Synthesis of Multicolor Core/Shell NaLuF4:Yb3+/Ln3+@CaF2 Upconversion Nanocrystals
Abstract
:1. Introduction
2. Results and Discussion
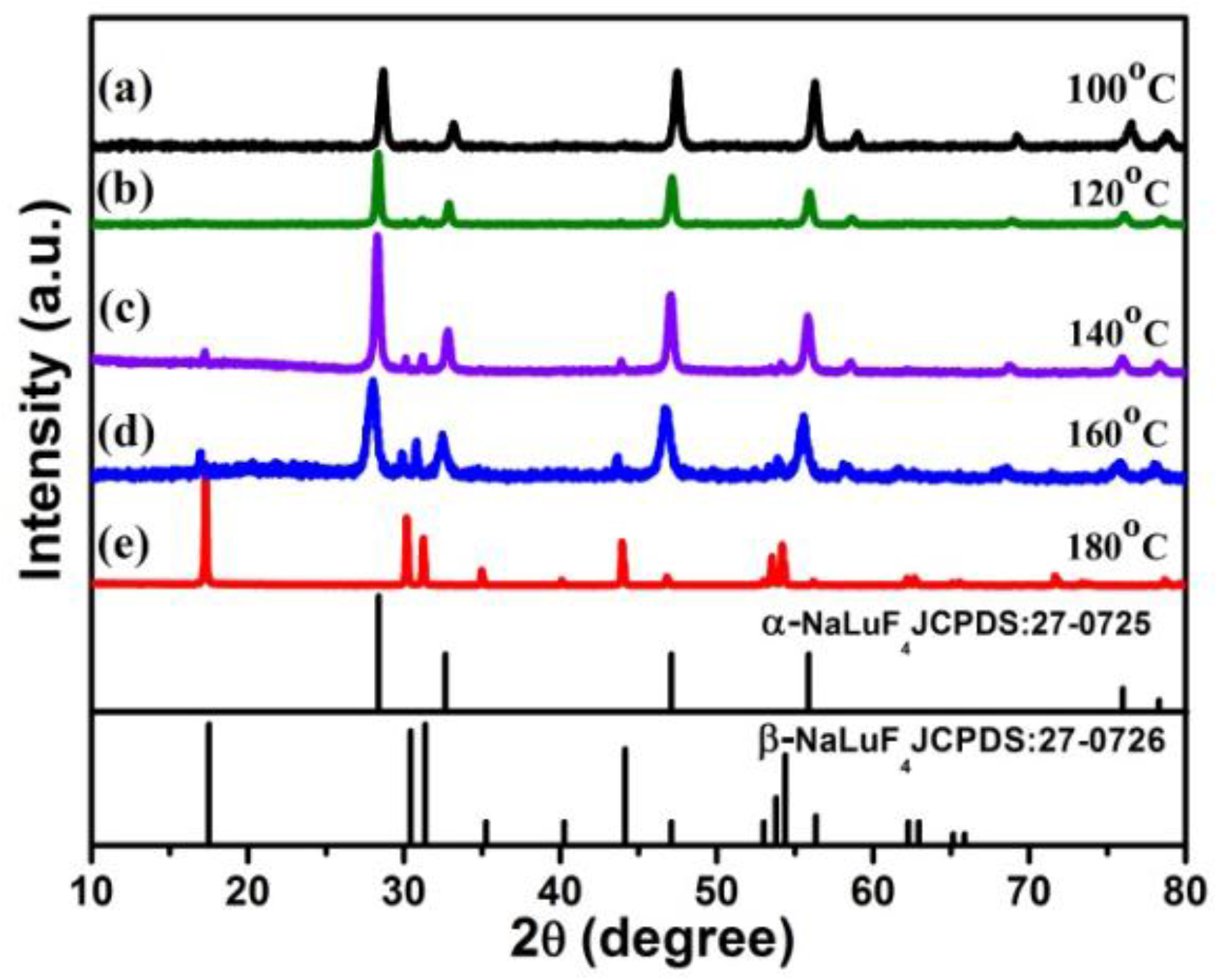
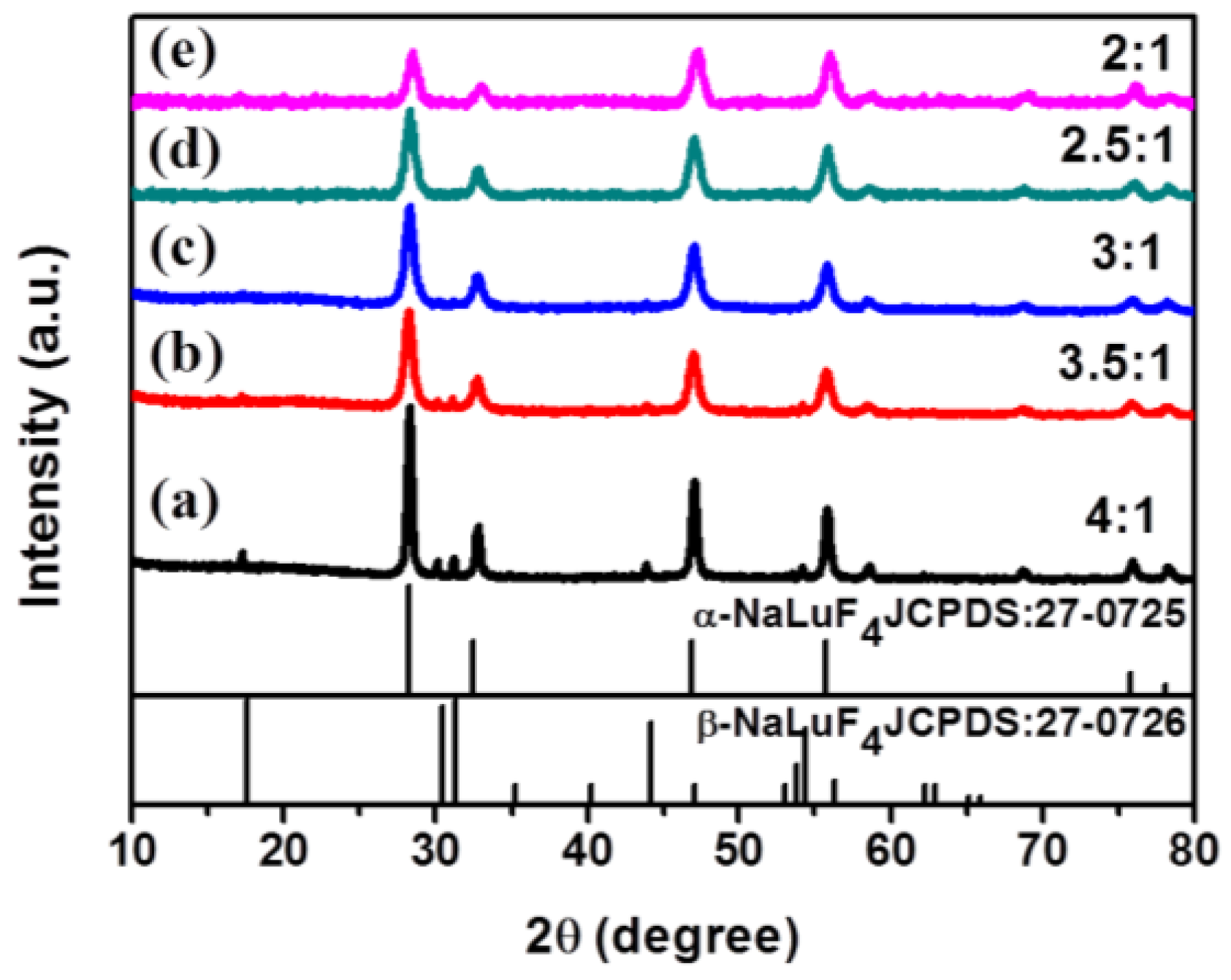
2.1. Synthesis of α-NaLuF4:Yb3+/Ln3+ (Ln = Er, Tm, Ho) or α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
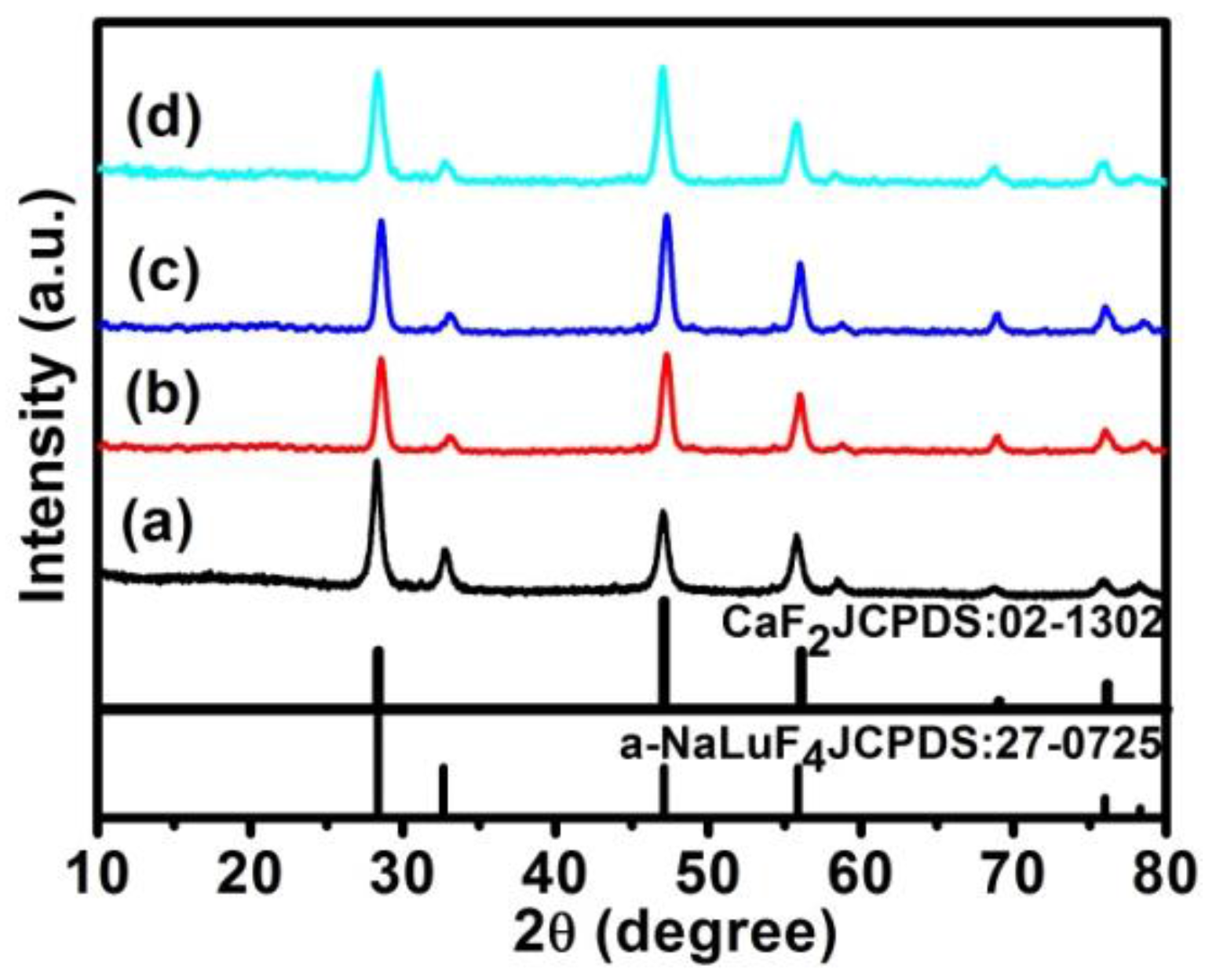
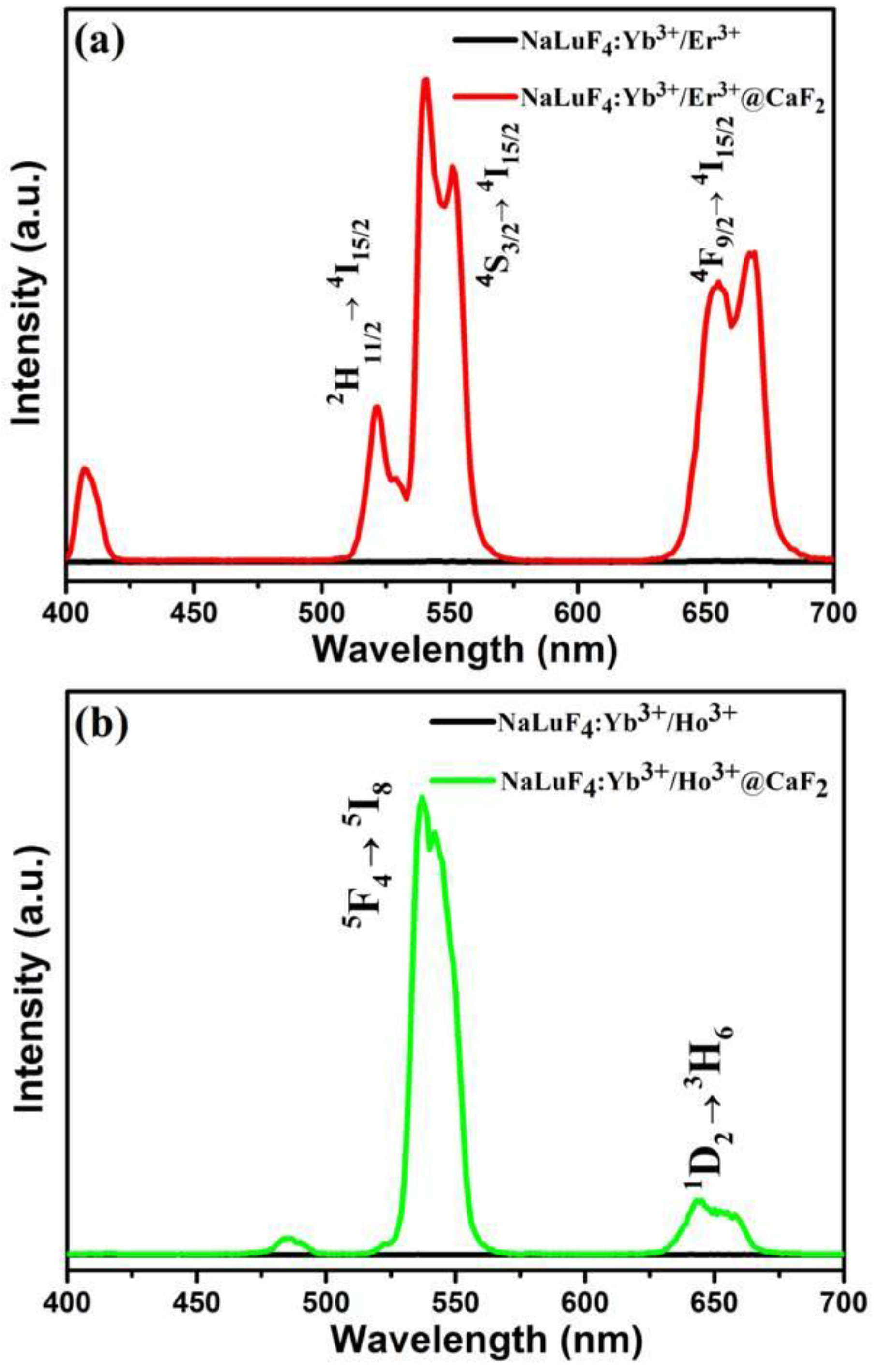
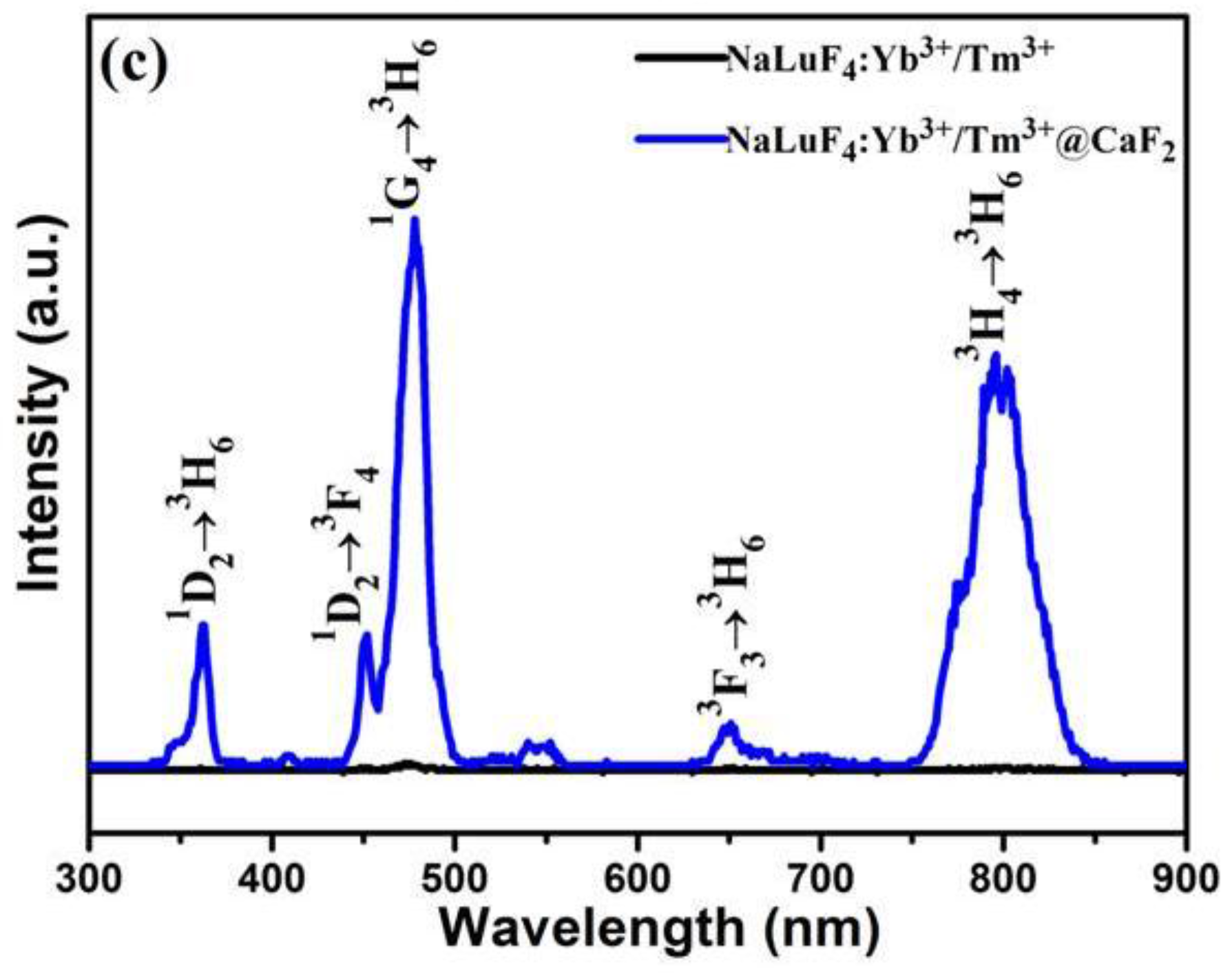
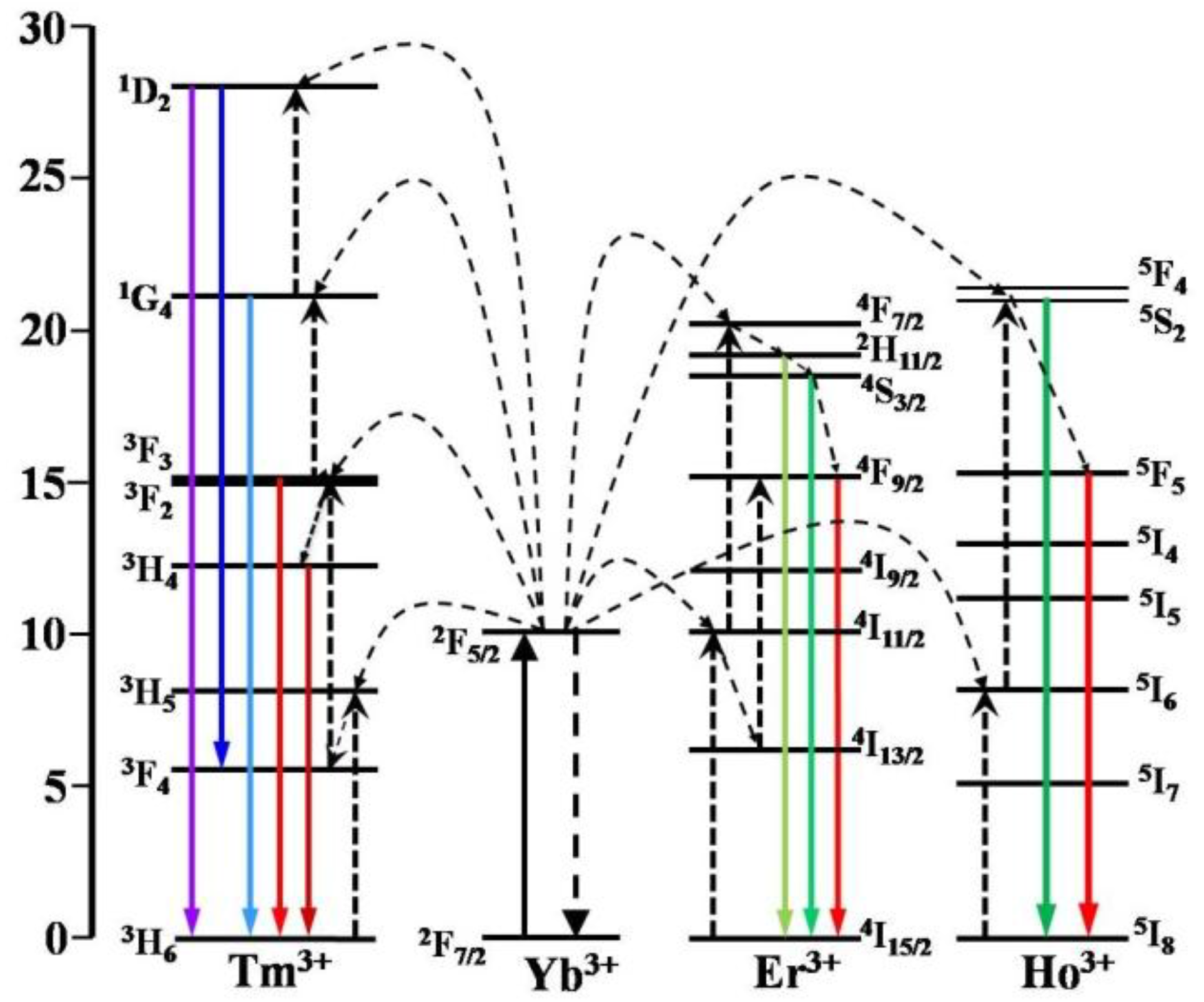
2.2. Characterization of α-NaLuF4:Yb3+/Ln3+@CaF2 (Ln = Er, Tm, or Ho) Core/Shell UCNCs
3. Materials and Methods
3.1. Materials
3.2. Hydrothermal Synthesis of α-NaLuF4:Yb3+/Ln3+ UCNCs
3.3. Thermal Decomposition Synthesis of α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
3.4. Thermal Decomposition Synthesis of α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suyver, J.F.; Aebischer, A.; Biner, D.; Gerner, P.; Grimm, J.; Heer, S.; Krämer, K.W.; Reinhard, C.; Güdel, H.U. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt. Mater. 2005, 27, 1111–1130. [Google Scholar] [CrossRef]
- Suyver, J.F.; Grimm, J.; van Veen, M.K.; Biner, D.; Kramer, K.W.; Gudel, H.U. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+. J. Lumin. 2006, 117, 1–12. [Google Scholar] [CrossRef]
- Gnach, A.; Bednarkiewicz, A. Lanthanide-doped up-converting nanoparticles: Merits and challenges. Nano Today 2012, 7, 532–563. [Google Scholar] [CrossRef]
- Naccache, R.; Rodríguez, E.M.; Bogdan, N.; Sanz-Rodríguez, F.; de la Cruz, M.D.I.; de la Fuente, Á.J.; Vetrone, F.; Jaque, D.; Solé, J.G.; Capobianco, J.A. High resolution fluorescence imaging of cancers using lanthanide ion-doped upconverting nanocrystals. Cancers 2012, 4, 1067–1105. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.L.; Xing, G.M.; Li, S.J.; et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Heer, S.; Koempe, K.; Guedel, H.U.; Haase, M. Highly efficient multicolor upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Lim, S.F.; Riehn, R.; Ryu, W.S.; Khanarian, N.; Tung, C.K.; Tank, D.; Austin, R.H. In vivo and scanning electron microscopy imaging of upconverting nanophosphors in Caenorhabditis elegans. Nano Lett. 2006, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. 2005, 44, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Jing, Z.; Liu, J.; Wei, F.; Li, F. Iridium-complex-modified upconversion nanophosphors for effective LRET detection of cyanide anions in pure water. Adv. Funct. Mater. 2012, 22, 2667–2672. [Google Scholar] [CrossRef]
- Miyazaki, D.; Lasher, M.; Fainman, Y. Fluorescent volumetric display excited by a single infrared beam. Appl. Opt. 2005, 44, 5281–5285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, X.; Schartner, E.P.; Lonescu, P.; Zhang, R.; Nguyen, T.L.; Jin, D.; Ebendorff-Heidepriem, H. Upconversion nanocrystal-doped glass: A new paradigm for photonic materials. Adv. Opt. Mater. 2016, 4, 1507–1517. [Google Scholar] [CrossRef]
- Deng, R.; Qin, F.; Chen, R.; Huang, W.; Hong, M.; Liu, X. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 2015, 10, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Yin, D.; Cao, X.; Wang, C.; Song, K.; Liu, B.; Zhang, L.; Han, Y.; Wu, W. Synthesis of NaLuF4-based nanocrystals and large enhancement of upconversion luminescence of NaLuF4:Gd, Yb, Er by coating an active shell for bioimaging. Dalton Trans. 2014, 43, 14001–14008. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Korinek, A.; Blasiak, A.B.; Tomanek, B.; van Veggel, F.C.J.M. Cation Exchange: A Facile Method to Make NaYF4:Yb,Tm-NaGdF4 Core-Shell Nanoparticles with a Thin, Tunable, and Uniform Shell. Chem. Mater. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Yang, T.; Feng, W.; Li, C.; Li, F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wu, X.; Chen, Z.; Hu, P.; Yan, H.; Tang, Z.; Xi, Z.; Liu, Y. Uniform NaLuF4 nanoparticles with strong upconversion luminescence for background-free imaging of plant cells and ultralow power detecting of trace organic dyes. Mater. Res. Bull. 2016, 73, 6–13. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Liu, Q.; Feng, W.; Yang, P.; Li, F. Cubic sub-20 nm NaLuF4-based upconversion nanophosphors for high-contrast bioimaging in different animal species. Biomaterials 2012, 33, 3733–3742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, X.; Hu, S.; Hu, P.; Yan, H.; Tang, Z.; Liu, Y. Multicolor upconversion NaLuF4 fluorescent nanoprobe for plant cell imaging and detection of sodium fluorescein. J. Mater. Chem. C 2015, 3, 153–161. [Google Scholar] [CrossRef]
- Gao, W.; Dong, J.; Liu, J.H.; Yan, X.W. Enhancement of red upconversion emission of cubic phase NaLuF4:Yb3+/Ho3+/Ce3+ nanocrystals. Mater. Res. Bull. 2016, 80, 256–262. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, W.; Yang, T.S.; Yi, T.; Li, F.Y. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 2013, 114, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, Y.; Ma, P.; Kang, X.; Cheng, Z.; Li, C.; Lin, J. One-step synthesis of small-sized and water-soluble NaREF4 upconversion nanoparticles for in vitro cell imaging and drug delivery. Chem. Eur. J. 2013, 19, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Liu, X. Unraveling epitaxial habits in the NaLnF4 system for color multiplexing at the single-particle level. Angew. Chem. Int. Ed. 2016, 55, 5718–5722. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, J.; Zhai, X.; Zhao, D.; Qin, W. Facile synthesis of β-NaLuF4:Yb/Tm hexagonal nanoplates with intense ultraviolet upconversion luminescence. CrystEngComm 2011, 13, 3782–3787. [Google Scholar] [CrossRef]
- Wang, L.; Lan, M.; Liu, Z.; Qin, G.; Wu, C.; Wang, X.; Qin, W.; Huang, W.; Huang, L. Enhanced deep-ultraviolet upconversion emission of Gd3+ sensitized by Yb3+ and Ho3+ in β-NaLuF4 microcrystals under 980 nm excitation. J. Mater. Chem. C 2013, 1, 2485–2490. [Google Scholar] [CrossRef]
- Zheng, K.Z.; Liu, Z.Y.; Lv, C.J.; Qin, W.P. Temperature sensor based on the UV upconversion luminescence of Gd3+ in Yb3+–Tm3+–Gd3+ codoped NaLuF4 microcrystals. J. Mater. Chem. C 2013, 1, 5502–5507. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, P.; Yuan, Q.; Xu, X.; Lei, P.; Liu, X.; Su, Y.; Dong, L.; Feng, J.; Zhang, H. Nd3⁺-sensitized NaLuF4 luminescent nanoparticles for multimodal imaging and temperature sensing under 808 nm excitation. Nanoscale 2015, 7, 17861–17870. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, S.L.; Liang, Z.Q.; Han, M.; Xu, Z. Optimized upconversion emission of NaLuF4:Er, Yb nanocrystals codoped with Gd3+ ions and its mechanism. J. Alloy. Compd. 2014, 593, 30–33. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, S.L.; Liang, Z.Q.; Yang, Y.X.; Zhang, J.J.; Xu, Z. The color tuning and mechanism of upconversion emission from green to red in NaLuF4:Yb3+/Ho3+ nanocrystals by codoping with Ce3+. J. Alloy. Compd. 2016, 659, 146–151. [Google Scholar] [CrossRef]
- Zheng, K.Z.; He, G.H.; Song, W.Y.; Bi, X.Q.; Qin, W.P. A strategy for enhancing the sensitivity of optical thermometers in β-NaLuF4:Yb3+/Er3+ nanocrystals. J. Mater. Chem. C 2015, 3, 11589–11594. [Google Scholar] [CrossRef]
- Lin, H.; Xu, D.K.; Teng, D.D.; Yang, S.H.; Zhang, Y.L. Shape-controllable synthesis and enhanced upconversion luminescence of Li+ doped β-NaLuF4:Yb3+,Ln3+ (Ln = Tm, Ho) microcrystals. New J. Chem. 2015, 39, 2565–2572. [Google Scholar] [CrossRef]
- Lin, H.; Xu, D.K.; Li, A.M.; Teng, D.D.; Yang, S.H.; Zhang, Y.L. Tuning of structure and enhancement of upconversion luminescence in NaLuF4:Yb3+, Ho3+ crystals. Phys. Chem. Chem. Phys. 2015, 17, 19515–19526. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Quan, Z.W.; Yang, P.P.; Huang, S.S.; Lian, H.Z.; Lin, J. Shape-controllable synthesis and upconversion properties of lutetium fluoride (doped with Yb3+/Er3+) microcrystals by hydrothermal process. J. Phys. Chem. C 2008, 112, 13395–13404. [Google Scholar] [CrossRef]
- Lin, H.; Xu, D.K.; Li, A.M.; Teng, D.D.; Yang, S.H.; Zhang, Y.L. Morphology evolution and pure red upconversion mechanism of β-NaLuF4 crystals. Sci. Rep. 2016, 6, 28051. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.J.; Wang, H.B.; Lu, W.; Yi, Z.G.; Rao, L.; Liu, H.R.; Hao, J.H. Dual-modal upconversion fluorescent/X-ray imaging using ligand-free hexagonal phase NaLuF4:Gd/Yb/Er nanorods for blood vessel visualization. Biomaterials 2014, 35, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.Y.; Hao, S.W.; Yang, C.H. Sub-6 nm hexagonal core/shell NaGdF4 nanocrystals with enhanced upconversion photoluminescence. Nanoscale 2017, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Qiu, P.; Wang, K.; Fu, H.; Gao, G.; He, R.; Cui, D. Shape-controllable synthesis of hydrophilic NaLuF4:Yb,Er nanocrystals by a surfactant-assistant two-phase system. Nanoscale Res. Lett. 2013, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.G.; Cao, H.Y.; Wu, X.F.; Zhan, S.P.; Wu, Q.Y.; Tang, Z.J.; Liu, Y.X. Upconversion luminescence and magnetic turning of NaLuF4:Yb3+/Tm3+/Gd3+ nanoparticles and their application for detecting acriflavine. J. Nanomater. 2016, 2016, 63479. [Google Scholar] [CrossRef]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty, I.B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.B.; Lu, Z.D.; Yin, Y.D.; McRae, C.; Piper, J.A.; Dawes, J.M.; Jin, D.Y.; Goldys, E.M. Upconversion luminescence with tunable lifetime in NaYF4:Yb, Er nanocrystals: Role of nanocrystal size. Nanoscale 2013, 5, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, X.L.; Lei, P.P.; Xu, X.; Dong, L.L.; Guo, L.M.; Yan, X.X.; Wang, P.; Song, S.Y.; Feng, J.; et al. Core–shell–shell heterostructures of α-NaLuF4: Yb/Er@NaLuF4:Yb@MF2 (M = Ca, Sr, Ba) with remarkably enhanced upconversion luminescence. Dalton Trans. 2016, 45, 11129–11136. [Google Scholar] [CrossRef] [PubMed]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Amuluru, L.; High, W.; Hiatt, K.M.; Ranville, J.; Shar, S.V.; Malik, B.; Swaminathan, S. Metal Deposition in Calcific Uremic Arteriolopathy. J. Am. Acad. Dermatol. 2009, 61, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.; Song, J.; Pandey, R.K.; Ågren, H.; Prasad, P.N.; et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.W.; Yang, L.M.; Qiu, H.L.; Fan, R.W.; Yang, C.H.; Chen, G.Y. Heterogeneous core/shell fluoride nanocrystals with enhanced upconversion photoluminescence for in vivo bioimaging. Nanoscale 2015, 7, 10775–10780. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, G.; Ohulchanskyy, T.Y.; Kesseli, S.J.; Buchholz, S.; Li, Z.; Prasad, P.N.; Han, G. Upconversion: Tunable Near Infrared to Ultraviolet Upconversion Luminescence Enhancement in (α-NaYF4:Yb,Tm)/CaF2 Core/Shell Nanoparticles for In situ Real-time Recorded Biocompatible Photoactivation. Small 2013, 9, 3213. [Google Scholar] [CrossRef] [PubMed]
- Prorok, K.; Bednarkiewice, A.; Cichy, B.; Gnach, A.; Misiak, M.; Sobczyk, M.; Strek, W. The impact of shell host (NaYF4/CaF2) and shell deposition methods on the up-conversion enhancement in Tb3⁺, Yb3⁺ codoped colloidal α-NaYF4 core-shell nanoparticles. Nanoscale 2014, 6, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Sun, L.D.; Xiao, J.W.; Feng, W.; Zhou, J.C.; Shen, J.; Yan, C.H. Rare-earth Nanoparticles with enhanced upconverison emission and suppressed rare-earth-ion leakage. Chem. Eur. J. 2012, 18, 5558–5564. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Nadort, A.; Zhao, J.B.; Goldys, E.M. Lanthanide upconversion luminescence at the nanoscale: Fundamentals and optical properties. Nanoscale 2016, 8, 13099–13130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. Heavy equipment operator training via virtual modeling technologies. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hao, S.; Yang, C.; Chen, G. Synthesis of Multicolor Core/Shell NaLuF4:Yb3+/Ln3+@CaF2 Upconversion Nanocrystals. Nanomaterials 2017, 7, 34. https://doi.org/10.3390/nano7020034
Li H, Hao S, Yang C, Chen G. Synthesis of Multicolor Core/Shell NaLuF4:Yb3+/Ln3+@CaF2 Upconversion Nanocrystals. Nanomaterials. 2017; 7(2):34. https://doi.org/10.3390/nano7020034
Chicago/Turabian StyleLi, Hui, Shuwei Hao, Chunhui Yang, and Guanying Chen. 2017. "Synthesis of Multicolor Core/Shell NaLuF4:Yb3+/Ln3+@CaF2 Upconversion Nanocrystals" Nanomaterials 7, no. 2: 34. https://doi.org/10.3390/nano7020034
APA StyleLi, H., Hao, S., Yang, C., & Chen, G. (2017). Synthesis of Multicolor Core/Shell NaLuF4:Yb3+/Ln3+@CaF2 Upconversion Nanocrystals. Nanomaterials, 7(2), 34. https://doi.org/10.3390/nano7020034







