Hydrothermal Synthesis of Nanooctahedra MnFe2O4 onto the Wood Surface with Soft Magnetism, Fire Resistance and Electromagnetic Wave Absorption
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. One-Pot Hydrothermal Synthesis of MW
2.3. Characterizations
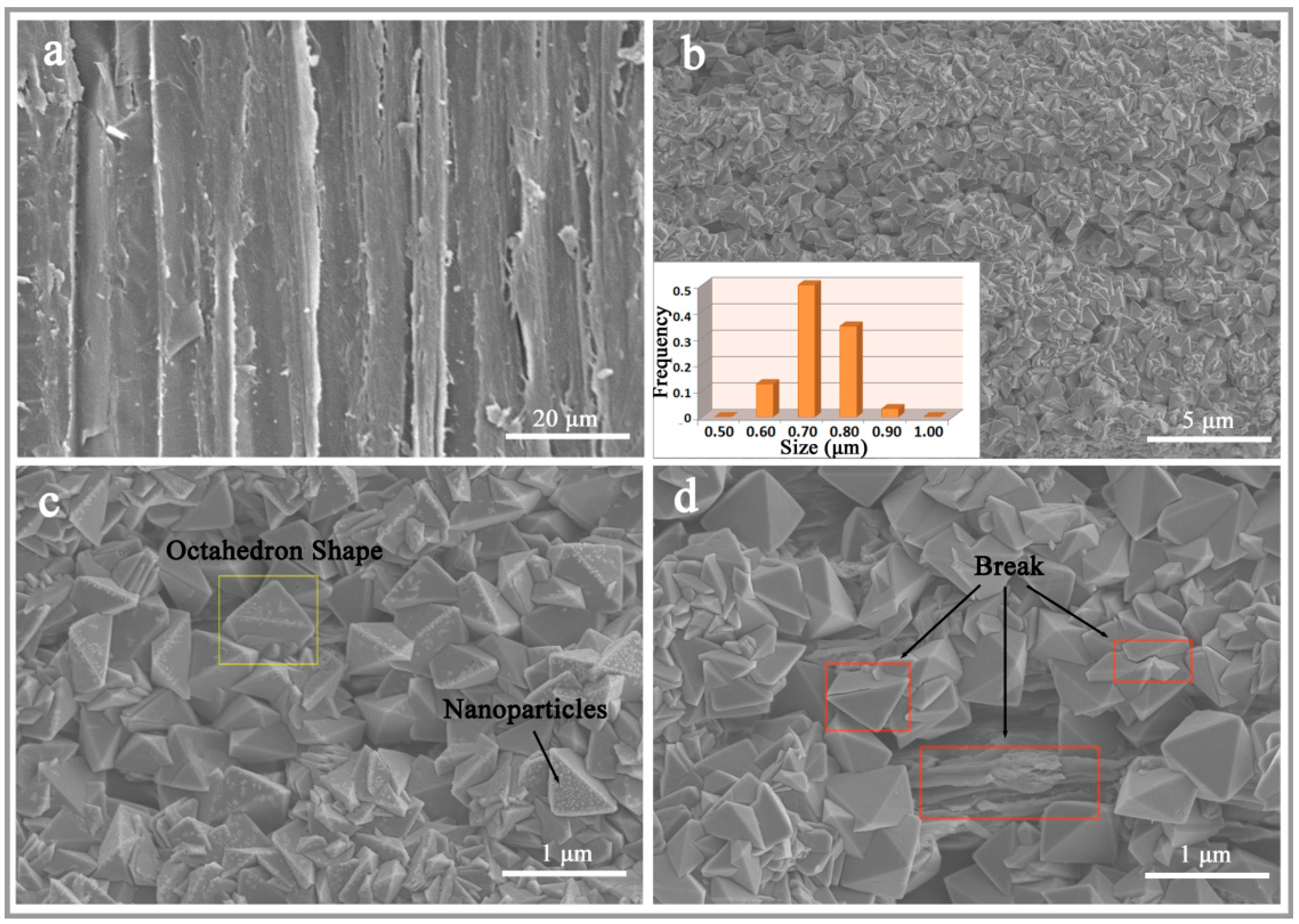
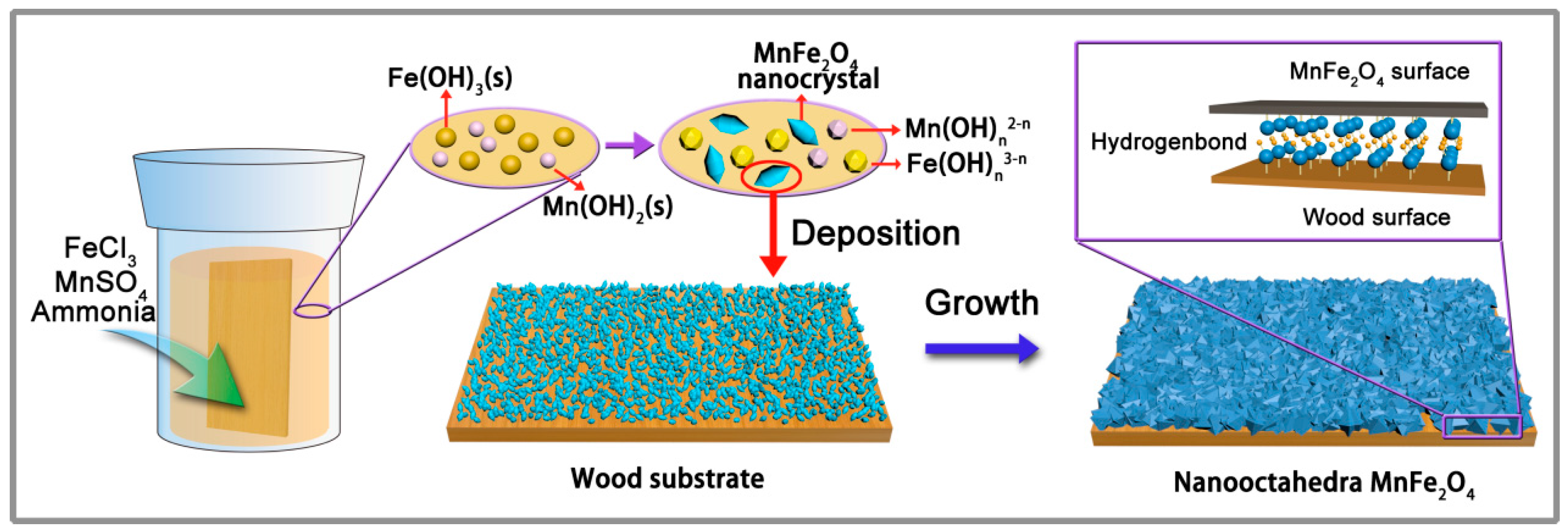
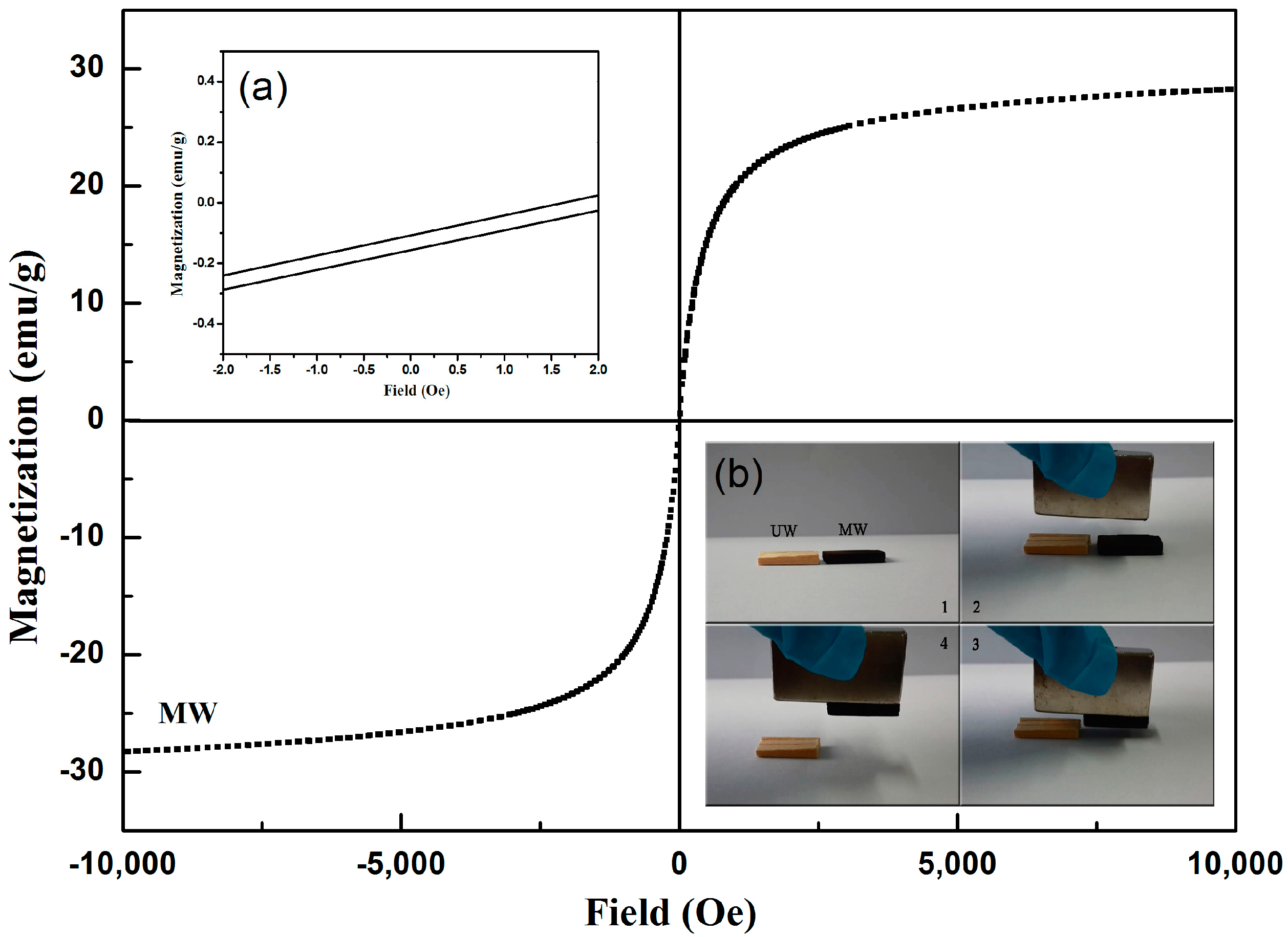
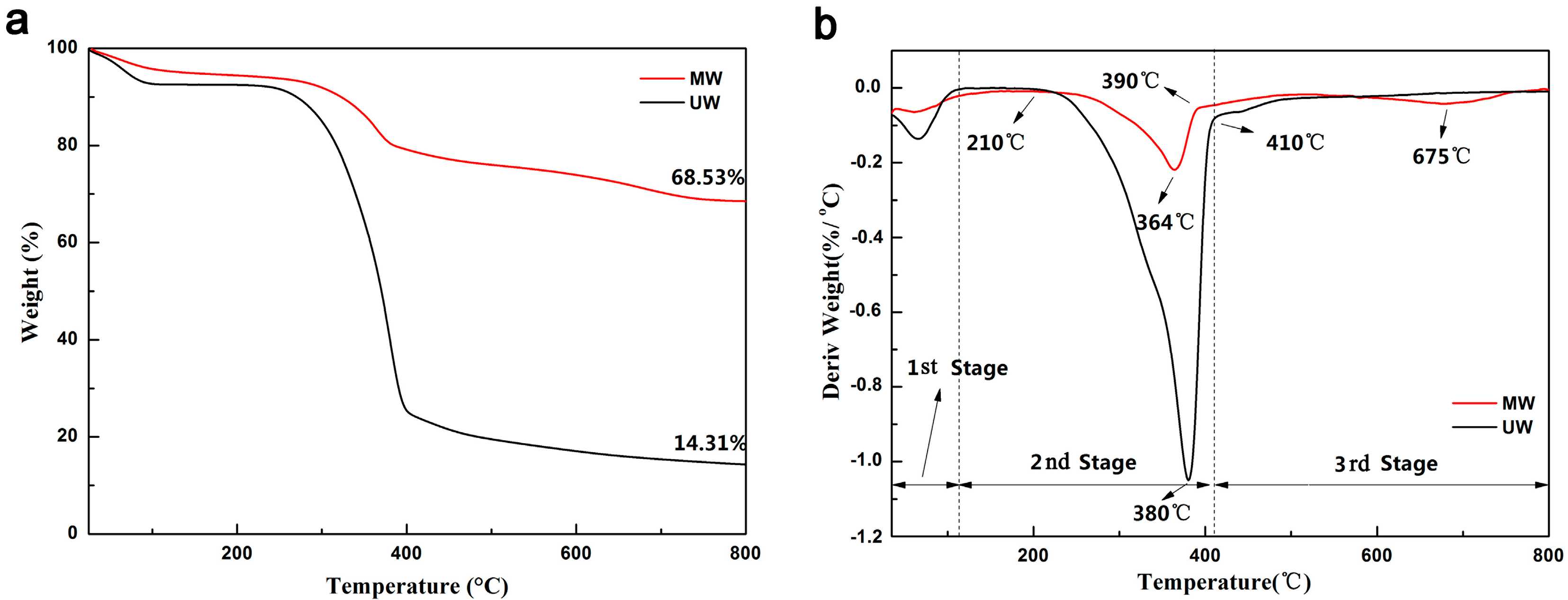
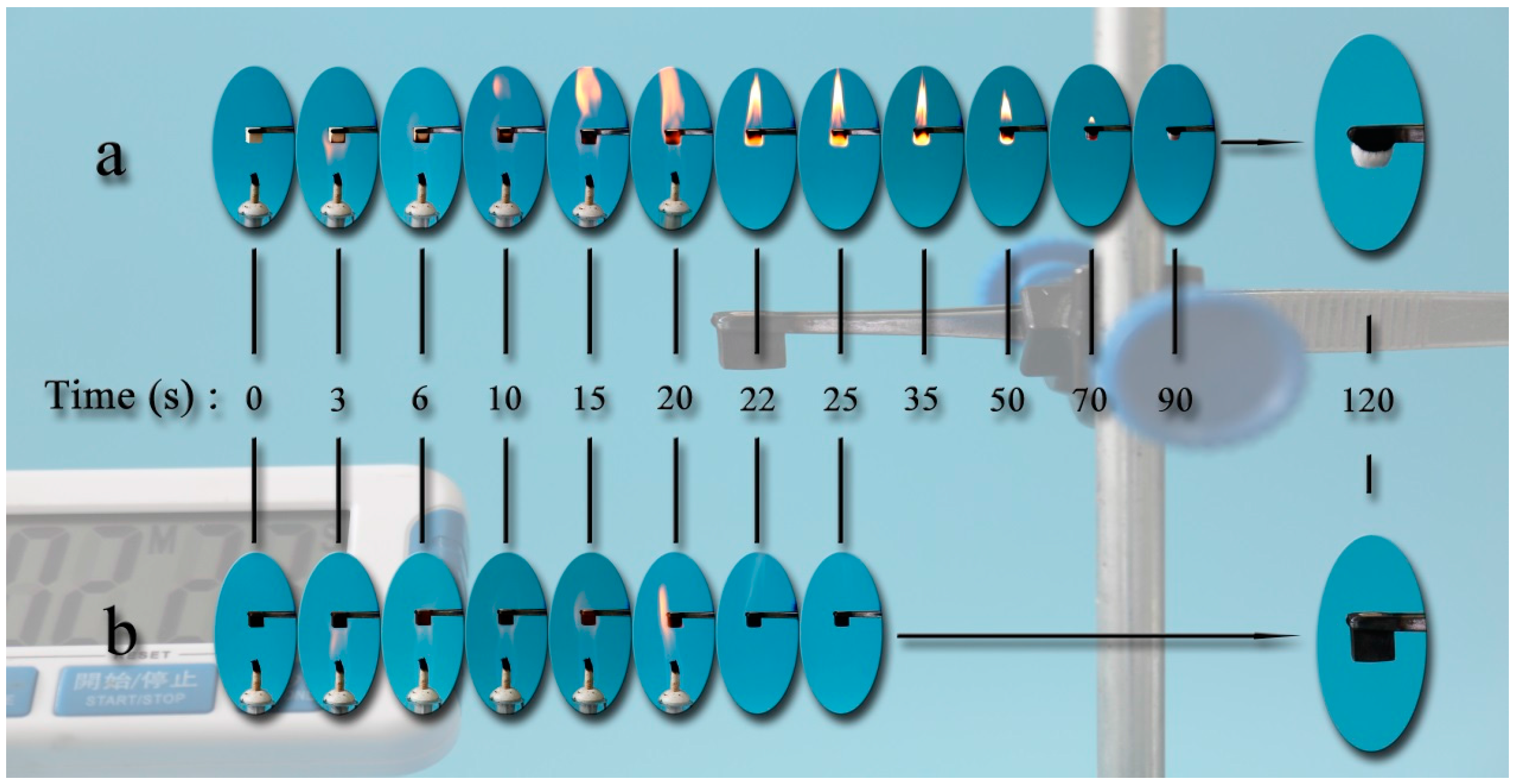
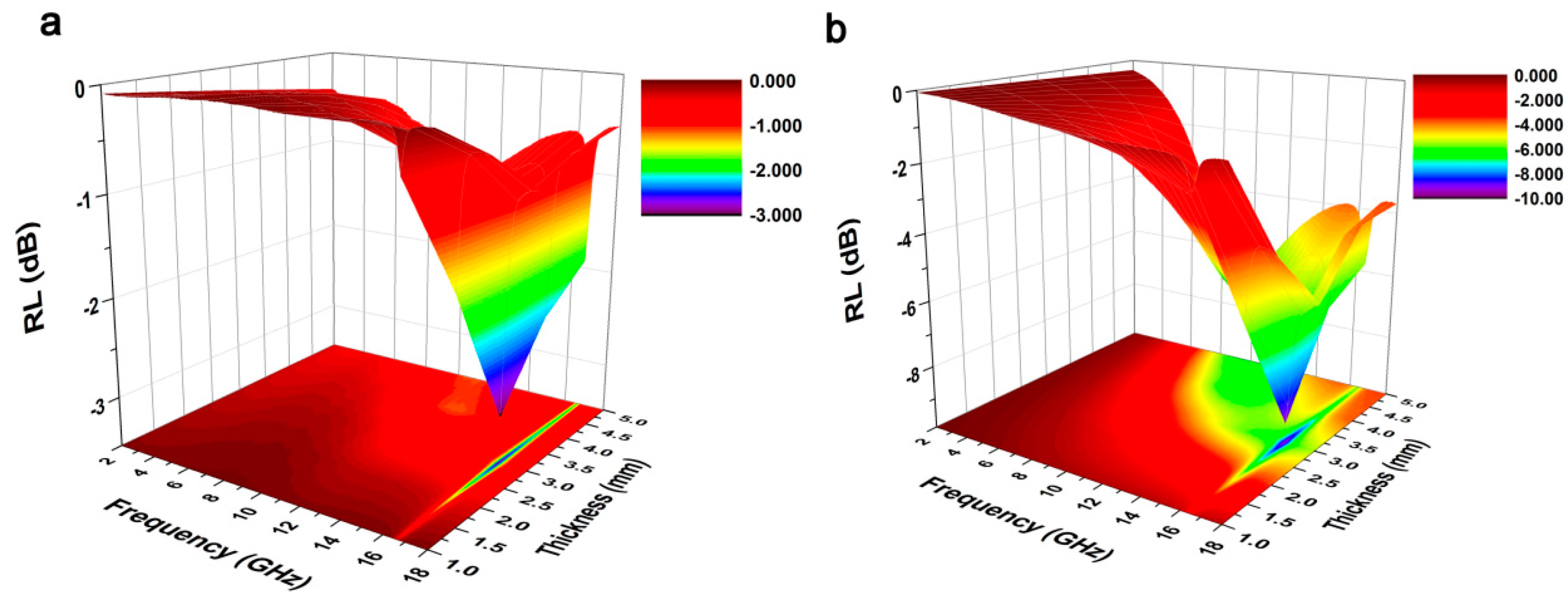
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devi, R.; Maji, T. Interfacial effect of surface modified TiO2 and SiO2 nanoparticles reinforcement in the properties of wood polymer clay nanocomposites. J. Taiwan Inst. Chem. E 2013, 44, 505–514. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Liu, Y. Growth of hydrophobic TiO2 on wood surface using a hydrothermal method. J. Mater. Sci. 2011, 46, 7706–7712. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, K.; Konwar, B.; Maji, T. Synergistic effect of nanoTiO2 and nanoclay on mechanical, flame retardancy, UV stability, and antibacterial properties of wood polymer composites. Polym. Bull. 2013, 70, 1397–1413. [Google Scholar] [CrossRef]
- Habisreutinger, S.; Schmidt-Mende, L.; Stolarczyk, J. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Kamimuta, Y.; Ogawa, A.; Watanabe, Y.; Migita, S.; Mizubayashi, W.; Morita, Y.; Takahashi, M.; Ota, H.; Nabatame, T. Experimental evidence for the flatband voltage shift of high–k metal-oxide-semiconductor devices due to the dipole formation at the high–k/SiO2 interface. Appl. Phys. Lett. 2008, 92, 132907. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.; Weng, B.; Xu, Y. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Preparation of Au/CeO2 exhibiting strong surface plasmon resonance effective for selective or chemoselective oxidation of alcohols to aldehydes or ketones in aqueous suspensions under irradiation by green light. J. Am. Chem. Soc. 2012, 134, 14526–14533. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Molian, P. Design of laser micromachined single crystal 6H–SiC diaphragms for high-temperature micro-electro-mechanical-system pressure sensors. Mater. Des. 2011, 32, 127–132. [Google Scholar] [CrossRef]
- Luis, D.L.S.V.; Angel Bustamante, D.; Llandro, J.; Suzuki, S.; Mitrelias, T.; Bellido Quispe, R. Attaching thiolated superconductor grains on gold surfaces for nanoelectronics applications. Jpn. J. Appl. Phys. 2010, 49, 093102. [Google Scholar]
- Pecholt, B.; Molian, P. Nanoindentation of laser micromachined 3C-SiC thin film micro-cantilevers. Mater. Des. 2011, 32, 3414–3420. [Google Scholar] [CrossRef]
- Luis, D.L.S.V.; Angel Bustamante, D.; Quispe, R.B.; Santibañez, W.F.; Aguiar, J.A.; Barnes, C.H.W.; Majima, Y. The Irreversibility Line and Curie-Weiss Temperature of the Superconductor LaCaBaCu3-x (BO3)x with x = 0.2 and 0.3. Phys. Proced. 2012, 36, 354–359. [Google Scholar]
- Luis, D.L.D.V.; Angel Bustamante, D.; Gonzalez, J.C.; Juan Feijoo, L.; Ana Osorio, A.; Mitrelias, T. Magnetic properties of the superconductor LaCaBaCu3o7. Open Supercond. J. 2010, 2, 19–27. [Google Scholar]
- Spinelli, P.; Polman, A. Prospects of near-field plasmonic absorption enhancement in semiconductor materials using embedded Ag nanoparticles. Opt. Express 2012, 20, A641–A654. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Zhang, Z.; Pan, F. Effect of heat treatment on electromagnetic shielding effectiveness of ZK60 magnesium alloy. Mater. Des. 2012, 42, 327–333. [Google Scholar] [CrossRef]
- Tshabalala, M.; Kingshott, P.; VanLandingham, M.; Plackett, D. Surface chemistry and moisture sorption properties of wood coated with multifunctional alkoxysilanes by sol-gel process. J. Appl. Polym. Sci. 2003, 88, 2828–2841. [Google Scholar] [CrossRef]
- Hao, J.; Xu, Z.; Chu, R.; Zhang, Y.; Chen, Q.; Fu, P.; Li, W.; Li, G.; Yin, Q. Characterization of (K0.5Na0.5) NbO3 powders and ceramics prepared by a novel hybrid method of sol–gel and ultrasonic atomization. Mater. Des. 2010, 31, 3146–3150. [Google Scholar] [CrossRef]
- Wang, H.; Zuo, R.; Ji, X.; Xu, Z. Effects of ball milling on microstructure and electrical properties of sol–gel derived (Bi0.5Na0.5)0.94Ba0.06TiO3 piezoelectric ceramics. Mater. Des. 2010, 31, 4403–4407. [Google Scholar] [CrossRef]
- Fischer, A.; Pettigrew, K.; Rolison, D.; Stroud, R.; Long, J. Incorporation of homogeneous, nanoscale MnO2 within ultraporous carbon structures via self-limiting electroless deposition: implications for electrochemical capacitors. Nano Lett. 2007, 7, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Tjeerdsma, B.; Militz, H. Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry heat-treated wood. Holz. Roh. Werkst. 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, Y.; Peng, L. Hydrothermal synthesis of ZrW2O8 nanorods and its application in ZrW2O8/Cu composites with controllable thermal expansion coefficients. Mater. Des. 2014, 54, 989–994. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Xia, X.; Shi, S.; Lu, Y.; Wang, X.; Gu, C.; Tu, J. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sources 2013, 239, 157–163. [Google Scholar] [CrossRef]
- Ezeigwe, E.; Khiew, P.; Siong, C.; Tan, M. Solvothermal synthesis of NiCo2O4, nanocomposites on liquid-phase exfoliated graphene as an electrode material for electrochemical capacitors. J. Alloys Compd. 2016, 693, 1133–1142. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Gong, J.; Li, Q.; Li, G. α-Fe2O3 Nanorings Prepared by a Microwave–Assisted Hydrothermal Process and Their Sensing Properties. Adv. Mater. 2007, 19, 2324–2329. [Google Scholar] [CrossRef]
- Pant, H.; Pant, B.; Sharma, R.; Amarjargal, A.; Kim, H.; Park, C.; Tijing, L.; Kim, C. Antibacterial and photocatalytic properties of Ag/TiO2/ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Kotutha, I.; Swatsitang, E.; Meewassana, W.; Maensiri, S. One-pot hydrothermal synthesis, characterization, and electrochemical properties of rGO/MnFe2O4 nanocomposites. Jpn. J. Appl. Phys. 2015, 54, 06FH10. [Google Scholar] [CrossRef]
- Phumying, S.; Labuayai, S.; Swatsitang, E.; Amornkitbamrung, V.; Maensiri, S. Nanocrystalline spinel ferrite (MFe2O4, M = Ni, Co, Mn, Mg, Zn) powders prepared by a simple aloe vera plant-extracted solution hydrothermal route. Mater. Res. Bull. 2013, 48, 2060–2065. [Google Scholar] [CrossRef]
- Tian, L.; Zhuang, Q.; Li, J.; Wu, C.; Shi, Y.; Sun, S. The production of self-assembled Fe2O3–graphene hybrid materials by a hydrothermal process for improved Li-cycling. Electrochim. Acta 2012, 65, 153–158. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Fan, B.; Wang, H.; Sun, Q.; Jin, C.; Zhang, H. One-step solvothermal deposition of ZnO nanorod arrays on a wood surface for robust superamphiphobic performance and superior ultraviolet resistance. Sci. Rep. 2016, 6, 35505. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Mohseni, S.; Asadnia, A.; Kerdari, H. Synthesis and microwave absorbing properties of polyaniline/MnFe2O4 nanocomposite. J. Alloys Compd. 2011, 509, 4682–4687. [Google Scholar] [CrossRef]
- Hong, D.; Yamada, Y.; Nagatomi, T.; Takai, Y.; Fukuzumi, S. Catalysis of nickel ferrite for photocatalytic water oxidation using [Ru (bpy)3]2+ and S2O82–. J. Am. Chem. Soc. 2012, 134, 19572–19575. [Google Scholar] [CrossRef] [PubMed]
- Guivar, J.A.R.; Bustamante, A.; Flores, J.; Santillan, M.M.; Osorio, A.M.; Martínez, A.I.; Valladares, L.D.L.S.; Barnes, C.H.W. Mössbauer study of intermediate superparamagnetic relaxation of maghemite (γ-Fe2O3) nanoparticles. Hyperfine Interact. 2014, 224, 89–97. [Google Scholar] [CrossRef]
- León, F.L.; Coaquira, J.A.; Martínez, M.A.; Goya, G.F.; Mantilla, J.; Sousa, M.H.; Valladares, L.L.; Barnes, C.H.; Morais, P.C. Structural and magnetic properties of core-shell Au/Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 41732. [Google Scholar] [CrossRef] [PubMed]
- Guivar, J.A.R.; Martínez, A.I.; Anaya, A.O.; Valladares, L.D.L.S.; Félix, L.L.; Dominguez, A.B. Structural and Magnetic Properties of Monophasic Maghemite (γ-Fe2O3) Nanocrystalline Powder. Adv. Nanopart. 2014, 3, 114. [Google Scholar] [CrossRef]
- Gao, J.; Dai, S.; Li, T. Electronic states of epitaxial thin films of La0.9Sn0.1MnO3 and La0.9Ca0.1MnO3. Phys. Rev. B 2003, 67, 153403. [Google Scholar] [CrossRef]
- Goodarz Naseri, M.; Saion, E.B.; Kamali, A. An overview on nanocrystalline ZnFe2O4, MnFe2O4, and CoFe2O4 synthesized by a thermal treatment method. ISRN Nanotechnol. 2012, 2012, 604241. [Google Scholar] [CrossRef]
- Andrade, P.L.; Silva, V.A.J.; Maciel, J.C.; Santillan, M.M.; Moreno, N.O.; Valladares, L.D.L.S.; Bustamante, A.; Pereira, S.M.B.; Silva, M.P.C.; Aguiar, J.A. Preparation and characterization of cobalt ferrite nanoparticles coated with fucan and oleic acid. Hyperfine Interact. 2014, 224, 217–225. [Google Scholar] [CrossRef]
- Luis, D.L.S.V.; Llandro, J.; Lee, D.; Mitrelias, T.; Palfreyman, J.J.; Hayward, T.J.; Cooper, J.; Bland, J.A.C.; Barnes, C.H.W.; Juan, L.A.C. Magnetic measurements of suspended functionalised ferromagnetic beads under DC applied fields. J. Magn. Magn. Mater. 2009, 321, 2129–2134. [Google Scholar]
- León-Félix, L.; Chaker, J.; Parise, M.; Coaquira, J.A.H.; Valladares, L.D.L.S.; Bustamante, A.; Garg, V.K.; Oliveira, A.C.; Morais, P.C. Synthesis and characterization of uncoated and gold-coated magnetite nanoparticles. Hyperfine Interact. 2014, 224, 179–188. [Google Scholar] [CrossRef]
- Lee, D.; Seo, J.; Valladares, L.D.L.S.; Quispe, O.A.; Barnes, C.H.W. Magnetic and structural properties of yellow europium oxide compound and Eu(OH)3. J. Solid State Chem. 2015, 228, 141–145. [Google Scholar] [CrossRef]
- Llamazares, J.L.S.; Quintananedelcos, A.; Ríosjara, D.; Sánchezvaldes, C.F.; Garcíafernández, T.; García, C. The effect of low temperature thermal annealing on the magnetic properties of Heusler Ni-Mn-Sn melt-spun ribbons. J. Magn. Magn. Mater. 2016, 401, 38–43. [Google Scholar] [CrossRef]
- Caloz, C.; Itoh, T. Application of the transmission line theory of left-handed (LH) materials to the realization of a microstrip “LH line”. In Proceedings of the Antennas and Propagation Society International Symposium, San Antonio, TX, USA, 16–21 June 2002; pp. 412–415. [Google Scholar]

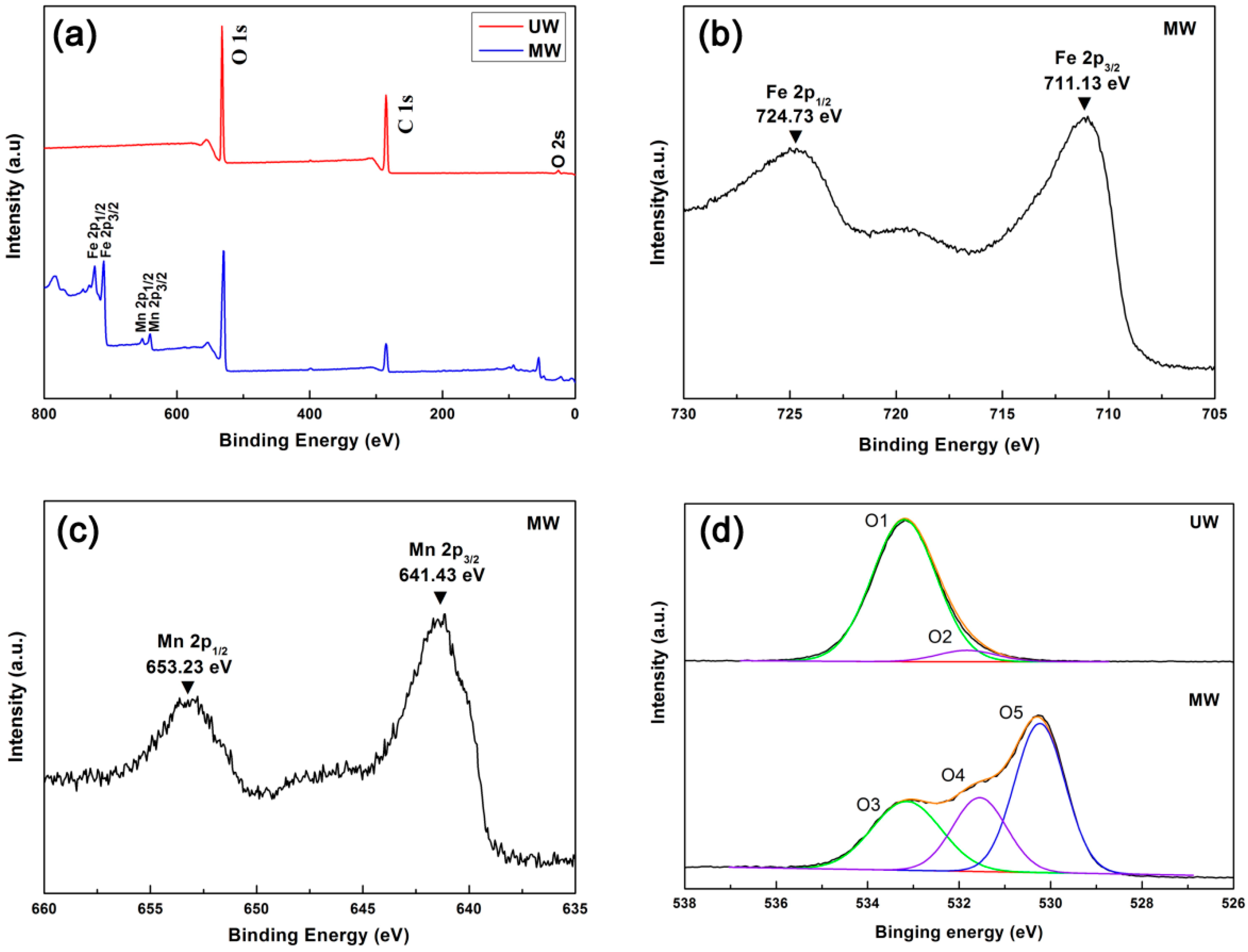
| UW | MW | Assignment | ||
|---|---|---|---|---|
| The excited electron O1s | Binding energy (eV) | The excited electron O1s | Binding energy (eV) | |
| O1 | 533.18 | O3 | 533.15 | C–O |
| O2 | 531.83 | O4 | 531.55 | O–H, C=O |
| O5 | 530.23 | Mn–O, Fe–O | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yao, Q.; Wang, C.; Ma, Z.; Sun, Q.; Fan, B.; Jin, C.; Chen, Y. Hydrothermal Synthesis of Nanooctahedra MnFe2O4 onto the Wood Surface with Soft Magnetism, Fire Resistance and Electromagnetic Wave Absorption. Nanomaterials 2017, 7, 118. https://doi.org/10.3390/nano7060118
Wang H, Yao Q, Wang C, Ma Z, Sun Q, Fan B, Jin C, Chen Y. Hydrothermal Synthesis of Nanooctahedra MnFe2O4 onto the Wood Surface with Soft Magnetism, Fire Resistance and Electromagnetic Wave Absorption. Nanomaterials. 2017; 7(6):118. https://doi.org/10.3390/nano7060118
Chicago/Turabian StyleWang, Hanwei, Qiufang Yao, Chao Wang, Zhongqing Ma, Qingfeng Sun, Bitao Fan, Chunde Jin, and Yipeng Chen. 2017. "Hydrothermal Synthesis of Nanooctahedra MnFe2O4 onto the Wood Surface with Soft Magnetism, Fire Resistance and Electromagnetic Wave Absorption" Nanomaterials 7, no. 6: 118. https://doi.org/10.3390/nano7060118
APA StyleWang, H., Yao, Q., Wang, C., Ma, Z., Sun, Q., Fan, B., Jin, C., & Chen, Y. (2017). Hydrothermal Synthesis of Nanooctahedra MnFe2O4 onto the Wood Surface with Soft Magnetism, Fire Resistance and Electromagnetic Wave Absorption. Nanomaterials, 7(6), 118. https://doi.org/10.3390/nano7060118






