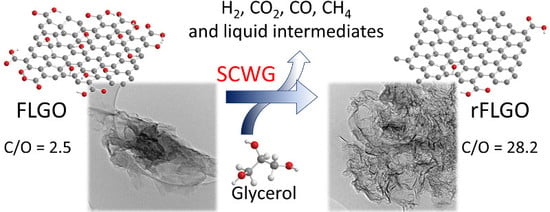
Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol
Abstract
1. Introduction
2. Results and Discussion
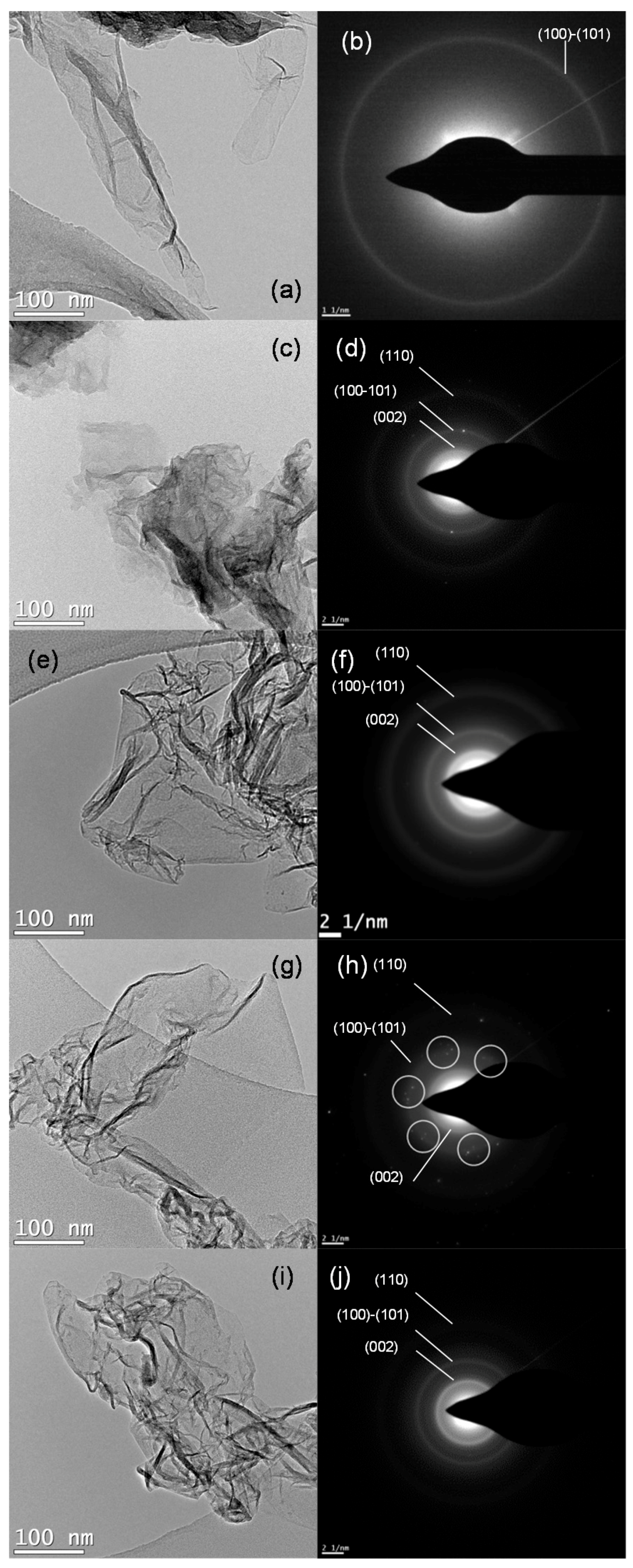
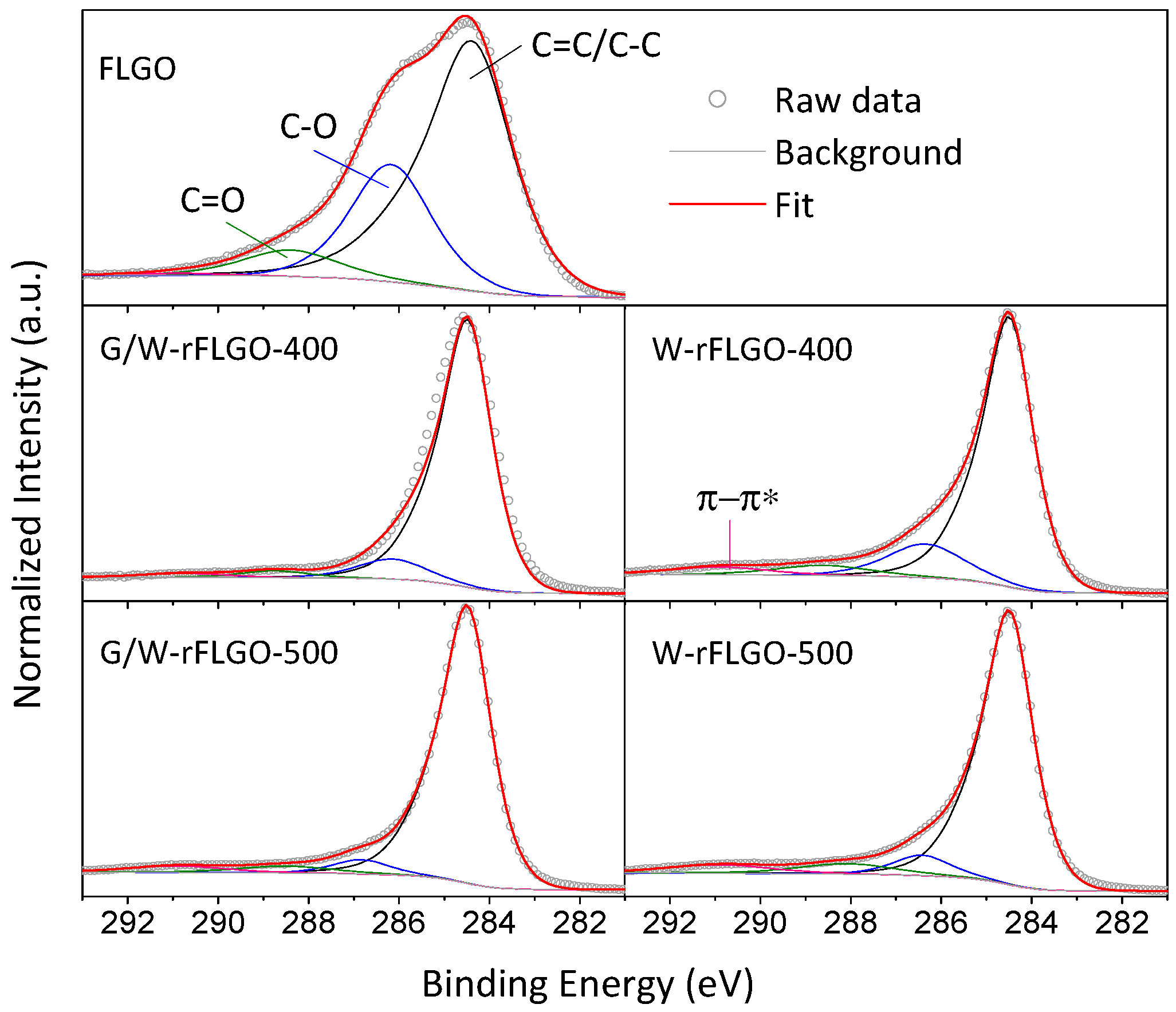
2.1. Reduced Graphene Oxide Characterization
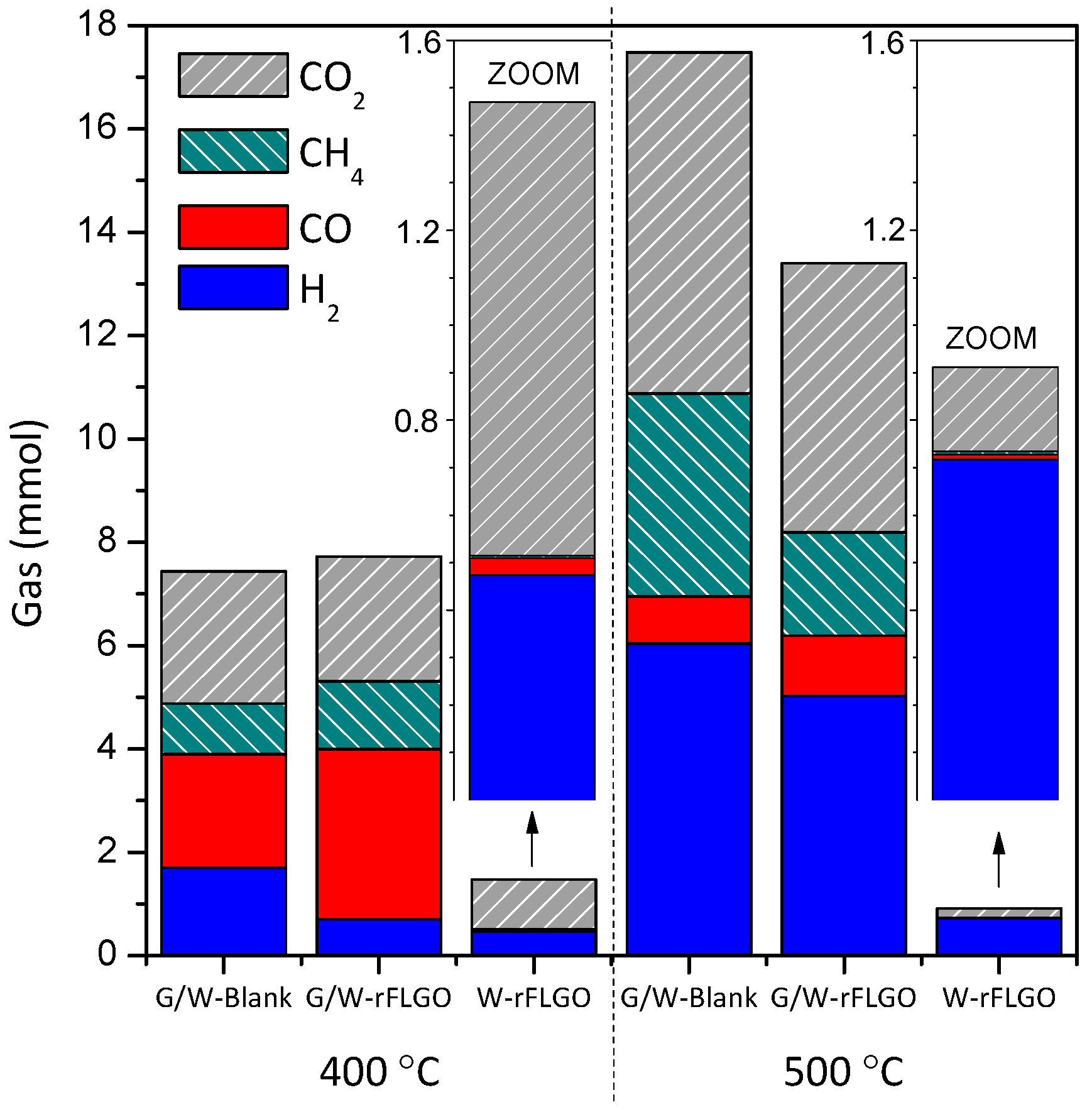
2.2. Products of the Supercritical Water Gasification of Glycerol
3. Materials and Methods
3.1. Graphene Oxide Obtention by Multi-Wall Carbon Nanotubes Unzipping
3.2. Reduction of FLGO by Supercritical Water Gasification of Glycerol
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Liu, S.; Zhi, L. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 2012, 8, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. Sur le poids atomique du graphite. Ann. Chim. Phys. 1960, 59, 466–472. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonson, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Seville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Guo, L.; Xia, H.; Chen, Q.D.; Feng, J.; Sun, H.B. Photoreduction of graphene oxides: Methods, properties, and applications. Adv. Opt. Mater. 2014, 2, 10–28. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon 2015, 94, 224–242. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef] [PubMed]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Chung, J.S. Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J. Mater. Chem. 2011, 21, 3371–3377. [Google Scholar] [CrossRef]
- Wang, Z.G.; Li, P.J.; Chen, Y.F.; He, J.R.; Zheng, B.J.; Liu, J.B.; Qi, F. The green synthesis of reduced graphene oxide by the ethanol-thermal reaction and its electrical properties. Mater. Lett. 2014, 116, 416–419. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Navarro, C.; Coronado, E.; Marti-Gastaldo, C.; Sanchez-Royo, J.F.; Gomez, M.G. Influence of the pH on the synthesis of reduced graphene oxide under hydrothermal conditions. Nanoscale 2012, 4, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.N.; Liu, Y.B.; Yuan, N.Y.; Ding, G.Q.; Fan, Y.; Yu, C.T. The influence of temperature, time and concentration on the dispersion of reduced graphene oxide prepared by hydrothermal reduction. Diam. Relat. Mater. 2012, 21, 11–15. [Google Scholar] [CrossRef]
- Shi, J.L.; Du, W.C.; Yin, Y.X.; Guo, Y.G.; Wan, L.J. Hydrothermal reduction of three-dimensional graphene oxide for binder-free flexible supercapacitors. J. Mater. Chem. A 2014, 2, 10830–10834. [Google Scholar] [CrossRef]
- Diez, N.; Sliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem. Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Mungse, H.P.; Sharma, O.P.; Sugimura, H.; Khatri, O.P. Hydrothermal deoxygenation of graphene oxide in sub- and supercritical water. RSC Adv. 2014, 4, 22589–22595. [Google Scholar] [CrossRef]
- Balaji, S.S.; Sathish, M. Supercritical fluid processing of nitric acid treated nitrogen doped graphene with enhanced electrochemical supercapacitance. RSC Adv. 2014, 4, 52256–52262. [Google Scholar] [CrossRef]
- Liu, C.; Hu, G. Highly efficient reduction of graphene oxide by sub/supercritical water and their application for thermal interface materials. Appl. Therm. Eng. 2015, 90, 193–198. [Google Scholar] [CrossRef]
- Rangappa, D.; Sone, K.; Wang, M.; Gautam, U.K.; Golberg, D.; Itoh, H.; Ichihara, M.; Honma, I. Rapid and direct conversion of graphite crystals into high-yielding, good-quality graphene by supercritical fluid exfoliation. Chem. Eur. J. 2010, 16, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- Budi Nursanto, E.; Nugroho, A.; Hong, S.-A.; Kim, S.J.; Yoon Chung, K.; Kim, J. Facile synthesis of reduced graphene oxide in supercritical alcohols and its lithium storage capacity. Green Chem. 2011, 13, 2714–2718. [Google Scholar] [CrossRef]
- Kong, C.Y.; Song, W.-L.; Meziani, M.J.; Tackett Ii, K.N.; Cao, L.; Farr, A.J.; Anderson, A.; Sun, Y.-P. Supercritical fluid conversion of graphene oxides. J. Supercrit. Fluids 2012, 61, 206–211. [Google Scholar] [CrossRef]
- Liu, S.; Chen, K.; Fu, Y.; Yu, S.; Bao, Z. Reduced graphene oxide paper by supercritical ethanol treatment and its electrochemical properties. Appl. Surf. Sci. 2012, 258, 5299–5303. [Google Scholar] [CrossRef]
- Seo, M.; Yoon, D.; Hwang, K.S.; Kang, J.W.; Kim, J. Supercritical alcohols as solvents and reducing agents for the synthesis of reduced graphene oxide. Carbon 2013, 64, 207–218. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. Supercritical CO2 processing to improve the electrochemical properties of graphene oxide. J. Supercrit. Fluids 2016, 118, 119–127. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefin. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerol: An update. Energy Convers. Manag. 2009, 50, 2600–2604. [Google Scholar] [CrossRef]
- Markočič, E.; Kramberger, B.; van Bennekom, J.G.; Jan Heeres, H.; Vos, J.; Knez, Ž. Glycerol reforming in supercritical water: A short review. Renew. Sustain. Energy Rev. 2013, 23, 40–48. [Google Scholar] [CrossRef]
- Xu, X.; Matsumura, Y.; Stenberg, J.; Antal, M.J., Jr. Carbon-catalyzed gasification of organic feedstocks in supercritical water. Ind. Eng. Chem. Res. 1996, 35, 2522–2530. [Google Scholar] [CrossRef]
- Bühler, W.; Dinjus, E.; Ederer, H.J.; Kruse, A.; Mas, C. Ionic reactions and pyrolysis of glycerol as competing reaction pathways in near- and supercritical water. J. Supercrit. Fluids 2002, 22, 37–53. [Google Scholar] [CrossRef]
- Kersten, S.R.A.; Potic, B.; Prins, W.; Van Swaaij, W.P.M. Gasification of model compounds and wood in hot compressed water. Ind. Eng. Chem. Res. 2006, 45, 4169–4177. [Google Scholar] [CrossRef]
- May, A.; Salvadó, J.; Torras, C.; Montané, D. Catalytic gasification of glycerol in supercritical water. Chem. Eng. J. 2010, 160, 751–759. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Brilman, D.W.F.; Van Swaaij, W.P.M.; Kersten, S.R.A. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind. Eng. Chem. Res. 2010, 49, 1113–1122. [Google Scholar] [CrossRef]
- Van Bennekom, J.G.; Venderbosch, R.H.; Assink, D.; Heeres, H.J. Reforming of methanol and glycerol in supercritical water. J. Supercrit. Fluids 2011, 58, 99–113. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Serrera, A.; Galera, S.; Ollero, P. Experimental study of the supercritical water reforming of glycerol without the addition of a catalyst. Energy 2013, 56, 193–206. [Google Scholar] [CrossRef]
- Pairojpiriyakul, T.; Kiatkittipong, W.; Assabumrungrat, S.; Croiset, E. Hydrogen production from supercritical water reforming of glycerol in an empty inconel 625 reactor. Int. J. Hydrogen Energy 2014, 39, 159–170. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Cao, C.; Yin, J.; Lu, Y.; Zhang, X. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor. Int. J. Hydrog. Energy 2012, 37, 5559–5568. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Yin, J.; Jin, H. Supercritical water gasification of glycerol: Intermediates and kinetics. J. Supercrit. Fluids 2013, 78, 95–102. [Google Scholar] [CrossRef]
- Watanabe, M.; Iida, T.; Aizawa, Y.; Aida, T.M.; Inomata, H. Acrolein synthesis from glycerol in hot-compressed water. Bioresour. Technol. 2007, 98, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hanna, M.A.; Marx, D.B.; Sun, R. Optimization of hydrogen production from supercritical water gasification of crude glycerol-byproduct of biodiesel production. Int. J. Energy Res. 2013, 37, 1600–1609. [Google Scholar] [CrossRef]
- Schubert, M.; Müller, J.B.; Vogel, F. Continuous hydrothermal gasification of glycerol mixtures: Autothermal operation, simultaneous salt recovery, and the effect of K3PO4 on the catalytic gasification. Ind. Eng. Chem. Res. 2014, 53, 8404–8415. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Kozinski, J.A. Supercritical water gasification of glycerol and methanol mixtures as model waste residues from biodiesel refinery. Chem. Eng. Res. Des. 2016, 113, 17–27. [Google Scholar] [CrossRef]
- Ott, L.; Bicker, M.; Vogel, H. Catalytic dehydration of glycerol in sub- and supercritical water: A new chemical process for acrolein production. Green Chem. 2006, 8, 214–220. [Google Scholar] [CrossRef]
- Byrd, A.J.; Pant, K.K.; Gupta, R.B. Hydrogen production from glycerol by reforming in supercritical water over Ru/Al2O3 catalyst. Fuel 2008, 87, 2956–2960. [Google Scholar] [CrossRef]
- Pairojpiriyakul, T.; Croiset, E.; Kiatkittipong, W.; Kiatkittipong, K.; Arpornwichanop, A.; Assabumrungrat, S. Hydrogen production from catalytic supercritical water reforming of glycerol with cobalt-based catalysts. Int. J. Hydrog. Energy 2013, 38, 4368–4379. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Aguilera, P.G.; Ollero, P. Hydrogen production from supercritical water reforming of glycerol over Ni/Al2O3-SiO2 catalyst. Energy 2015, 84, 634–642. [Google Scholar] [CrossRef]
- Van Bennekom, J.G.; Kirillov, V.A.; Amosov, Y.I.; Krieger, T.; Venderbosch, R.H.; Assink, D.; Lemmens, K.P.J.; Heeres, H.J. Explorative catalyst screening studies on reforming of glycerol in supercritical water. J. Supercrit. Fluids 2012, 70, 171–181. [Google Scholar] [CrossRef]
- Van Bennekom, J.G.; Venderbosch, R.H.; Assink, D.; Lemmens, K.P.J.; Heeres, H.J. Bench scale demonstration of the supermethanol concept: The synthesis of methanol from glycerol derived syngas. Chem. Eng. J. 2012, 207–208, 245–253. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Foroughi, H.; Dai, T.; Azadi, F.; Farnood, R. Catalytic reforming of activated sludge model compounds in supercritical water using nickel and ruthenium catalysts. Appl. Catal. B 2013, 134–135, 265–273. [Google Scholar] [CrossRef]
- Chakinala, A.G.; van Swaaij, W.P.M.; Kersten, S.R.A.; de Vlieger, D.; Seshan, K.; Brilman, D.W.F. Catalytic reforming of glycerol in supercritical water over bimetallic Pt-Ni catalyst. Ind. Eng. Chem. Res. 2013, 52, 5302–5312. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Aguilera, P.G.; Ollero, P. Supercritical water reforming of glycerol: Performance of ru and ni catalysts on Al2O3 support. Energy 2016, 96, 561–568. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Ollero, P. Turnover rates for the supercritical water reforming of glycerol on supported Ni and Ru catalysts. Fuel 2016, 180, 417–423. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark Alexander, V.; Olivier James, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and langmuir-blodgett films. Nat. Nano 2008, 3, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Feret, F.R. Determination of the crystallinity of calcined and graphitic cokes by X-ray diffraction. Analyst 1998, 123, 595–600. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The role of intercalated water in multilayered graphene oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, N.; Kim, B.G.; Jung, D.S.; Im, K.; Hur, J.; Choi, J.W. Restacking-inhibited 3D reduced graphene oxide for high performance supercapacitor electrodes. ACS Nano 2013, 7, 9366–9374. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Ruiz-Vargas, C.S.; van der Zande, A.M.; Whitney, W.S.; Levendorf, M.P.; Kevek, J.W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y.; et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 2011, 469, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of graphitic ab stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, X.; Guo, L.; Zhu, C.; Cao, C.; Wu, Z. Experimental investigation on methanation reaction based on coal gasification in supercritical water. Int. J. Hydrog. Energy 2017, 42, 4636–4641. [Google Scholar]
- Jin, H.; Guo, L.; Guo, J.; Ge, Z.; Cao, C.; Lu, Y. Study on gasification kinetics of hydrogen production from lignite in supercritical water. Int. J. Hydrog. Energy 2015, 40, 7523–7529. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of glycerol to alkyl-aromatics over zeolites. Energy Fuels 2010, 24, 3804–3809. [Google Scholar] [CrossRef]
- Jang, H.-S.; Bae, K.; Shin, M.; Kim, S.M.; Kim, C.-U.; Suh, Y.-W. Aromatization of glycerol/alcohol mixtures over zeolite H-ZSM-5. Fuel 2014, 134, 439–447. [Google Scholar] [CrossRef]
- Jin, C.; Lan, H.; Peng, L.; Suenaga, K.; Iijima, S. Deriving carbon atomic chains from graphene. Phys. Rev. Lett. 2009, 102, 205501. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of Fe-Mo catalysts. Int. J. Hydrog. Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Moliner, R.; Suelves, I. On the oxidation degree of few-layer graphene oxide sheets obtained from chemically oxidized multiwall carbon nanotubes. Carbon 2015, 81, 405–417. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Unzipping of multi-wall carbon nanotubes with different diameter distributions: Effect on few-layer graphene oxide obtention. Appl. Surf. Sci. 2017, 424, 101–110. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Galvez, E.M.; Suelves, I. Graphene quantum dots from fishbone carbon nanofibers. RSC Adv. 2016, 6, 48504–48514. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Arcelus-Arrillaga, P.; Puron, H.; Millan, M. Selective catalytic steam cracking of anthracene using mesoporous Al2O3 supported ni-based catalysts doped with Na, Ca or K. Appl. Catal. A 2013, 459, 17–25. [Google Scholar] [CrossRef]
- Nist Chemistry Webbook, Nist Standard Reference Database Number 69. Available online: http://webbook.nist.gov (accessed on 11 December 2017).
- Biscoe, J. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
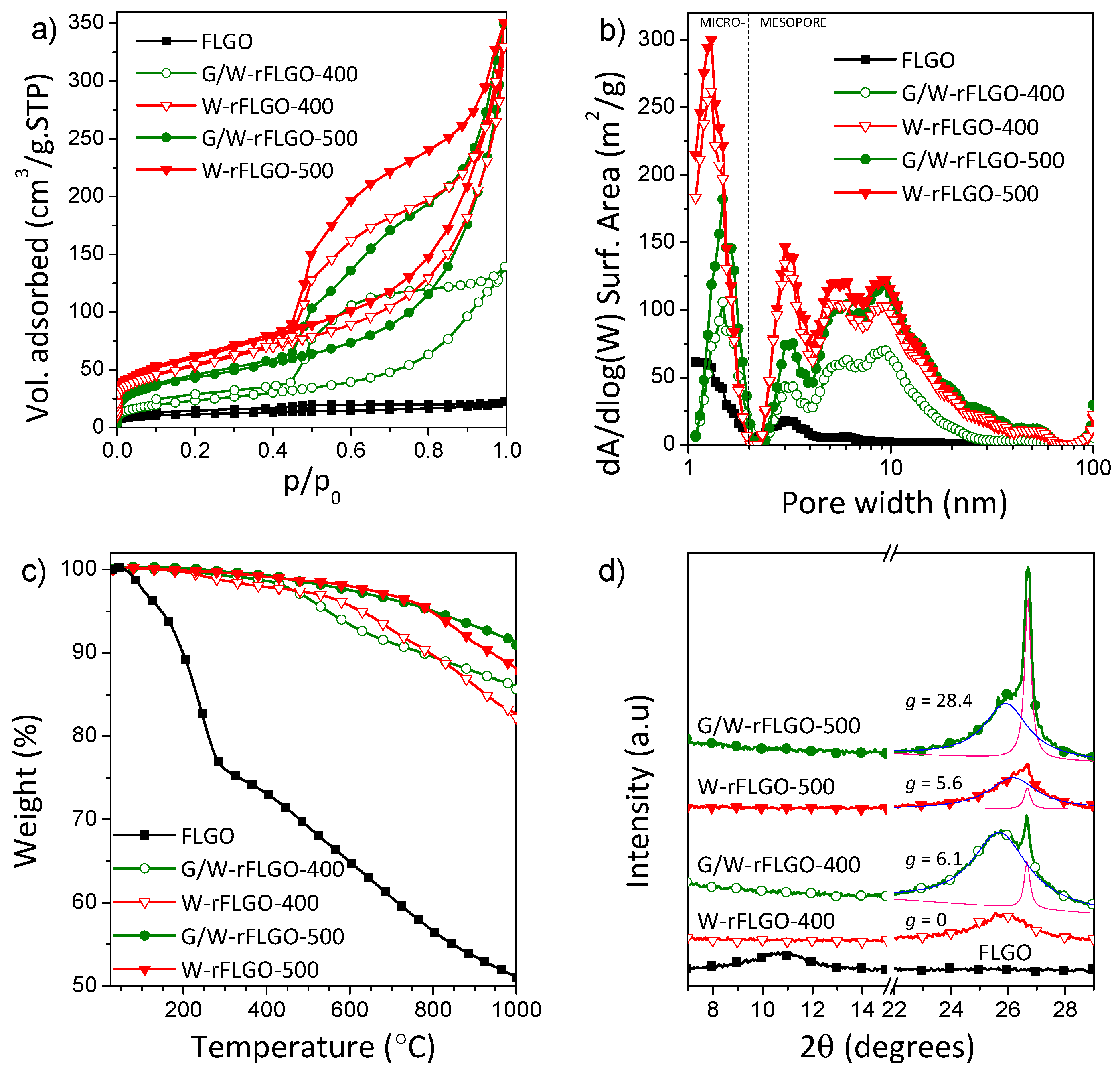
| SBET [m2 g−1] 1 | Smic [m2 g−1] 2 | Vt [cm3 g−1] 3 | |
|---|---|---|---|
| FLGO | 42 | 11 | 0.035 |
| W-rFLGO-400 | 191 | 42 | 0.510 |
| W-rFLGO-500 | 215 | 47 | 0.542 |
| G/W-rFLGO-400 | 84 | 16 | 0.216 |
| G/W-rFLGO-500 | 155 | 26 | 0.540 |
| XPS Analysis [at. %] 1 | EDX Analysis [at. %] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C=C/C–C | C–O | C=O | π-π* | C | O | C/O | C | O | C/O | |
| FLGO | 47.6 | 45.4 | 6.7 | 0.3 | 68.6 | 30.3 | 2.3 | 69.9 | 27.8 | 2.5 |
| W-rFLGO-400 | 76.1 | 16.3 | 4.2 | 3.4 | 85.5 | 14.5 | 5.9 | 88.1 | 11.2 | 7.9 |
| W-rFLGO-500 | 80.9 | 7.3 | 6.4 | 5.5 | 88.8 | 11.2 | 8.0 | 90.8 | 8.3 | 10.9 |
| G/W-rFLGO-400 | 85.4 | 9.2 | 3.1 | 2.3 | 91.0 | 9.0 | 10.2 | 92.6 | 7.4 | 12.4 |
| G/W-rFLGO-500 | 86.3 | 5.1 | 4.6 | 4.0 | 91.9 | 8.1 | 11.4 | 96.6 | 3.4 | 28.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, D.; Arcelus-Arrillaga, P.; Millan, M.; Pinilla, J.L.; Suelves, I. Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol. Nanomaterials 2017, 7, 447. https://doi.org/10.3390/nano7120447
Torres D, Arcelus-Arrillaga P, Millan M, Pinilla JL, Suelves I. Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol. Nanomaterials. 2017; 7(12):447. https://doi.org/10.3390/nano7120447
Chicago/Turabian StyleTorres, Daniel, Pedro Arcelus-Arrillaga, Marcos Millan, José Luis Pinilla, and Isabel Suelves. 2017. "Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol" Nanomaterials 7, no. 12: 447. https://doi.org/10.3390/nano7120447
APA StyleTorres, D., Arcelus-Arrillaga, P., Millan, M., Pinilla, J. L., & Suelves, I. (2017). Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol. Nanomaterials, 7(12), 447. https://doi.org/10.3390/nano7120447