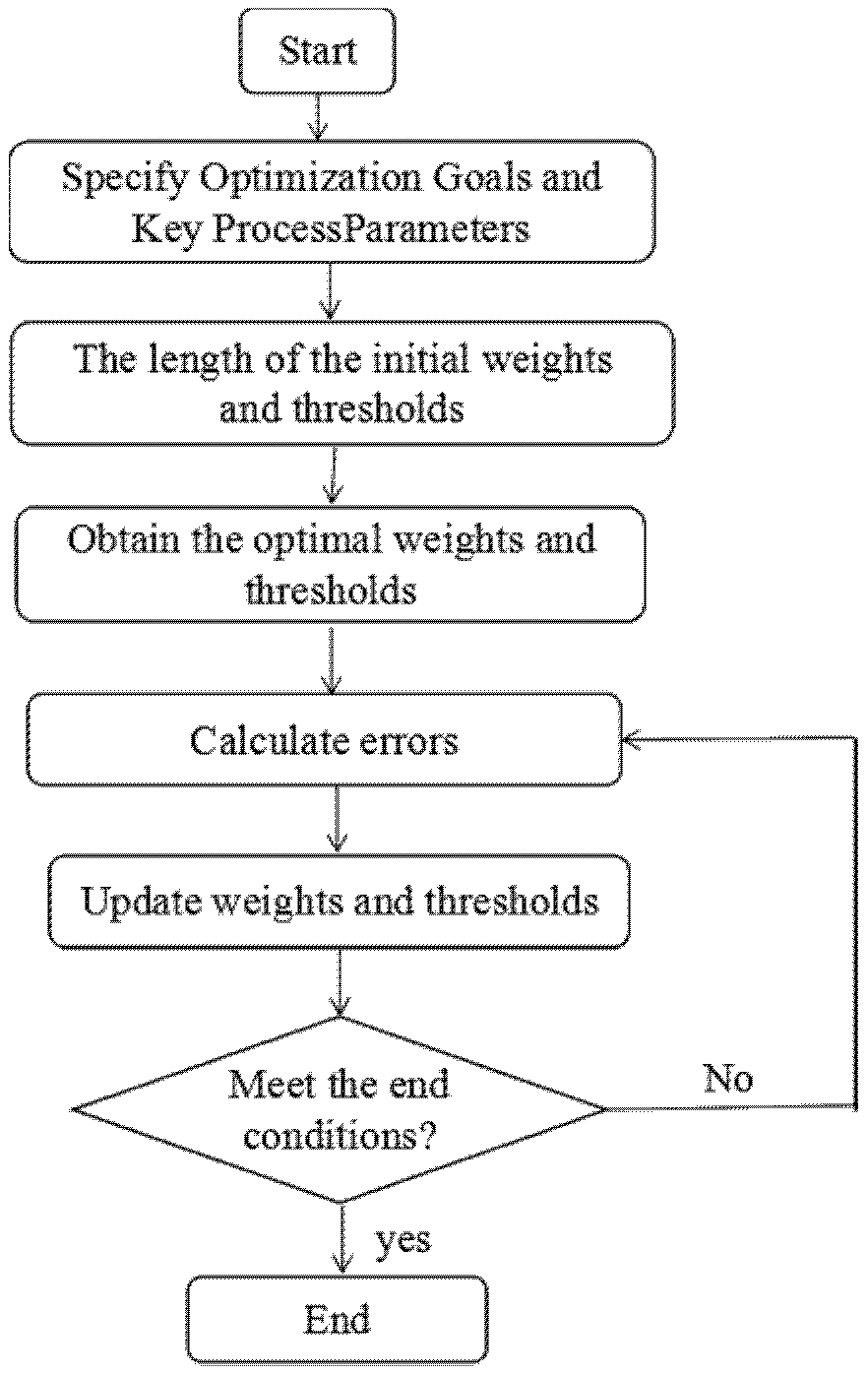
2.6. ANN Modeling and ANN-GA Optimization
3. Results and Discussion
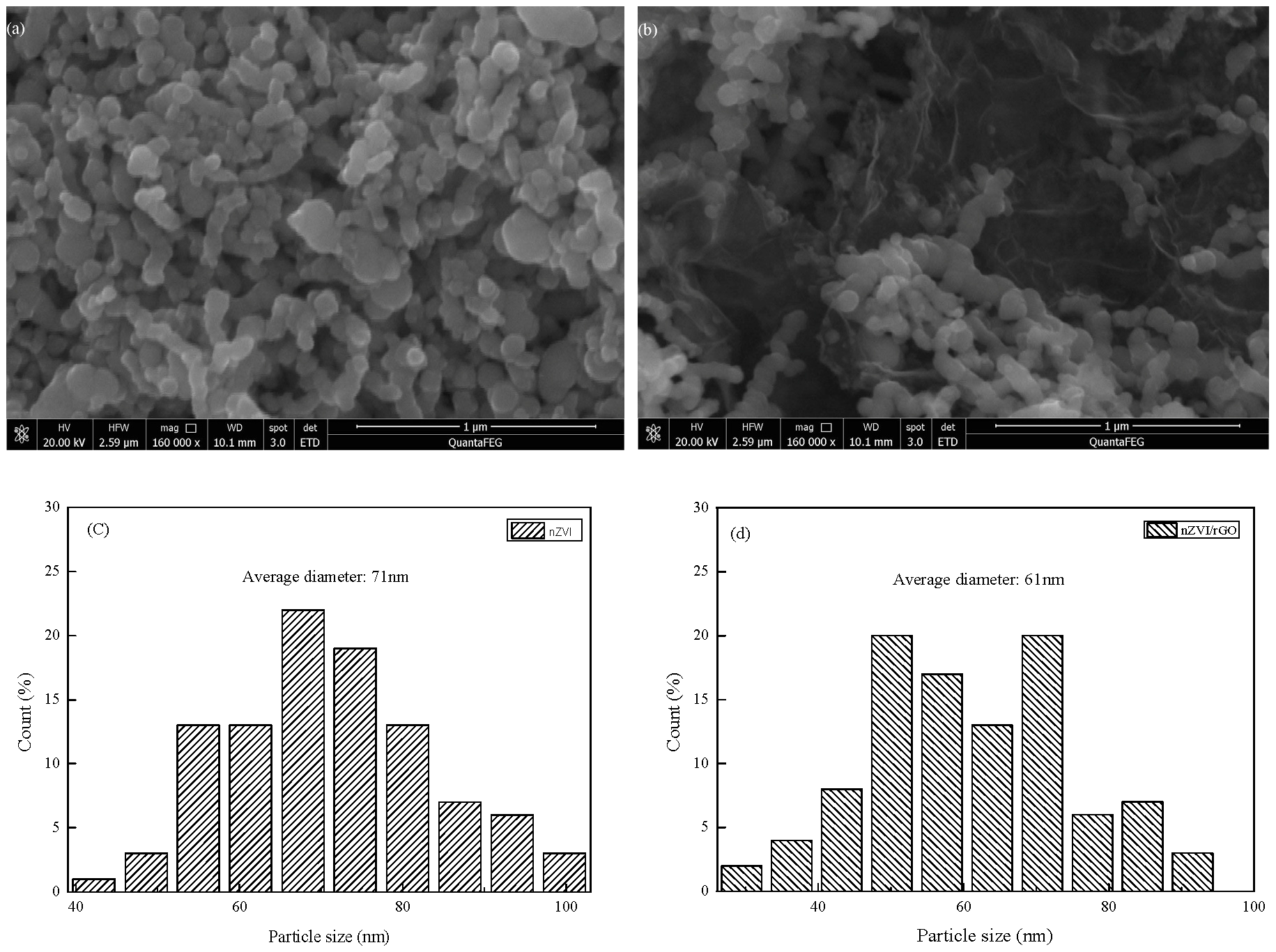
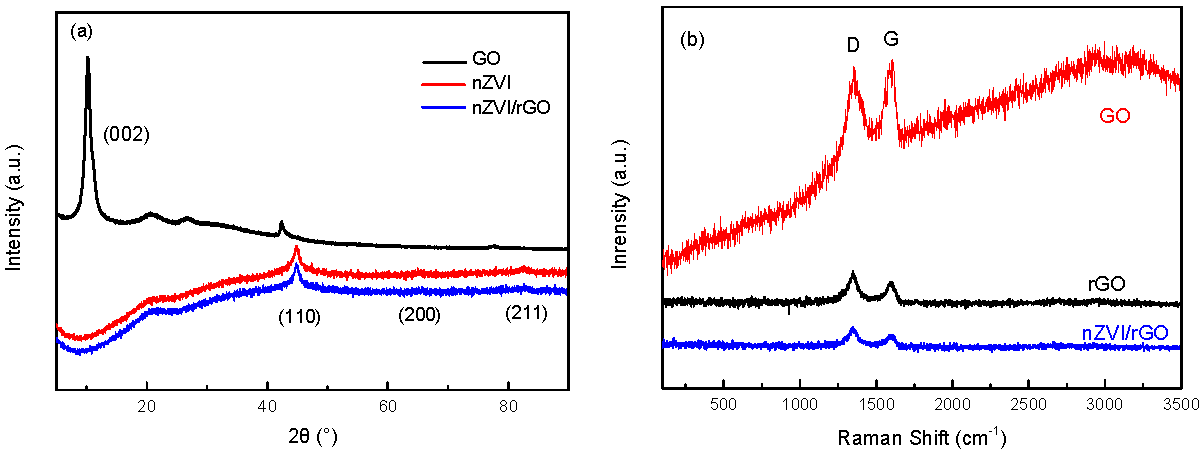
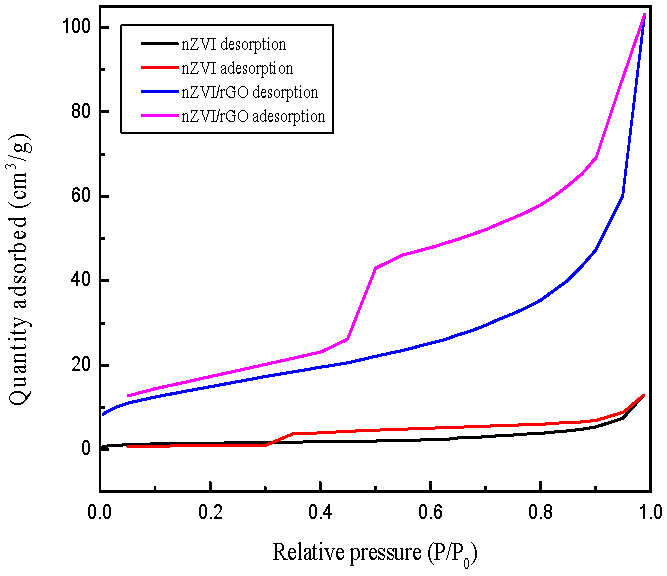
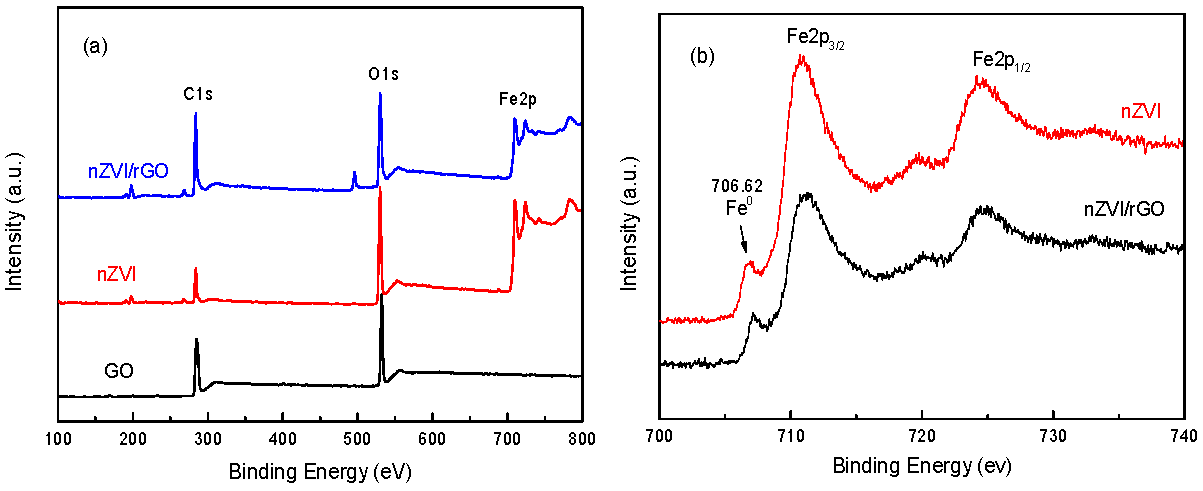
3.1. Characterization of nZVI and nZVI/rGO
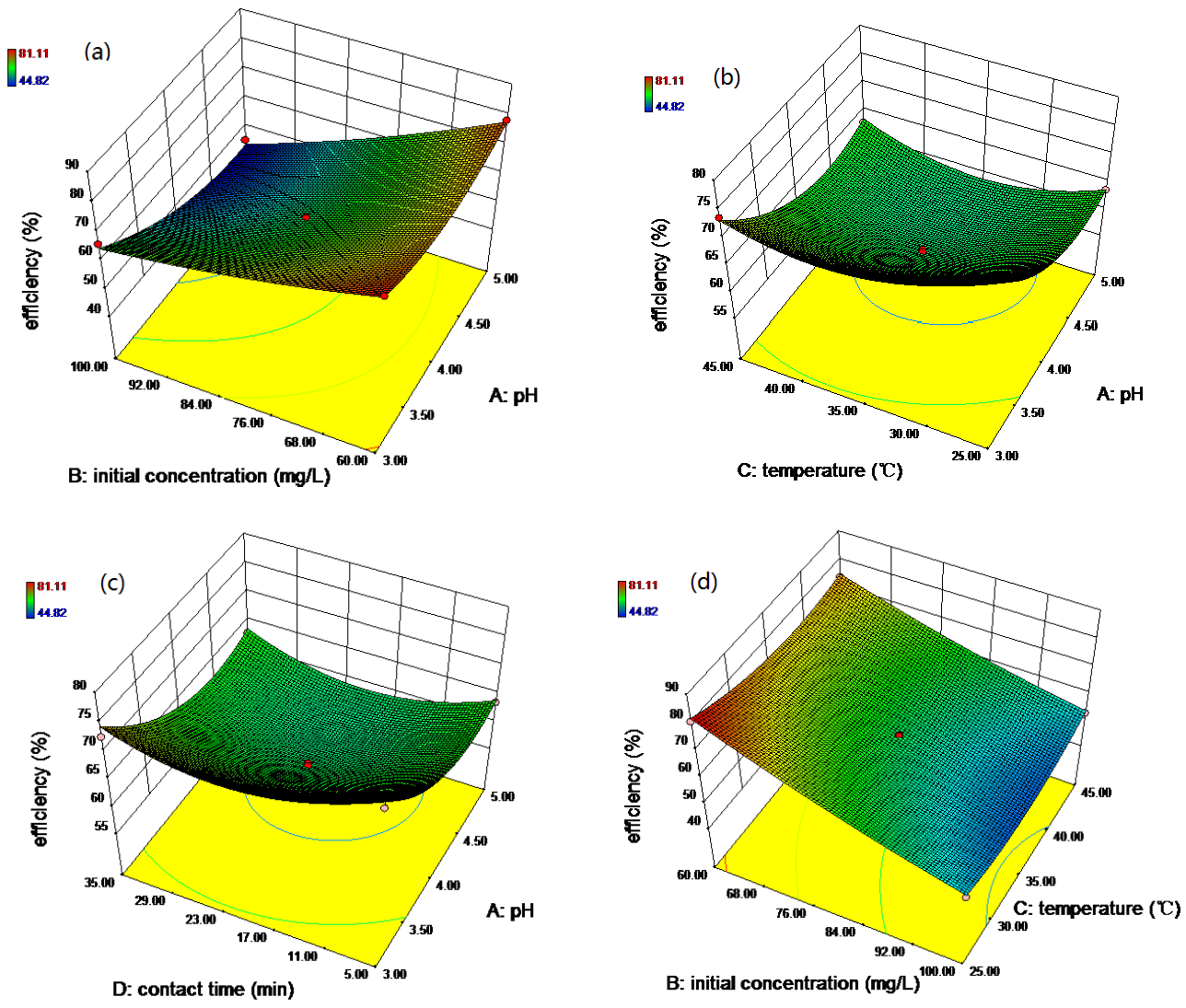
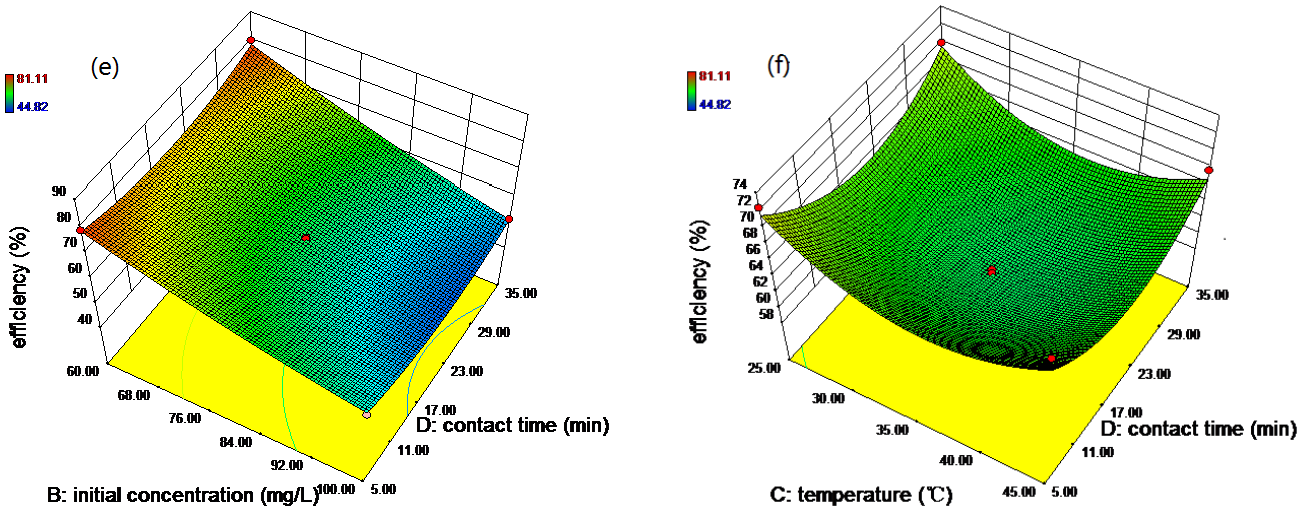
3.2. RSM-BBD Modeling
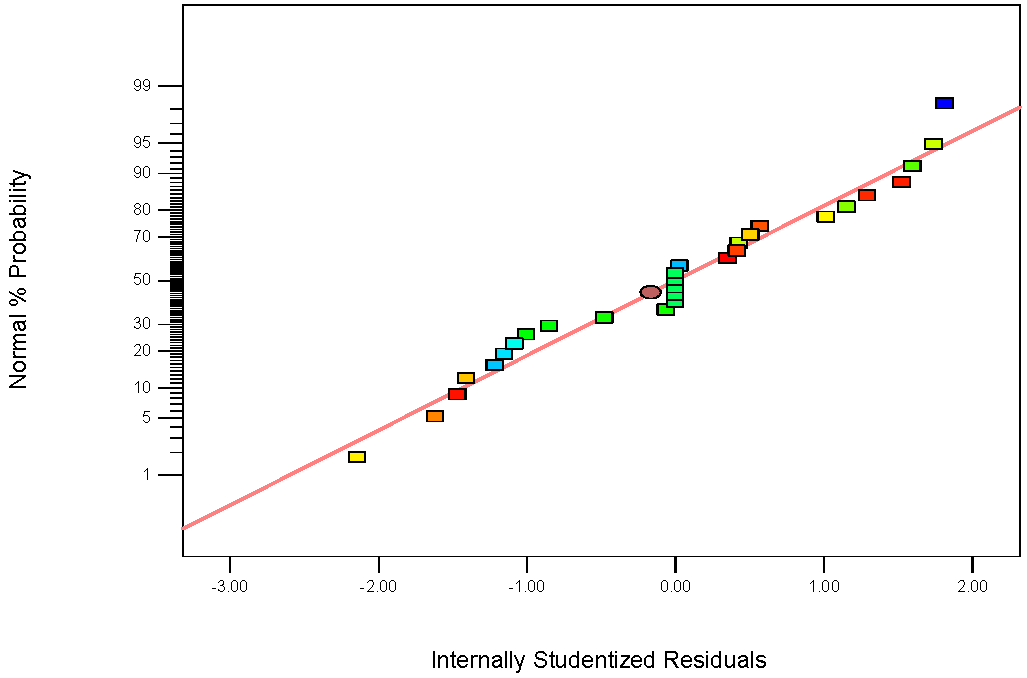
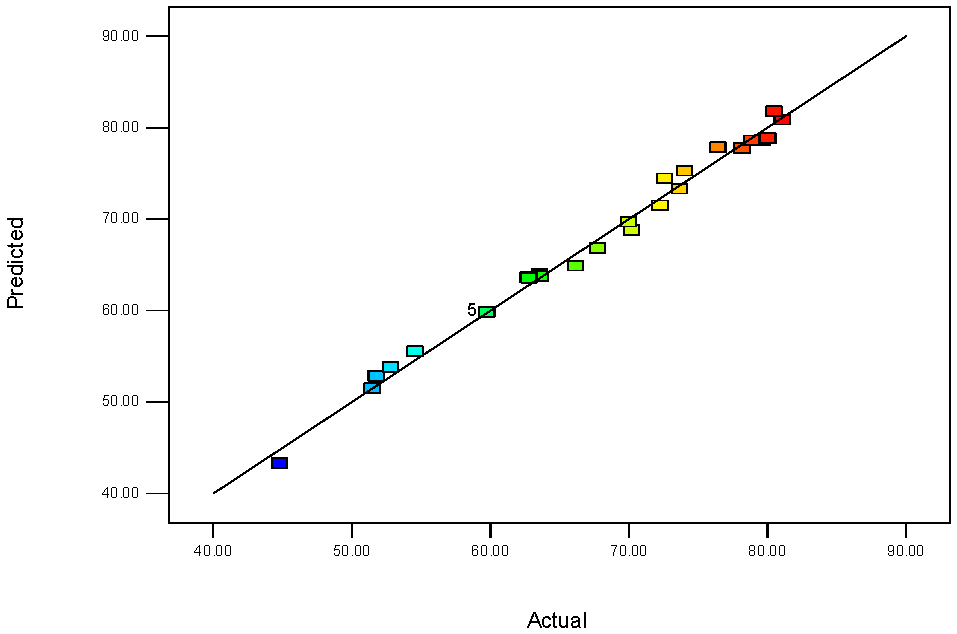
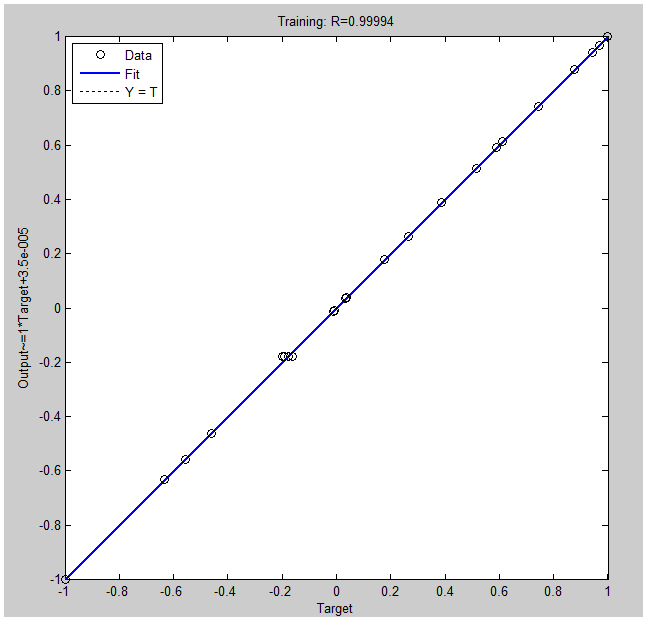
3.3. ANN Modeling
3.4. Optimization for the Removal of Rh B by RSM and ANN-GA
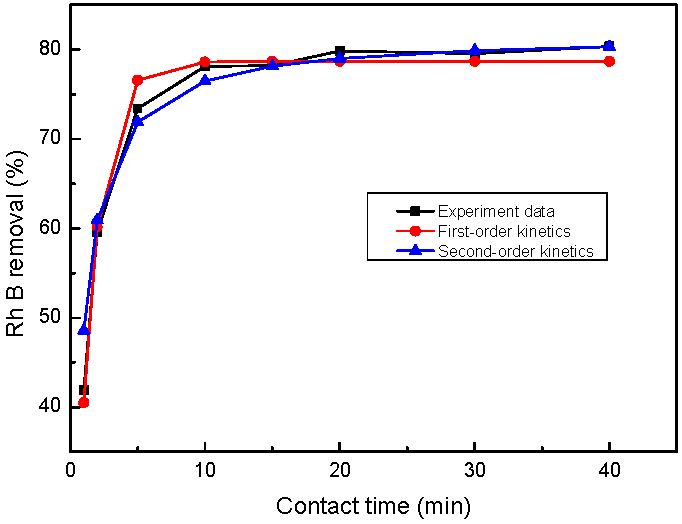
3.5. Equilibrium Adsorption Isotherm and Kinetics Studies
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgment
Author Contributions
Conflicts of Interest
References
- Hou, M.F.; Ma, C.X.; Zhang, W.D.; Tang, X.Y.; Fan, Y.N.; Wan, H.F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Experimental design and response surface modeling for optimization of Rhodamine B removal from water by magnetic nanocomposite. Chem. Eng. J. 2010, 165, 151–160. [Google Scholar] [CrossRef]
- Lei, D.Y.; Li, B.; Wang, Q.; Wu, B.; Ma, L.; Xu, H. Removal of Neutral Red from aqueous solution using Pleurotus ostreatus nanoparticles by response surface methodology. Desalin. Water Treat. 2015, 54, 2794–2805. [Google Scholar] [CrossRef]
- Dong, X.; Ding, W.; Zhang, X.; Liang, X. Mechanism and kinetics model of degradation of synthetic dyes by UV–vis/H2O2/ferrioxalate complexes. Dyes Pigm. 2007, 74, 470–476. [Google Scholar] [CrossRef]
- Wong, Y.; Yu, J. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 1999, 33, 3512–3520. [Google Scholar] [CrossRef]
- Banate, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile dye containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Slimane, M.; Oualid, H.; Fethi, S.; Mahdi, C.; Christian, P. Influence of bicarbonate and carbonate ions on sonochemical degradation of rhodamine B in aqueous phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar]
- Lu, L.; Zhao, M.; Liang, S.C.; Zhao, L.Y.; Li, D.B.; Zhang, B.B. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. J. Appl. Microbiol. 2009, 107, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chien, J.T.; Ho, G.H.; Yang, J.; Chen, B.H. Equilibrium and kinetic studies on sorption of basic dyes by a natural biopolymer poly(gamma-glutamic acid). Biochem. Eng. J. 2006, 31, 204–215. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Karthika, C.; Vennilamani, N.; Pattabhi, S. Activated carbon from industrial solid waste as an adsorbent for the removal of Rhodamine-B from aqueous solution: Kinetic and equilibrium studies. Chemosphere 2005, 60, 1009–1017. [Google Scholar] [CrossRef]
- Nestmann, E.R.; Douglas, G.R.; Matula, T.I.; Grant, C.E.; Kowbel, D.J. Mutagenic activity of rhodamine dyes and their impurities as detected by mutation induction in Salmonella and DNA damage in Chinese hamster ovary cells. Cancer Res. 1979, 39, 4412–4417. [Google Scholar]
- Bulut, E.; Özacar, M.; Şengil, İ.A. Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite. J. Hazard. Mater. 2008, 154, 613–622. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Hu, J.W.; Shi, X.D.; Fan, M.Y.; Luo, J.; Wei, X.H. Nanoscale zero-valent metals: A review of synthesis, characterization and applications to environmental remediation. Environ. Sci. Pollut. Res. 2016, 18, 17880–17900. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Y.; Li, T.; Hu, J.W.; Cao, R.S.; Wu, Q.; Wei, X.H.; Li, L.Y.; Shi, X.X.; Ruan, W.Q. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites used for Pb(II) removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef]
- Janiak, C. Metal nanoparticle synthesis in ionic liquids. Top. Organoment. Chem. 2015, 51, 17–54. [Google Scholar]
- Khan, M.S.; Ahmad, A.; Bangash, F.U.K.; Shah, S.S.; Khan, P. Removal of Basic Dye from Aqueous Solutions Using Nano Scale Zero Valent Iron (NZVI) as Adsorbent. J. Chem. Soc. Pak. 2013, 35, 744–748. [Google Scholar]
- Joo, S.H.; Feitz, A.J.; Waite, T.D. Oxidative degradation of the carbothioate herbicide molinate, using nanoscale zero-valent iron. Environ. Sci. Technol. 2004, 38, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Jin, Z.H.; Li, T.L.; Qi, X.H. Preperation of chitosan-stabilized Fe0 nanopartiicles for removel of hexavalent chromium in water. Sci. Total Environ. 2009, 407, 4994–5000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Jia, Y.F.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.; Chen, Z.L.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.L.; Zhu, K.R.; Wang, X.K. Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal. J. Taiwan Inst. Chem. Eng. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T. The removal of uranium onto carbon-supported nanoscale zero-valent iron particles. J. Nanopart. Res. 2014, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, H.J.; Zhang, Z.L.; Wu, Y.; Zhang, J.; Chen, S.W. Removal of As(III) and As(V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J. Hazard. Mater. 2014, 268, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Gu, X.G.; Li, L.; Lv, S.G.; Xu, Y.S.; Guo, X.H. Efficient degradation of trichloroethylene in water using persulfate activated by reduced graphene oxide-iron nanocomposite. Environ. Sci. Pollut. Res. 2015, 22, 17876–17885. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Zhou, Q.X. Preliminary study on effects of nanoscale amendments on hyperaccumulator Indian Marigold grow on co-contaminated soils. Adv. Mater. Res. 2014, 955–959, 243–247. [Google Scholar] [CrossRef]
- Duarte, B.P.M.; Saraiva, P.M. Hybrid models combining mechanistic models with adaptive regression splines and local stepwise regression. Ind. Eng. Chem. Res. 2003, 42, 99–107. [Google Scholar] [CrossRef]
- Achenie, L.; Butkus, M.A.; Grasso, D.; Schulthess, C.P.; Morris, T.; Hyde, J. A comparative study of neural network and mechanistic models for surface complexation. Adv. Environ. Res. 2001, 5, 137–143. [Google Scholar] [CrossRef]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Response surface modeling and optimization of chromium(VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. J. Hazard. Mater. 2009, 172, 818–825. [Google Scholar] [CrossRef]
- Kaan, Y.; Sevgi, D. Artificial neural network (ANN) approach for modeling of Pb(II) adsorption from aqueous solution by Antep pistachio (Pistacia Vera L.) shells. J. Hazard. Mater. 2008, 153, 1288–1300. [Google Scholar]
- Alam, Z.; Muyibi, S.A.; Toramae, J. Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J. Environ. Sci. 2007, 19, 674–677. [Google Scholar] [CrossRef]
- Arsand, D.R.; Kümmerer, K.; Martins, A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total Environ. 2013, 443, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Vavra, J.; Forbes, V.E. Effects of water quality parameters on agglomeration and dissolution of copper oxide nanoparticles (CuO-NPs) using a central composite circumscribed design. Sci. Total Environ. 2015, 521–522, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Avramović, J.M.; Veličković, A.V.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of sunflower oil ethanolysis catalyzed by calcium oxide: RSM versus ANN-GA. Energy Convers. Manag. 2015, 105, 1149–1156. [Google Scholar] [CrossRef]
- Rajendra, M.; Jena, PC.; Raheman, H. Prediction of optimized pretreatment process parameters for biodiesel production using ANN and GA. Fuel 2009, 88, 868–875. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H.; Geyik, F. Use of artificial neural networks and genetic algorithms for prediction of sorption of an azo-metal complex dye onto lentil straw. Bioresour. Technol. 2012, 129, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ma, Y.; Wan, J.; Zhang, H.; Wang, Y.; Chen, Y.; Yoo, C.; Guo, W. A hybrid genetic-neural algorithm for modeling the biodegradation process of DnBP in AAO system. Bioresour. Technol. 2011, 102, 8907–8913. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1939. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.; Kemp, K.C.; Chandra, V. Synthesis of nano zerovalent iron nanoparticles-Graphene composite for the treatment of lead contaminated water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Cao, H.Q.; Yin, G.; Lu, Y.X.; Yin, J.F. Cu2O@reduced Graphene oxide composite for removal of contaminants from water and supercapacitor. J. Mater. Chem. 2011, 21, 10645–10648. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Experimental design and response surface modeling for optimization of fluoroquinolone removal from aqueous solution by NaOH-modified rice husk. Desalin. Water Treat. 2016, 57, 16456–16465. [Google Scholar] [CrossRef]
- Madala, S.; Mudumala, V.N.R.; Vudagandla, S.; Abburi, K. Modified leaf biomass for Pb(II) removal from aqueous solution: Application of response surface methodology. Ecol. Eng. 2015, 83, 218–226. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, B. Modeling batch and column phosphate removal by hydrated ferric oxide-based nanocomposite using response surface methodology and artificial neural network. Chem. Eng. J. 2014, 249, 111–120. [Google Scholar] [CrossRef]
- Winiczenko, R.; Górnicki, K.; Kaleta, A.; Janaszek-Mańkowska, M. Optimisation of ANN topology for predicting the rehydrated apple cubes colour change using RSM and GA. Neural Comput. Appl. 2016. [Google Scholar] [CrossRef]
- Pal, M.P.; Vaidya, B.K.; Desai, K.M.; Joshi, R.M.; Nene, S.N.; Kulkarni, B.D. Media optimization for biosurfactant production by Rhodococcus erythropolis MTCC 2794: Artificial intelligence versus a statistical approach. J. Ind. Microbiol. Biotechnol. 2009, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman spectrunm of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.St.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- He, D.; Ma, J.; Collins, R.N.; Waite, T.D. Effect of structural transformation of nanoparticulate zero-valent iron on generation of reactive oxygen species. Environ. Sci. Technol. 2016, 50, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.A.; Kiser, J.R.; Kwon, H.; Kanel, S.R. Spectroscopic investigation of Cr(III)- and Cr(VI)-treated nanoscale zerovalent iron. Environ. Sci. Technol. 2007, 41, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Mohammadi, M.; Hassani, A.J.; Mohamed, A.R.; Najafpour, G.D. Removal of Rhodamine B from aqueous solution using palm shell-based activated carbon: Adsorption and kinetic studies. J. Chem. Eng. Data 2010, 55, 5777–5785. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Tamilarasan, R.; Dharmendirakumar, M. Adsorption, Kinetic, Equilibrium and Thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci. 2012, 3, 157–170. [Google Scholar]
- Patil, S.P.; Bethi, B.; Sonawane, G.H.; Shrivastava, V.S.; Sonawane, S. Efficient adsorption and photocatalytic degradation of Rhodamine B dye over Bi2O3-bentonite nanocomposites: A kinetic study. J. Ind. Eng. Chem. 2016, 34, 356–363. [Google Scholar] [CrossRef]
- Selvam, P.P.; Preethi, S.; Basakaralingam, P.; Thinakaran, N.; Sivasamy, A.; Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Hazard. Mater. 2008, 155, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, X.; Sun, Y.; Wang, Y.; Long, Y.; Jiang, J.; Xu, H. Removal of Rhodamine B from wastewater by modified Volvariella volvacea: Batch and column study. RSC. Adv. 2015, 5, 25337–25347. [Google Scholar] [CrossRef]



| Variable | Unit | Factors | Level | ||
|---|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |||
| Initial pH | A | 3 | 4 | 5 | |
| Initial concentration | mg/L | B | 60 | 80 | 100 |
| Temperature | °C | C | 25 | 35 | 45 |
| Contact time | min | D | 5 | 20 | 35 |
| Run | A | B (mg/L) | C (°C) | D (min) | Actual (%) | Predicted (%) | |
|---|---|---|---|---|---|---|---|
| RSM | ANN | ||||||
| 1 | 3 | 80 | 35 | 5 | 76.5 | 77.8 | 76.5 |
| 2 | 4 | 100 | 35 | 5 | 54.6 | 55.5 | 54.6 |
| 3 | 4 | 80 | 35 | 20 | 59.5 | 59.5 | 59.7 |
| 4 | 5 | 100 | 35 | 20 | 44.8 | 43.3 | 44.8 |
| 5 | 5 | 80 | 25 | 20 | 63.6 | 63.7 | 63.6 |
| 6 | 4 | 60 | 25 | 20 | 80.5 | 81.7 | 80.5 |
| 7 | 5 | 80 | 35 | 5 | 63.6 | 64.0 | 63.6 |
| 8 | 3 | 100 | 35 | 20 | 66.2 | 64.9 | 66.2 |
| 9 | 3 | 80 | 45 | 20 | 73.7 | 73.3 | 73.7 |
| 10 | 4 | 80 | 35 | 20 | 60.0 | 59.5 | 59.7 |
| 11 | 4 | 80 | 35 | 20 | 59.8 | 59.5 | 59.7 |
| 12 | 5 | 80 | 35 | 35 | 62.8 | 63.6 | 62.8 |
| 13 | 4 | 60 | 45 | 20 | 74.1 | 75.2 | 74.1 |
| 14 | 5 | 80 | 45 | 20 | 62.8 | 63.5 | 62.8 |
| 15 | 4 | 80 | 35 | 20 | 59.8 | 59.8 | 59.7 |
| 16 | 4 | 80 | 25 | 5 | 72.3 | 71.4 | 72.3 |
| 17 | 4 | 80 | 25 | 35 | 70.0 | 69.6 | 70.0 |
| 18 | 4 | 60 | 35 | 5 | 78.9 | 78.5 | 78.9 |
| 19 | 4 | 60 | 35 | 35 | 80.1 | 78.8 | 80.0 |
| 20 | 4 | 100 | 35 | 35 | 51.5 | 51.5 | 51.5 |
| 21 | 3 | 60 | 35 | 20 | 81.1 | 80.8 | 81.1 |
| 22 | 4 | 80 | 45 | 35 | 67.77 | 66.81 | 67.8 |
| 23 | 4 | 80 | 35 | 20 | 59.37 | 59.51 | 59.7 |
| 24 | 4 | 100 | 45 | 20 | 52.84 | 53.8 | 52.8 |
| 25 | 5 | 60 | 35 | 20 | 78.2 | 77.72 | 78.6 |
| 26 | 4 | 100 | 25 | 20 | 51.8 | 52.81 | 54.6 |
| 27 | 3 | 80 | 25 | 20 | 79.68 | 78.6 | 76.1 |
| 28 | 3 | 80 | 35 | 35 | 72.62 | 74.41 | 69.1 |
| 29 | 4 | 80 | 45 | 5 | 70.23 | 68.78 | 72.3 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2870.1 | 14 | 205.01 | 119.07 | <0.0001 |
| A | 455.35 | 1 | 455.35 | 264.48 | <0.0001 |
| B | 1903.1 | 1 | 1903.1 | 1105.38 | <0.0001 |
| C | 22.77 | 1 | 22.77 | 13.23 | 0.0027 |
| D | 10.74 | 1 | 10.74 | 6.24 | 0.0256 |
| AB | 85.19 | 1 | 85.19 | 49.48 | <0.0001 |
| AC | 6.71 | 1 | 6.71 | 3.90 | 0.0685 |
| AD | 2.28 | 1 | 2.28 | 1.32 | 0.2691 |
| BC | 14.03 | 1 | 14.03 | 8.15 | 0.0127 |
| BD | 4.60 | 1 | 4.60 | 2.67 | 0.1244 |
| CD | 6.40×10−3 | 1 | 6.40×10−3 | 3.72×10−3 | 0.9522 |
| A2 | 197.47 | 1 | 197.47 | 114.69 | <0.0001 |
| B2 | 17.61 | 1 | 17.61 | 10.23 | 0.0064 |
| C2 | 145.81 | 1 | 145.81 | 84.69 | <0.0001 |
| D2 | 156.93 | 1 | 156.93 | 91.15 | <0.0001 |
| Residual | 24.1 | 14 | 1.72 | ||
| Lack of Fit | 23.33 | 10 | 2.33 | ||
| Pure Error | 0.77 | 4 | 0.19 | ||
| Cor Total | 2894.2 | 28 |
| Process Variable | RSM Optimization | ANN-GA Optimization |
|---|---|---|
| Initial pH | 3.00 | 3.20 |
| Initial concentration (mg/L) | 60.00 | 60.00 |
| Temperature (°C) | 25.00 | 27.00 |
| Contact time (min) | 5.30 | 6.00 |
| Efficiency, model (%) | 95.2 | 90.0 |
| Efficiency, actual (%) | 87.4 | 86.4 |
| Isotherms | Equation | Parameters | Value of Parameters |
|---|---|---|---|
| Langmuir | = + RL = 1/(1 + KLC0) | KL (L/mg) | 0.0913 |
| qm (mg/g) | 87.72 | ||
| R2 | 0.9670 | ||
| RL | 0.0641–0.3539 | ||
| Freundlich | Kf (mg/g) | 21.11 | |
| 1/n | 0.3016 | ||
| R2 | 0.9773 | ||
| Temkin | qe = BlnA + BlnCe | A (L/g) | 2.988 |
| B | 13.711 | ||
| R2 | 0.9122 |
| Model | Equation | Parameters | Value of Parameters |
|---|---|---|---|
| First-order kinetics | k1 (1/min) | 0.72 | |
| qe (mg/g) | 56.65 | ||
| R2 | 0.9866 | ||
| Second-order kinetics | k2 (g/mg·min) | 2.49 × 10−2 | |
| qe (mg/g) | 58.82 | ||
| R2 | 0.9999 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Ruan, W.; Hu, J.; Fan, M.; Cao, R.; Wei, X. Optimizing the Removal of Rhodamine B in Aqueous Solutions by Reduced Graphene Oxide-Supported Nanoscale Zerovalent Iron (nZVI/rGO) Using an Artificial Neural Network-Genetic Algorithm (ANN-GA). Nanomaterials 2017, 7, 134. https://doi.org/10.3390/nano7060134
Shi X, Ruan W, Hu J, Fan M, Cao R, Wei X. Optimizing the Removal of Rhodamine B in Aqueous Solutions by Reduced Graphene Oxide-Supported Nanoscale Zerovalent Iron (nZVI/rGO) Using an Artificial Neural Network-Genetic Algorithm (ANN-GA). Nanomaterials. 2017; 7(6):134. https://doi.org/10.3390/nano7060134
Chicago/Turabian StyleShi, Xuedan, Wenqian Ruan, Jiwei Hu, Mingyi Fan, Rensheng Cao, and Xionghui Wei. 2017. "Optimizing the Removal of Rhodamine B in Aqueous Solutions by Reduced Graphene Oxide-Supported Nanoscale Zerovalent Iron (nZVI/rGO) Using an Artificial Neural Network-Genetic Algorithm (ANN-GA)" Nanomaterials 7, no. 6: 134. https://doi.org/10.3390/nano7060134
APA StyleShi, X., Ruan, W., Hu, J., Fan, M., Cao, R., & Wei, X. (2017). Optimizing the Removal of Rhodamine B in Aqueous Solutions by Reduced Graphene Oxide-Supported Nanoscale Zerovalent Iron (nZVI/rGO) Using an Artificial Neural Network-Genetic Algorithm (ANN-GA). Nanomaterials, 7(6), 134. https://doi.org/10.3390/nano7060134






