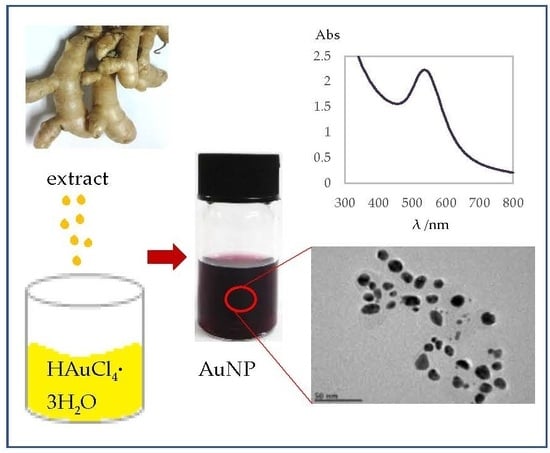
Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility
Abstract
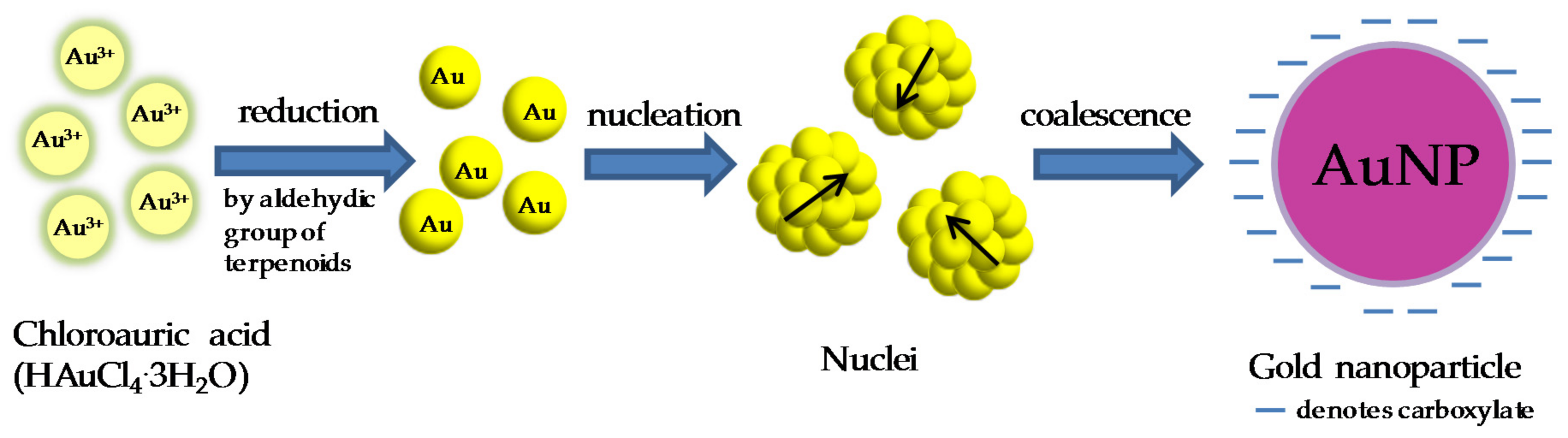
:1. Introduction
2. Results
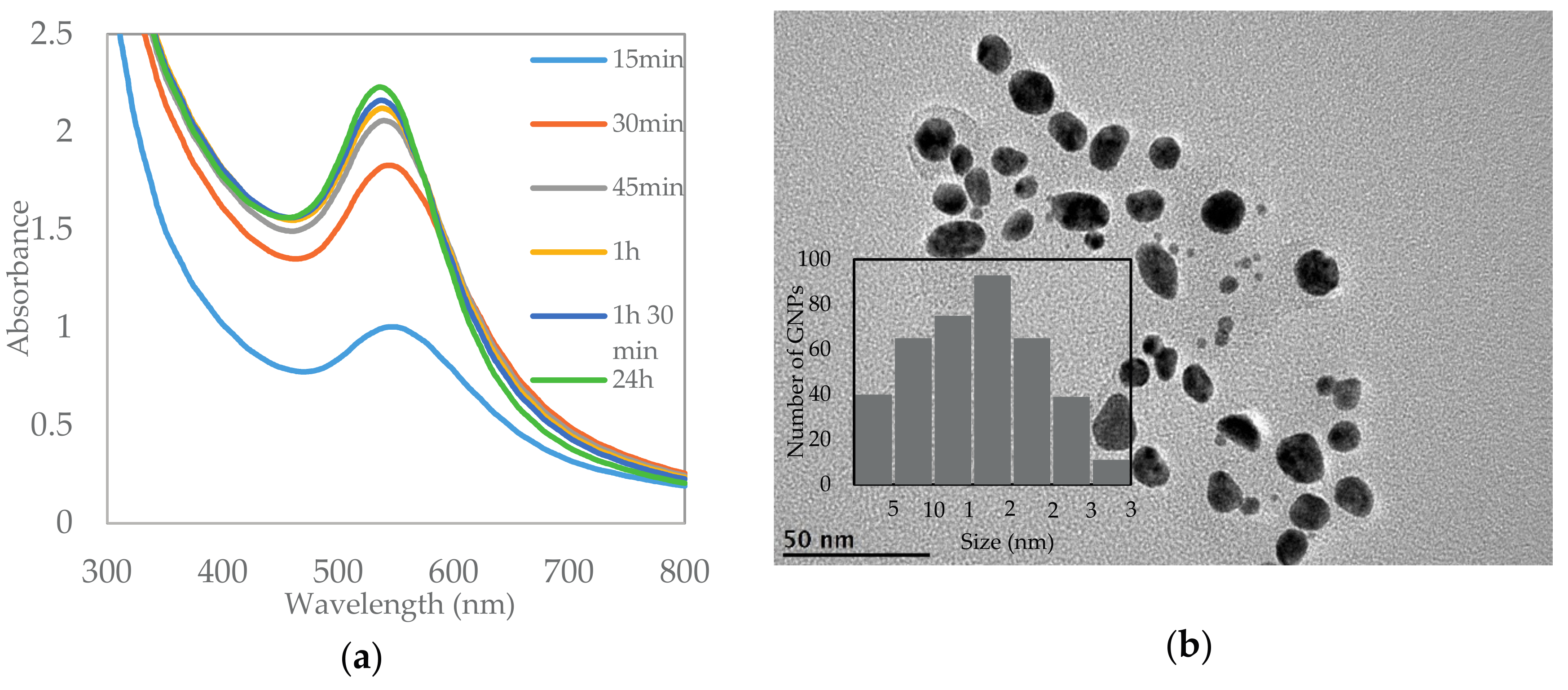
2.1. Characterization of AuNPs by Ultraviolet-Visible (UV-Vis) Spectroscopy and Transmission Electron Microscopy
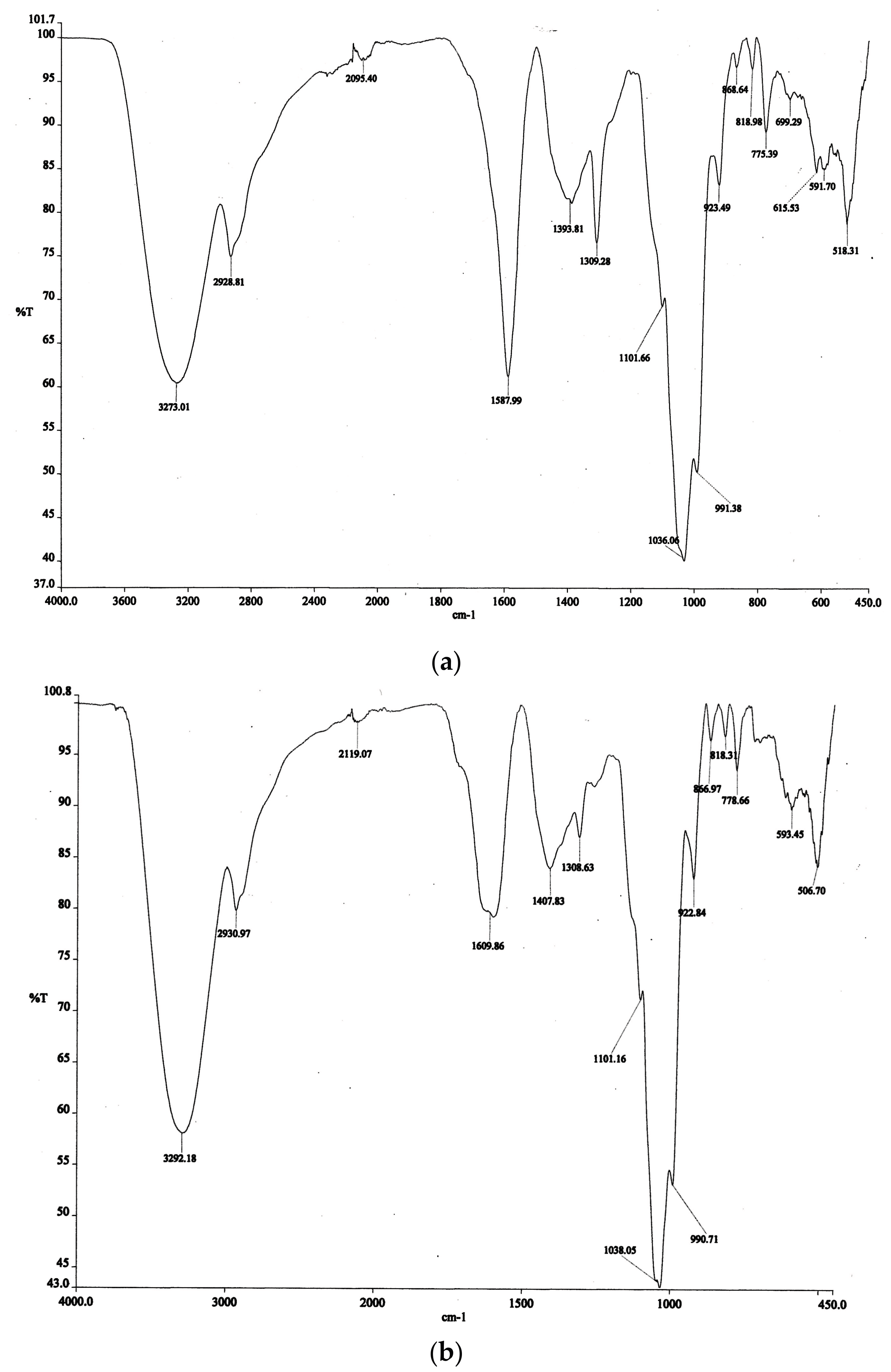
2.2. Fourier Transform Infrared Spectroscopy
2.3. Cyclic Voltammetry
2.4. In Vitro Stability Study
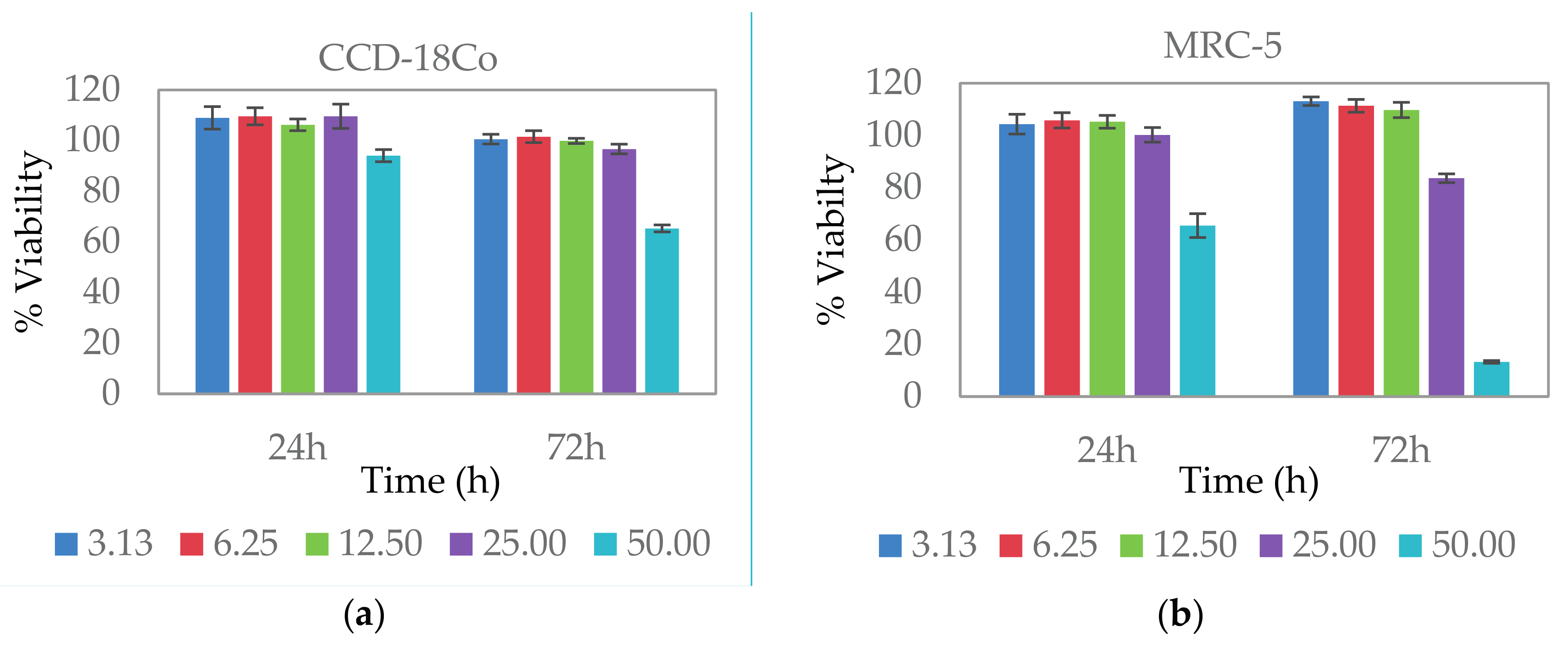
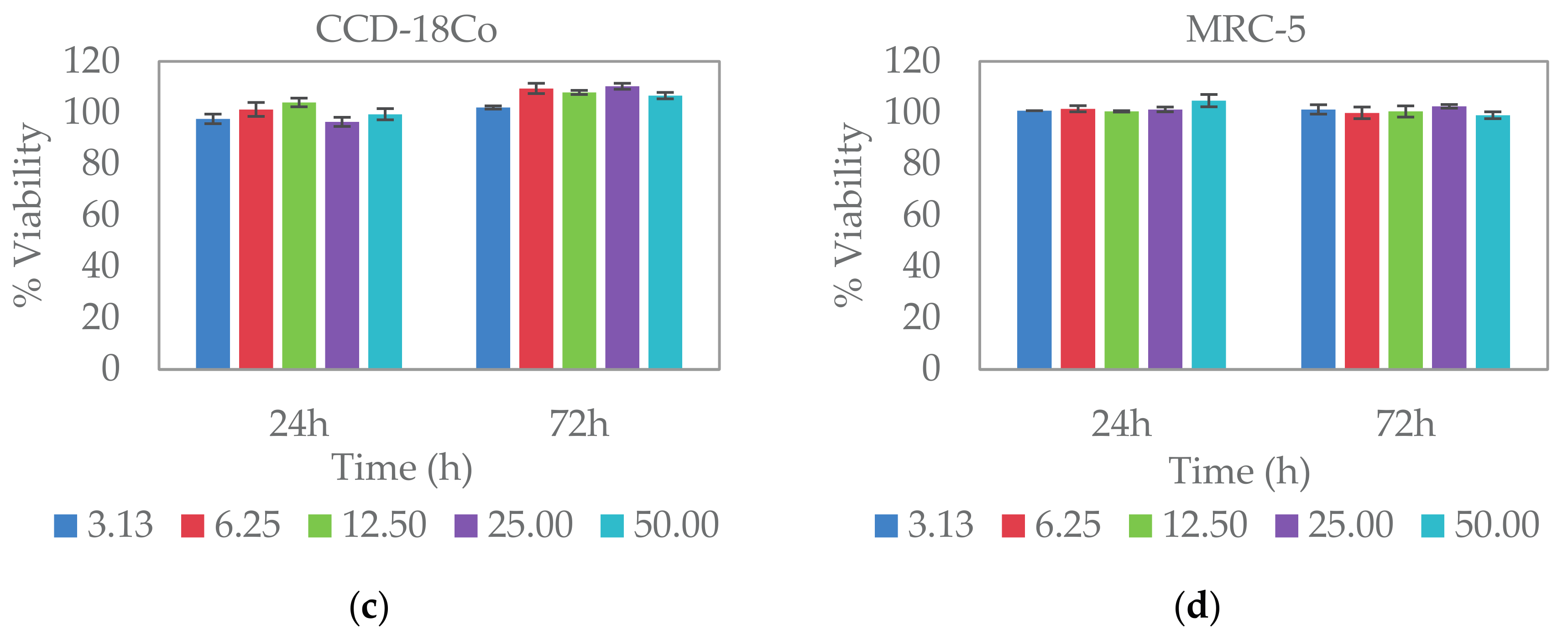
2.5. Cytocompatibility Study
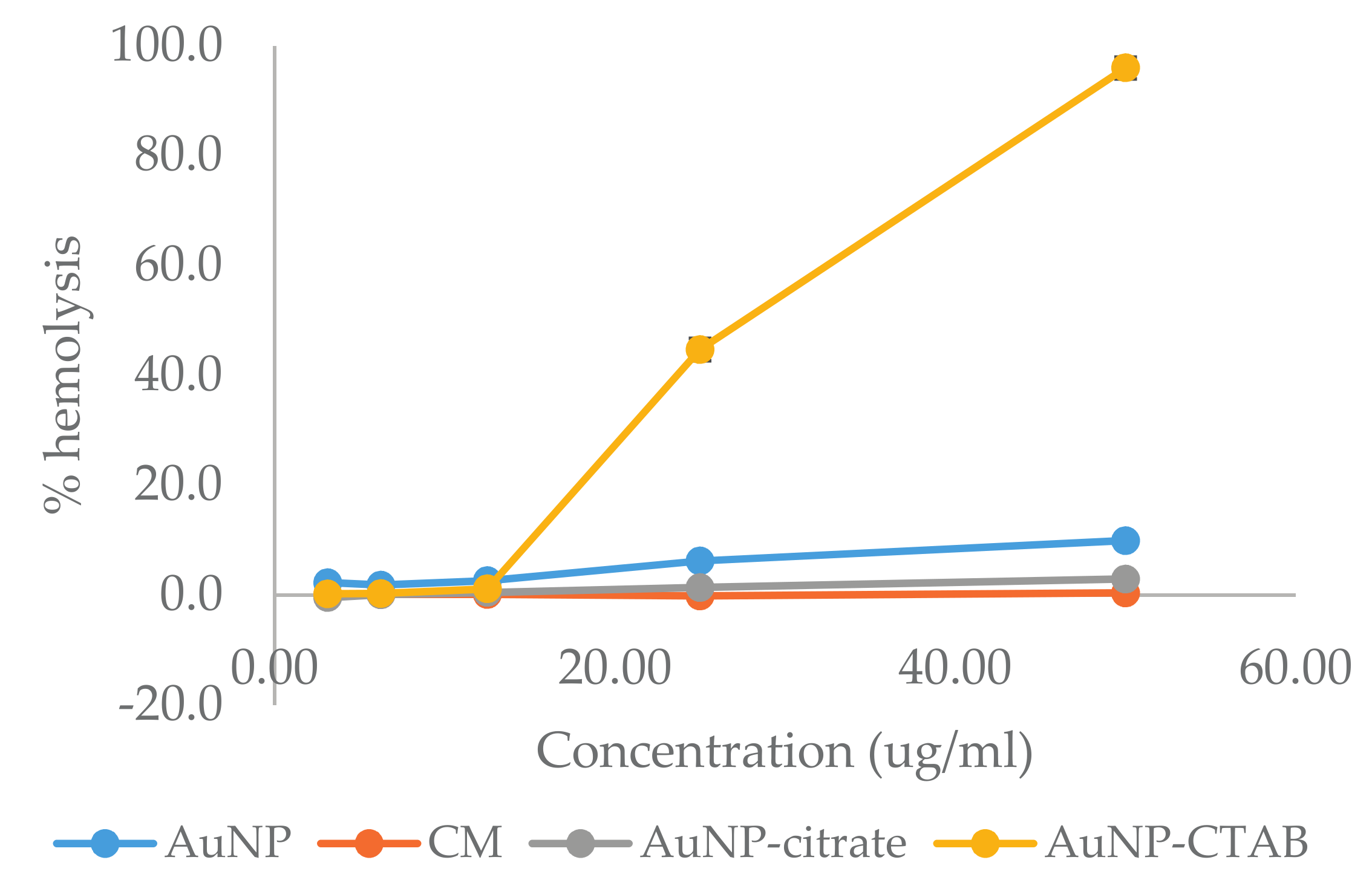
2.6. In Vitro Blood Compatibility
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Purification of AuNPs
4.3. Characterization of AuNPs
4.4. In Vitro Stability Study
4.5. Cytocompatibility Study
4.6. Hemocompatibility Test
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiao, P.F.; Zhou, H.Y.; Chen, L.X.; Yan, B. Cancer-targeting multifunctionalized gold nanoparticles in imaging and therapy. Curr. Med. Chem. 2011, 18, 2086–2102. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica–gold nanoparticles for atheroprotective management of plaques: Results of the nanom-fim trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel pegylated colloidal gold-rhtnf nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Chauhan, N.; Othman, S.F.; Khalilzad-Sharghi, V.; Ebeling, M.C.; Khan, S.; Jaggi, M.; Chauhan, S.C. Implications of protein corona on physico-chemical and biological properties of magnetic nanoparticles. Biomaterials 2015, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold nanoparticles: Synthesis, stability test, and application for the rice growth. J. Nanomater. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, J.-H.; Liu, T.; Xie, Z.; Yu, X.-F.; Li, W. Surface chemistry but not aspect ratio mediates the biological toxicity of gold nanorods in vitro and in vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef] [PubMed]
- Freese, C.; Uboldi, C.; Gibson, M.I.; Unger, R.E.; Weksler, B.B.; Romero, I.A.; Couraud, P.-O.; Kirkpatrick, C.J. Uptake and cytotoxicity of citrate-coated gold nanospheres: Comparative studies on human endothelial and epithelial cells. Part. Fibre Toxicol. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Ganesan, S. In vitro cytotoxicity assay on gold nanoparticles with different stabilizing agents. J. Nanomater. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology–a new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Sen, I.K.; Islam, S.S. Green synthesis of gold nanoparticles using gum polysaccharide of cochlospermum religiosum (katira gum) and study of catalytic activity. Physica E 2012, 45, 130–134. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using pseudomonas aeruginosa. Spectrochim. Acta Part A 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.K.; Govindaraju, K.; Manikandan, R.; Ahn, J.S.; Bae, E.Y.; Singaravelu, G. Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf. B 2010, 75, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Malek, S.N.A.; Lee, G.S.; Hong, S.L.; Yaacob, H.; Wahab, N.A.; Weber, J.-F.F.; Shah, S.A.A. Phytochemical and cytotoxic investigations of curcuma mangga rhizomes. Molecules 2011, 16, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nair, M.G. Labdane diterpenes in curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferation. Food Chem. 2011, 124, 527–532. [Google Scholar] [CrossRef]
- Philip, K.; Malek, S.N.; Sani, W.; Shin, S.K.; Kumar, S.; Lai, H.S.; Serm, L.G.; Rahman, S.N. Antimicrobial activity of some medicinal plants from malaysia. Am. J. Appl. Sci. 2009, 6, 1613. [Google Scholar]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Smith, B. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Sperling, R.A.; Parak, W. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. Lond. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Regmi, R.; Gumber, V.; Rao, V.S.; Kohli, I.; Black, C.; Sudakar, C.; Vaishnava, P.; Naik, V.; Naik, R.; Mukhopadhyay, A. Discrepancy between different estimates of the hydrodynamic diameter of polymer-coated iron oxide nanoparticles in solution. J. Nanopart. Res. 2011, 13, 6869–6875. [Google Scholar] [CrossRef]
- Caracciolo, G.; Callipo, L.; De Sanctis, S.C.; Cavaliere, C.; Pozzi, D.; Laganà, A. Surface adsorption of protein corona controls the cell internalization mechanism of DC-Chol–DHOL/DNA lipoplexes in serum. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine b colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Evani, S.J.; Ramasubramanian, A.K. Hemocompatibility of nanoparticles. In Nanobiomaterials Handbook; Sitharaman, B., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–17. [Google Scholar]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007. [Google Scholar]
- Rajan, A.; MeenaKumari, M.; Philip, D. Shape tailored green synthesis and catalytic properties of gold nanocrystals. Spectrochim. Acta Part A 2014, 118, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Kraehnert, R.; Radtke, M.; Reinholz, U.; Riesemeier, H.; Thünemann, A.F.; Emmerling, F. New Insights of the Nucleation and Growth Process of Gold Nanoparticles via in siTu Coupling of Saxs and Xanes. J. Phys. Conf. Ser. 2010, 247, 012051. [Google Scholar] [CrossRef]
- Aziz, M.A.; Kim, J.-P.; Oyama, M. Preparation of monodispersed carboxylate-functionalized gold nanoparticles using pamoic acid as a reducing and capping reagent. Gold Bull. 2014, 47, 127–132. [Google Scholar] [CrossRef]
- Du, S.; Kendall, K.; Toloueinia, P.; Mehrabadi, Y.; Gupta, G.; Newton, J. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J. Nanopart. Res. 2012, 14, 758. [Google Scholar] [CrossRef]
- Gosens, I.; Post, J.A.; de la Fonteyne, L.J.; Jansen, E.H.; Geus, J.W.; Cassee, F.R.; de Jong, W.H. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part. Fibre Toxicol. 2010, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carrillo-Carrion, C.; de Aberasturi, D.J.; Merk, V.; Barcikowski, S.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Kumar, S.; Dutta, P.K. Dibutyrylchitin nanoparticles as novel drug carrier. Int. J. Biol. Macromol. 2016, 82, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Foo, Y.Y.; Periasamy, V.; Malek, S.N.A. Green synthesis of gold nanoparticles using aqueous ethanol extract of curcuma mangga rhizomes as reducing agent. AIP Conf. Proc. 2015, 1657, 060008. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Nelson, C.E.; Shann, S.Y.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of ph-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. JoVE (J. Vis. Exp.) 2013, 73, e5016. [Google Scholar] [CrossRef] [PubMed]
- Singhal, J.P.; Ray, A.R. Synthesis of blood compatible polyamide block copolymers. Biomaterials 2002, 23, 1139–1145. [Google Scholar] [CrossRef]

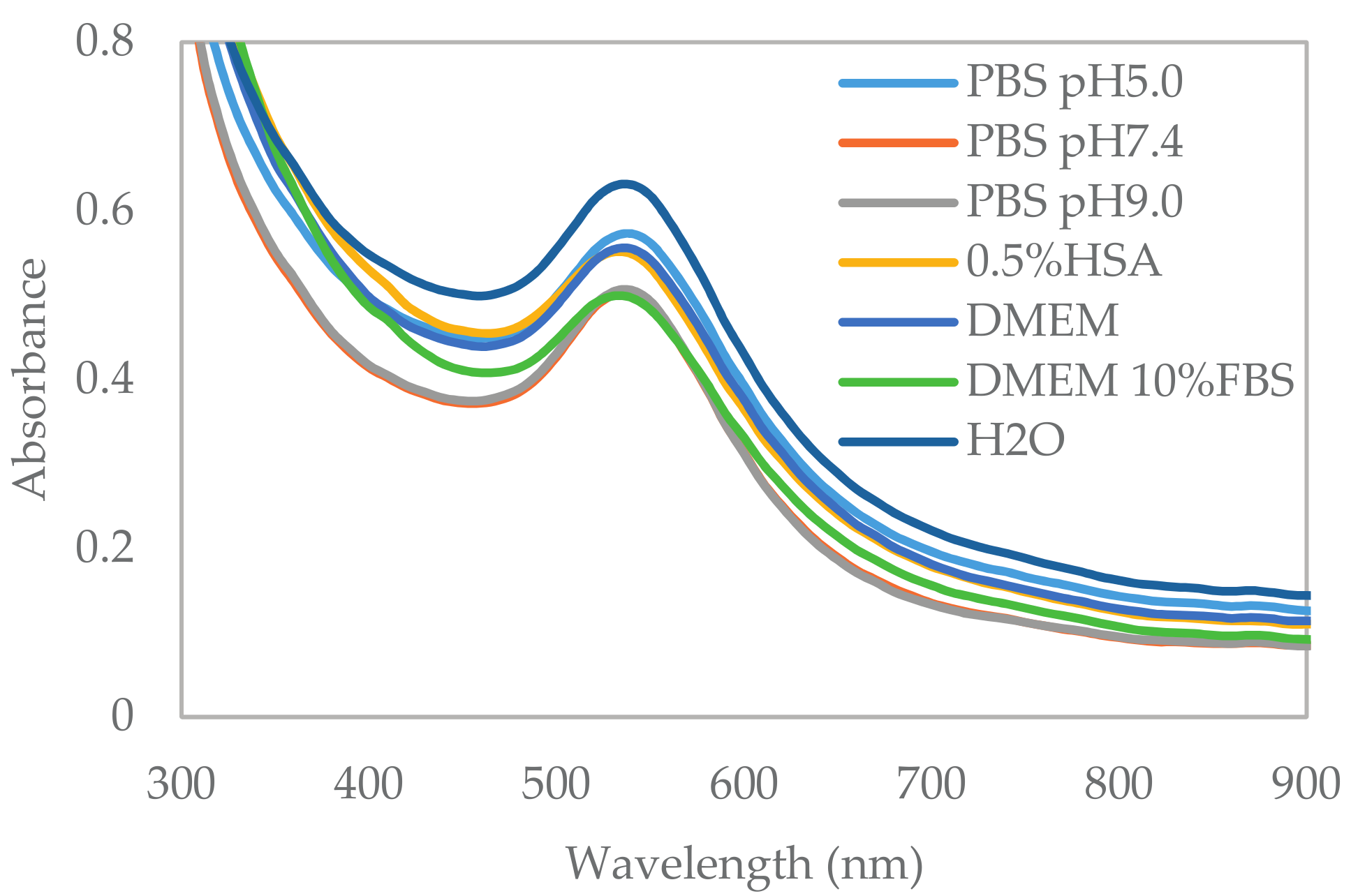
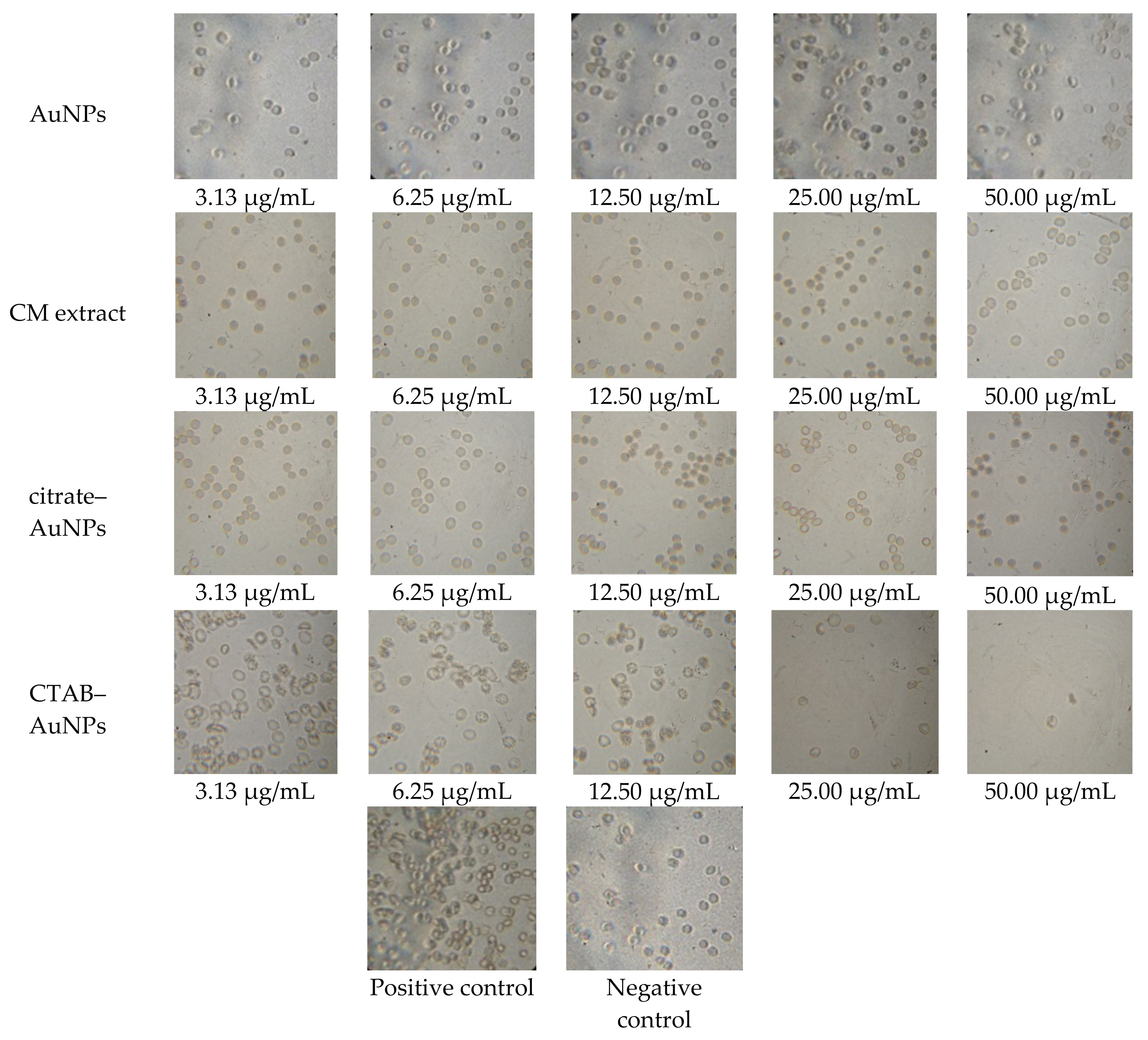
| Physiological Media | Zeta Potential (mV) | Size (nm) |
|---|---|---|
| PBS pH 5.0 | −26.4 ± 1.0 * | 82.9 ± 1.0 |
| PBS pH 7.4 | −29.2 ± 0.8 * | 55.4 ± 1.5 * |
| PBS pH 9.0 | −33.3 ± 0.6 * | 68.8 ± 2.0 * |
| 0.5% HSA | −34.4 ± 1.0 * | 77.3 ± 1.3 |
| DMEM | −20.9 ± 0.4 * | 75.8 ± 1.8 |
| DMEM with 10% FBS | −13.5 ± 0.1 * | 114.7 ± 1.4 * |
| Control | −38.2 ± 0.6 | 78.7 ± 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foo, Y.Y.; Periasamy, V.; Kiew, L.V.; Kumar, G.G.; Malek, S.N.A. Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility. Nanomaterials 2017, 7, 123. https://doi.org/10.3390/nano7060123
Foo YY, Periasamy V, Kiew LV, Kumar GG, Malek SNA. Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility. Nanomaterials. 2017; 7(6):123. https://doi.org/10.3390/nano7060123
Chicago/Turabian StyleFoo, Yiing Yee, Vengadesh Periasamy, Lik Voon Kiew, G. Gnana Kumar, and Sri Nurestri Abd Malek. 2017. "Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility" Nanomaterials 7, no. 6: 123. https://doi.org/10.3390/nano7060123
APA StyleFoo, Y. Y., Periasamy, V., Kiew, L. V., Kumar, G. G., & Malek, S. N. A. (2017). Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility. Nanomaterials, 7(6), 123. https://doi.org/10.3390/nano7060123