Exploring the Optical and Morphological Properties of Ag and Ag/TiO2 Nanocomposites Grown by Supersonic Cluster Beam Deposition
Abstract
1. Introduction
2. Results and Discussion
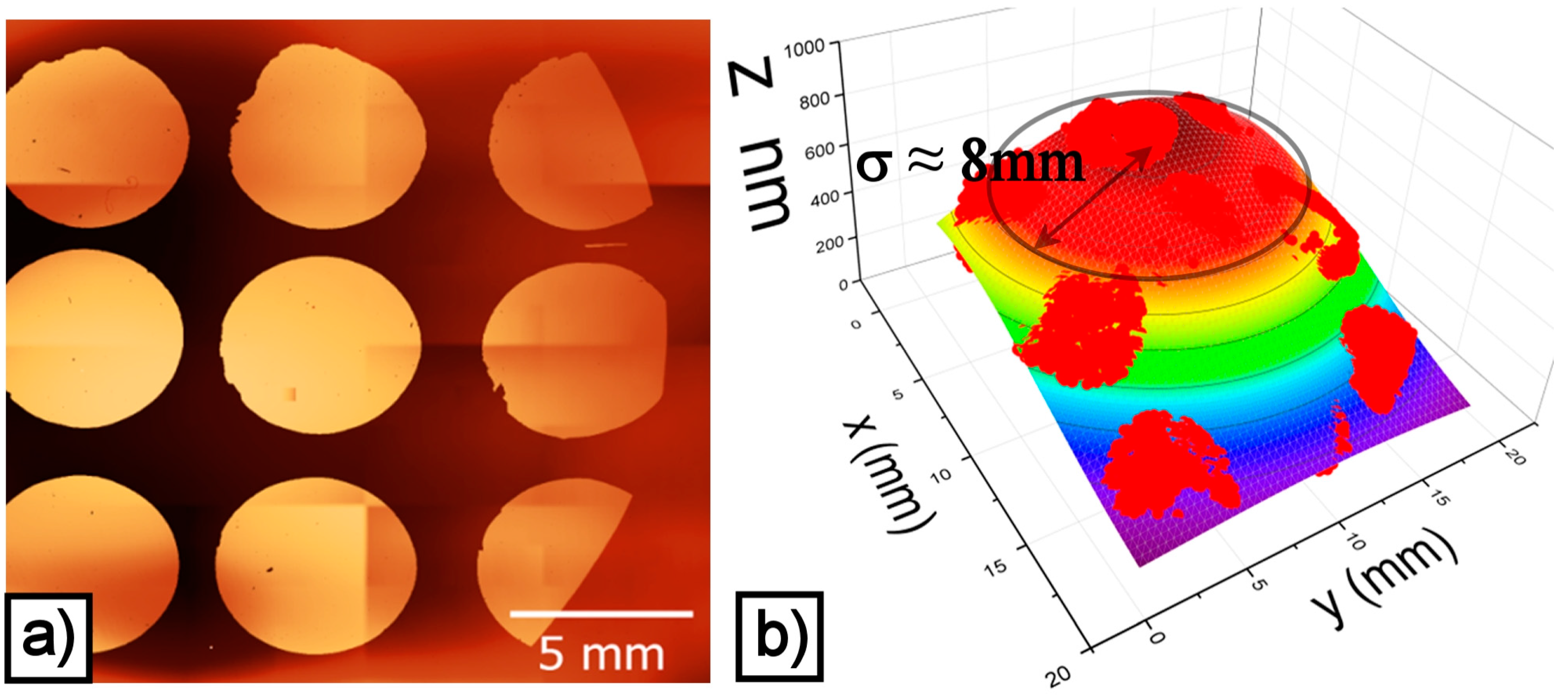
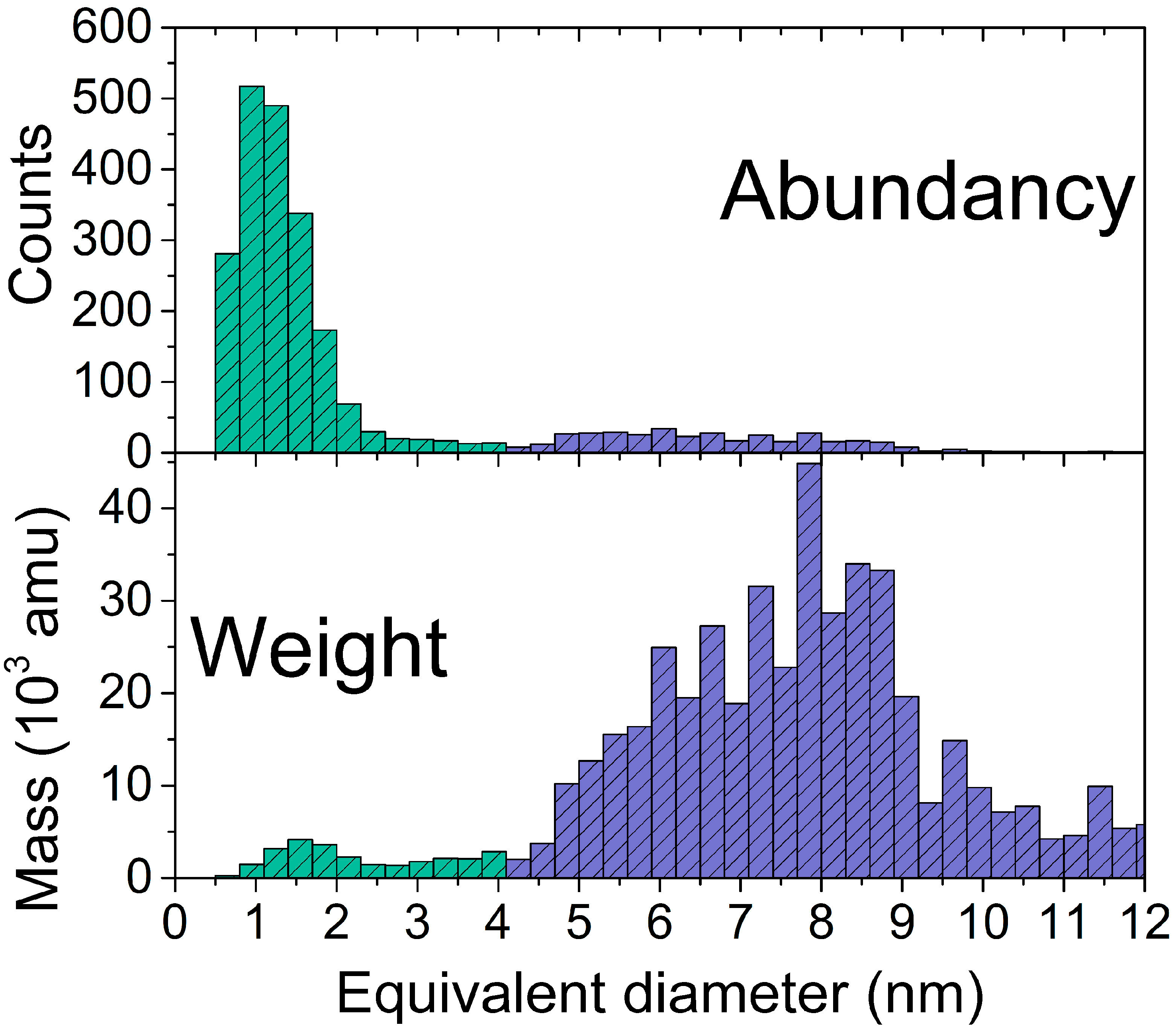
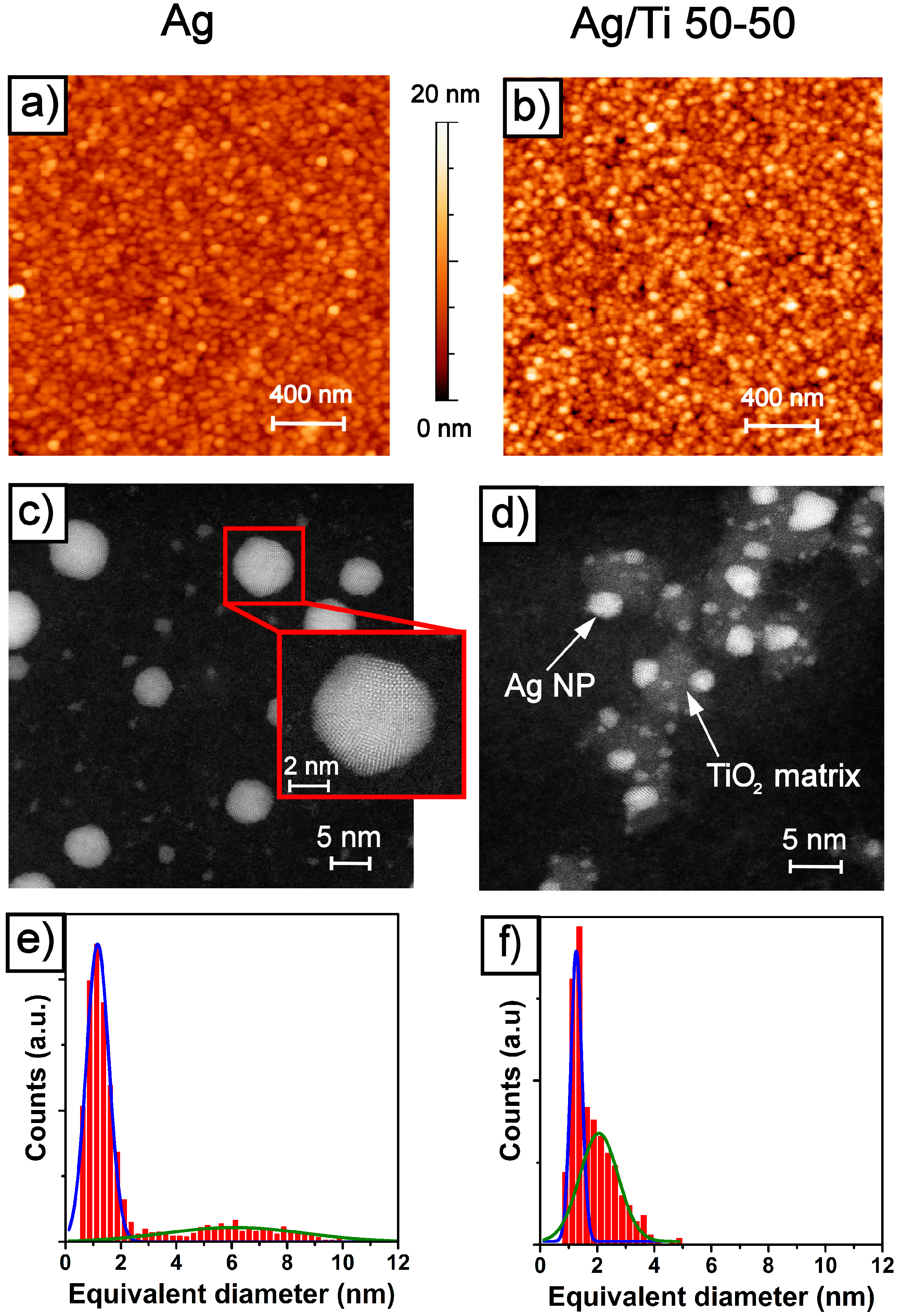
2.1. Morphology and Structure of the Ag and Ag/TiO2 (Ag/Ti 50-50) Films
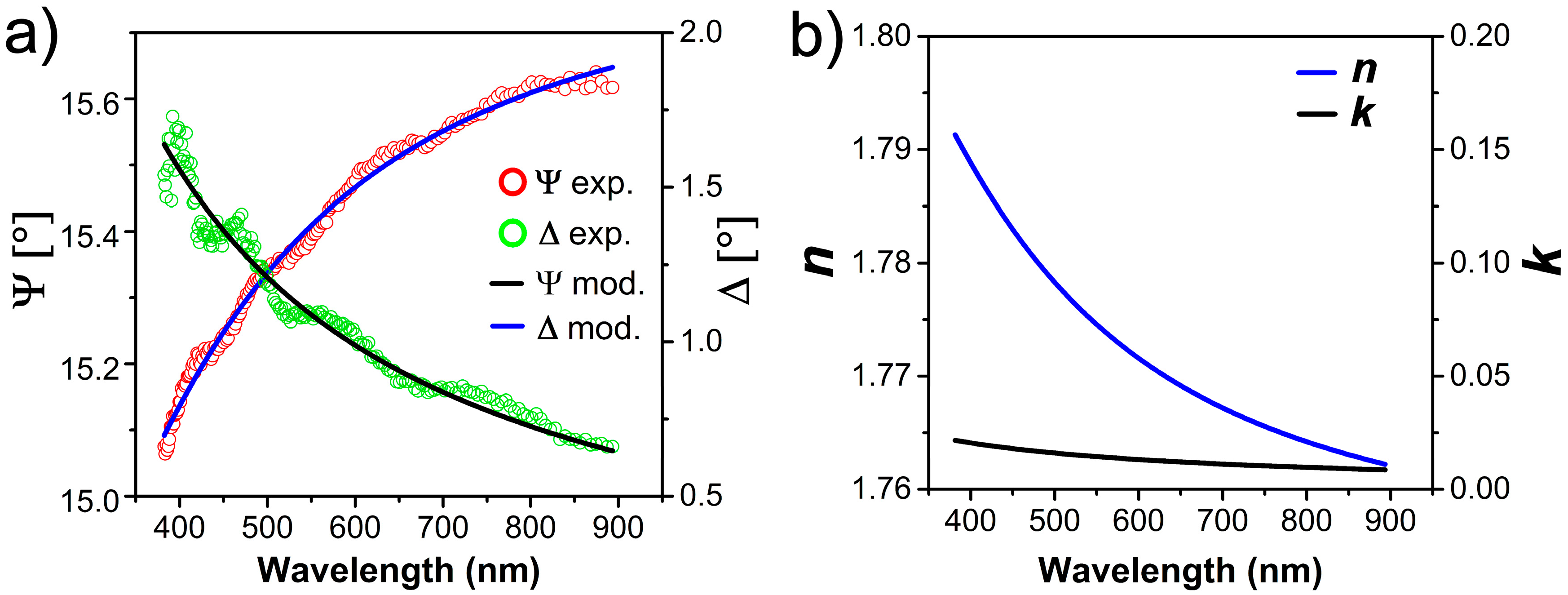
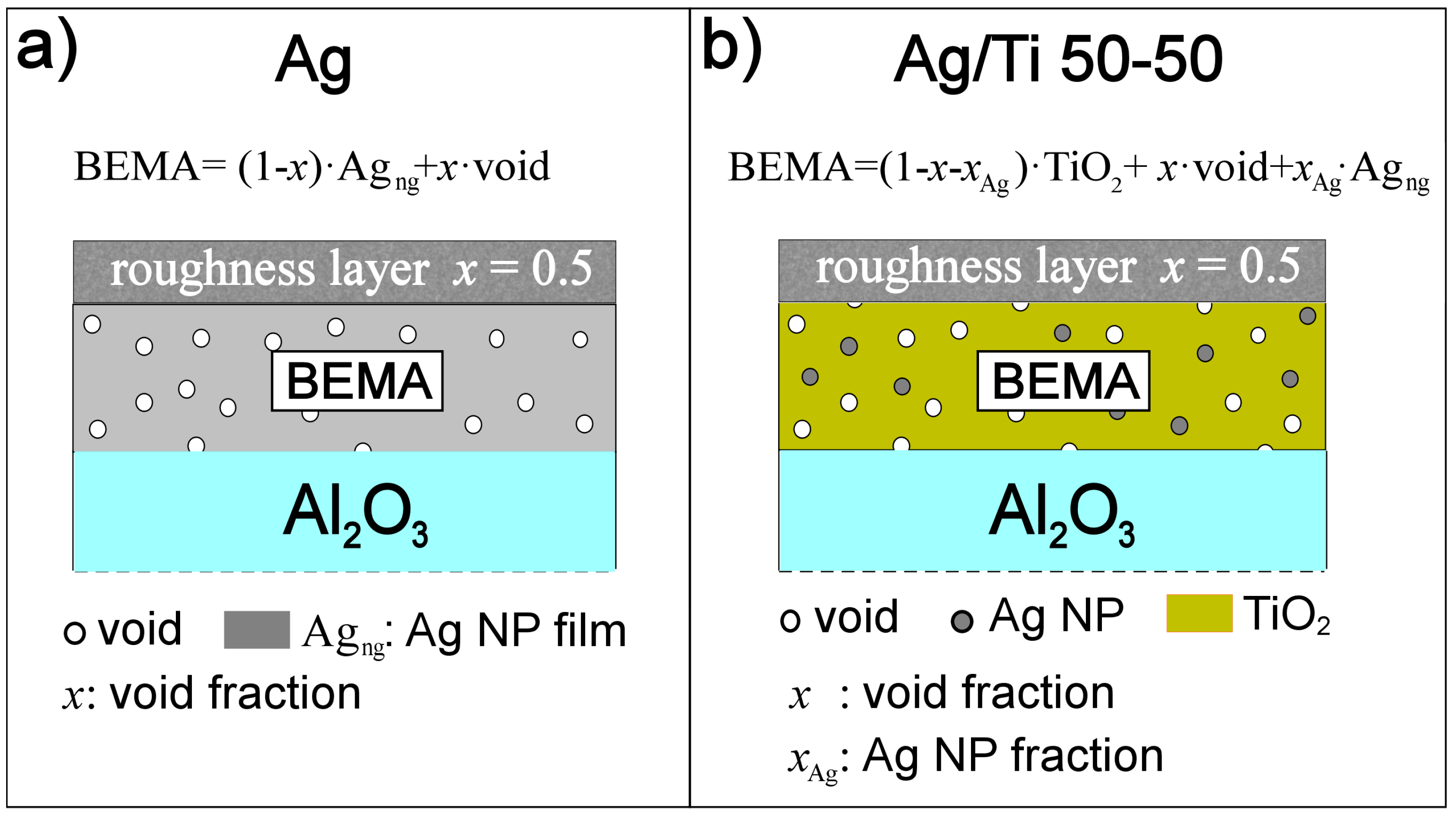
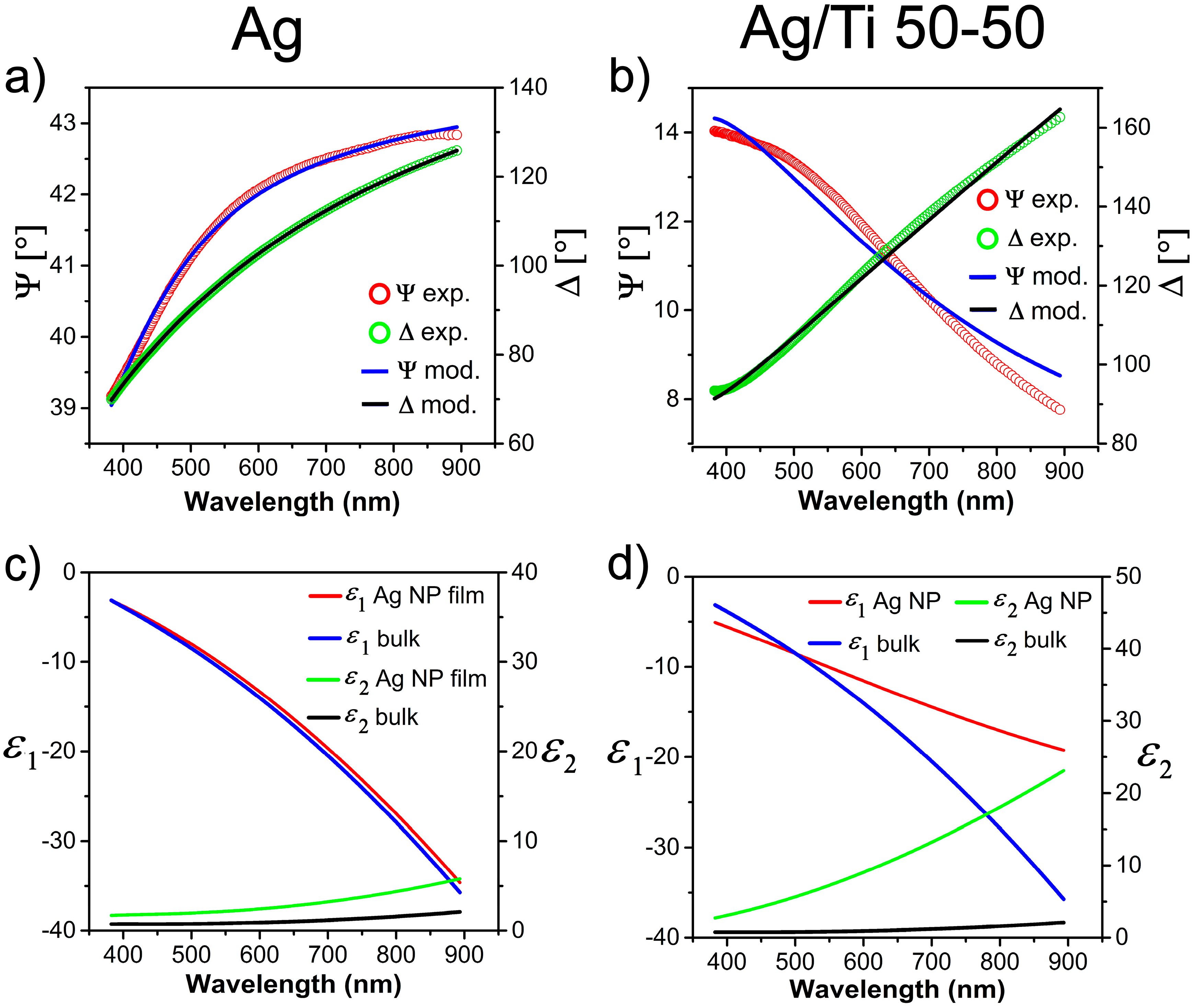
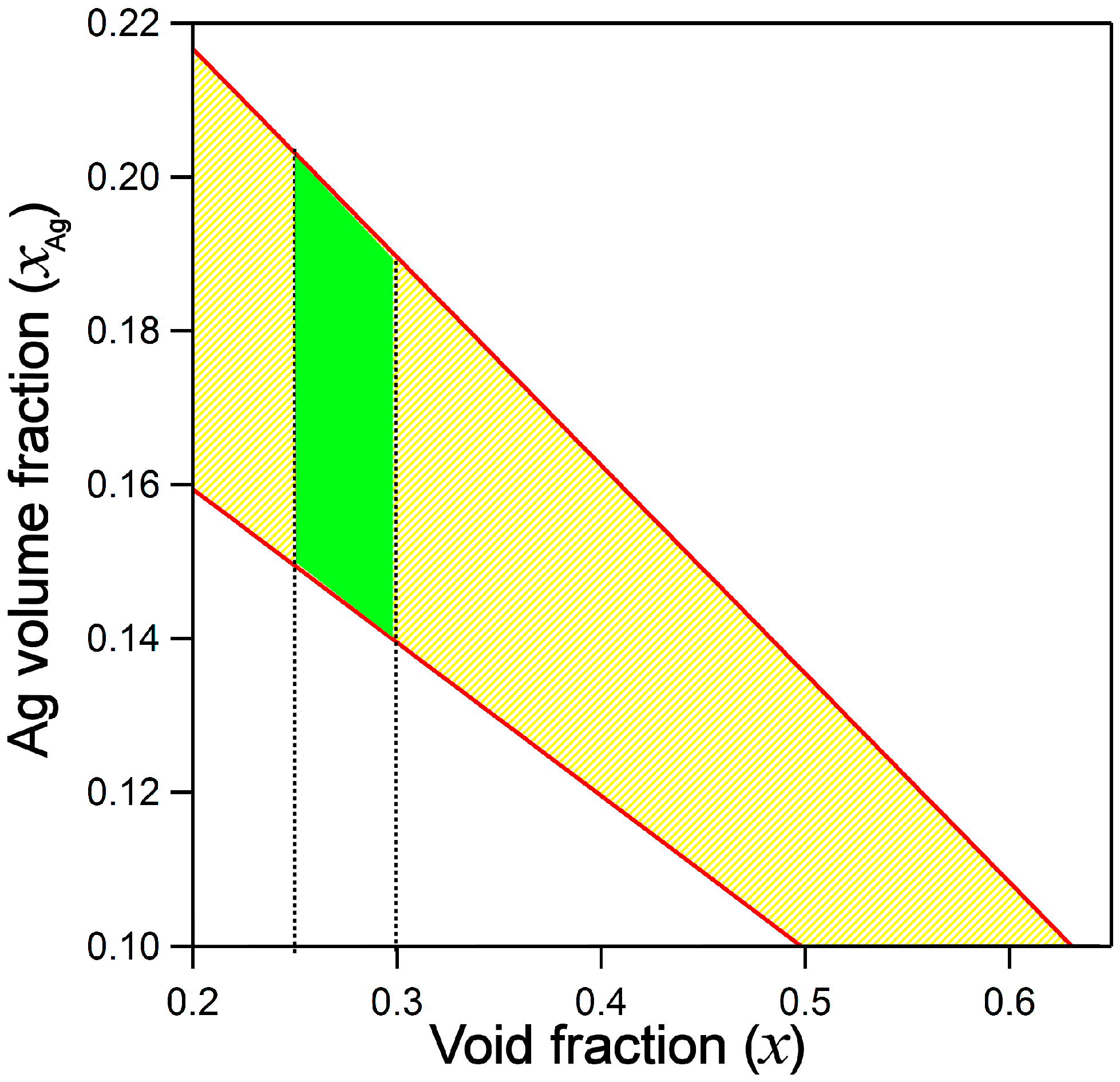
2.2. Optical Properties by Spectroscopic Ellipsometry (SE)
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
- = 1.179
- ρ = 6.7635 × 10−6 Ω∙cm; Drude term
- τ = 9.487 fs;
- A1 = 1.206883 + i × 1.3885; Interband oscillator harmonic terms
- ħΓ1 = 6.8152 eV;
- ħω1 = 4.137 eV;
- A2 = −0.674042 + i × 2.3861;
- ħΓ2 = 0.3806 eV;
- ħω2 = 4.011 eV;
- A3 = 4.525906 − i × 2.3944;
- ħΓ3 = 22.148 eV;
- ħω3 = 2.723 eV;
- Mean square error of the fit:
- MSE = 1.445
- Thickness = 87.2 ± 5 nm
- Roughness = 1.5 ± 0.2 nm
- ħΓ(R) = 0.13 ± 0.05 eV
- x = 17 ± 1%
- Mean square error of the fit:
- MSE = 9.187
- Thickness = 43 ± 2 nm
- Roughness = 2.06 ± 4 nm
- ħΓ(R) = 1.0 ± 0.1 eV
- xAg = 18.4 ± 0.7%
- x = 25 ± 5.2% for BEMA layer
- : i-th wavelength experimental N value
- : i-th wavelength model/fit N value
- : i-th wavelength experimental C value
- : i-th wavelenght model/fit C value
- i-th wavelength experimental S value
- i-th wavelenght model/fit S value
References
- Holade, Y.; Hickey, D.P.; Minteer, S.D. Halide-regulated growth of electrocatalytic metal nanoparticles directly onto a carbon paper electrode. J. Mater. Chem. A 2016, 4, 17154–17162. [Google Scholar] [CrossRef]
- Hu, J.E.; Liu, Z.; Eichhorn, B.W.; Jackson, G.S. CO-tolerance of nano-architectured Pt-Mo anode electrocatalysts for PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 11268–11275. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the Composition of AuPt Bimetallic Nanoparticles for Antibacterial Application. Angew. Chem. Int. Ed. Engl. 2014, 53, 8127–8131. [Google Scholar] [CrossRef] [PubMed]
- Botequim, D.; Maia, J.; Lino, M.M.F.; Lopes, L.M.F.; Simões, P.N.; Ilharco, L.M.; Ferreira, L. Nanoparticles and Surfaces Presenting Antifungal, Antibacterial and Antiviral Properties. Langmuir 2012, 28, 7646–7656. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Turgeon, S.; Busby, Y.; Tatoulian, M.; Pireaux, J.-J.; Mantovani, D. Controlled Distribution and Clustering of Silver in Ag-DLC Nanocomposite Coatings Using a Hybrid Plasma Approach. ACS Appl. Mater. Interfaces 2016, 8, 21020–21027. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, E.; De Cesari, S.; Landini, G.; Riccobono, E.; Pallecchi, L.; Rossolini, G.M.; Gavioli, L. Highly bactericidal Ag nanoparticle films obtained by cluster beam deposition. Nanomed. Nanotechnol. Biol. Med. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shin, S. Room temperature nanojoining of Cu-Ag core-shell nanoparticles and nanowires. J. Nanopart. Res. 2017, 19, 53. [Google Scholar] [CrossRef]
- Kaptay, G.; Janczak-Rusch, J.; Jeurgens, L.P.H. Melting Point Depression and Fast Diffusion in Nanostructured Brazing Fillers Confined Between Barrier Nanolayers. J. Mater. Eng. Perform. 2016, 25, 3275–3284. [Google Scholar] [CrossRef]
- Yliniemi, K.; Vahvaselkä, M.; Van Ingelgem, Y.; Baert, K.; Wilson, B.P.; Terryn, H.; Kontturi, K. The formation and characterisation of ultra-thin films containing Ag nanoparticles. J. Mater. Chem. 2008, 18, 199–206. [Google Scholar] [CrossRef]
- Nasiri, N.; Elmøe, T.D.; Liu, Y.; Qin, Q.H.; Tricoli, A. Self-assembly dynamics and accumulation mechanisms of ultra-fine nanoparticles. Nanoscale 2015, 7, 9859–9867. [Google Scholar] [CrossRef] [PubMed]
- Benetti, G.; Caddeo, C.; Melis, C.; Ferrini, G.; Giannetti, C.; Winckelmans, N.; Bals, S.; Van Bael, M.J.; Cavaliere, E.; Gavioli, L.; et al. Bottom-up Mechanical Nanometrology of Granular Ag Nanoparticles Thin Films. J. Phys. Chem. C 2017, 121, 22434–22441. [Google Scholar] [CrossRef]
- Walters, G.; Parkin, I.P. The incorporation of noble metal nanoparticles into host matrix thin films: Synthesis, characterisation and applications. J. Mater. Chem. 2009, 19, 574–590. [Google Scholar] [CrossRef]
- Chuang, M.; Chen, F. Synergistic Plasmonic Effects of Metal Nanoparticle—Decorated PEGylated Graphene Oxides in Polymer Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.U.; Yang, R.T. Corrected Horváth-Kawazoe equations for pore-size distribution. AIChE J. 2000, 46, 734–750. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Peli, S.; Cavaliere, E.; Benetti, G.; Gandolfi, M.; Chiodi, M.; Cancellieri, C.; Giannetti, C.; Ferrini, G.; Gavioli, L.; Banfi, F. Mechanical Properties of Ag Nanoparticle Thin Films Synthesized by Supersonic Cluster Beam Deposition. J. Phys. Chem. C 2016, 120, 4673–4681. [Google Scholar] [CrossRef]
- Wegner, K.; Piseri, P.; Tafreshi, H.V.; Milani, P. Cluster beam deposition: A tool for nanoscale science and technology. J. Phys. D Appl. Phys. 2006, 39, R439–R459. [Google Scholar] [CrossRef]
- Barborini, E.; Kholmanov, I.N.; Conti, A.M.; Piseri, P.; Vinati, S.; Milani, P.; Ducati, C. Supersonic cluster beam deposition of nanostructured titania. Eur. Phys. J. D 2003, 24, 277–282. [Google Scholar] [CrossRef]
- Chiodi, M.; Cheney, C.P.; Vilmercati, P.; Cavaliere, E.; Mannella, N.; Weitering, H.H.; Gavioli, L. Enhanced Dopant Solubility and Visible-Light Absorption in Cr-N Codoped TiO2 Nanoclusters. J. Phys. Chem. C 2012, 116, 311–318. [Google Scholar] [CrossRef]
- Fraters, B.D.; Cavaliere, E.; Mul, G.; Gavioli, L. Synthesis of photocatalytic TiO2 nano-coatings by Supersonic Cluster Beam Deposition. J. Alloys Compd. 2014, 615, 467–471. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Canteri, A.; Landini, G.; Rossolini, G.M.; Pallecchi, L.; Chiodi, M.; Van Bael, M.; Winckelmans, N.; Bals, S.; et al. Direct synthesis of antimicrobial coatings based on tailored bi-elemental nanoparticles. APL Mater. 2017, 5, 036105. [Google Scholar] [CrossRef]
- Barborini, E.; Kholmanov, I.; Piseri, P.; Ducati, C.; Bottani, C.; Milani, P. Engineering the Nanocrystalline Structure of TiO2 Films by Aerodynamically Filtered Cluster Deposition. Appl. Phys. Lett. 2002, 81, 3052–3054. [Google Scholar] [CrossRef]
- Asoro, M.A.; Damiano, J.; Ferreira, P.J. Size Effects on the Melting Temperature of Silver Nanoparticles: In-Situ TEM Observations. Microsc. Microanal. 2009, 15 (Suppl. 2), 706–707. [Google Scholar] [CrossRef]
- Lu, H.M.; Li, P.Y.; Cao, Z.H.; Meng, X.K. Size-, Shape- and Dimensionality-Dependent Melting Temperatures of Nanocrystals. J. Phys. Chem. C 2009, 113, 7598–7602. [Google Scholar] [CrossRef]
- Blackmore, C.E.; Rees, N.V.; Palmer, R.E. Modular construction of size-selected multiple core Pt–TiO2 nanoclusters for electro-catalysis. Phys. Chem. Chem. Phys. 2015, 17, 28005–28009. [Google Scholar] [CrossRef] [PubMed]
- Langlois, C.; Li, Z.L.; Yuan, J.; Alloyeau, D.; Nelayah, J.; Bochicchio, D.; Ferrando, R.; Ricolleau, C. Transition from core–shell to Janus chemical configuration for bimetallic nanoparticles. Nanoscale 2012, 4, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H. Ellipsometry: Principles and Applications; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-0-470-01608-4. [Google Scholar]
- Azzam, R.M.A.; Bashara, N.M. Ellipsometry and Polarized Light; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 24, 636–664. [Google Scholar] [CrossRef]
- Palik, E.D. (Ed.) Handbook of Optical Constants of Solids; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Hövel, H.; Fritz, S.; Hilger, A.; Kreibig, U.; Vollmer, M. Width of cluster plasmon resonances: Bulk dielectric functions and chemical interface damping. Phys. Rev. B 1993, 48, 18178. [Google Scholar] [CrossRef]
- Kooij, E.S.; Wormeester, H.E.A.; Martijn Brouwer, E.A.; Van Vroonhoven, E.; Van Silfhout, A.; Poelsema, B. Optical Characterization of Thin Colloidal Gold Films by Spectroscopic Ellipsometry. Langmuir 2002, 18, 4401–4413. [Google Scholar] [CrossRef]
- Ehrenreich, H.; Phillips, H.R. Optical properties of Ag and Cu. Phys. Rev. 1962, 128, 1622. [Google Scholar] [CrossRef]
- Blaber, M.G.; Arnold, M.D.; Ford, M.J. Search for the Ideal Plasmonic Nanoshell: The Effects of Surface Scattering and alternatives to Gold and Silver. J. Phys. Chem. C 2009, 8, 3041–3045. [Google Scholar] [CrossRef]
- Aspnes, D.E.; Kinsbron, E.; Bacon, D.D. Optical properties of Au: Sample effects. Phys. Rev. B 1980, 21, 3290. [Google Scholar] [CrossRef]
- Bisio, F.; Palombo, M.; Prato, M.; Cavalleri, O.; Barborini, E.; Vinati, S.; Franchi, M.; Mattera, L.; Canepa, M. Optical properties of cluster-assembled nanoporous gold films. Phys. Rev. B 2009, 80, 205428. [Google Scholar] [CrossRef]
- Bardotti, L.; Prével, B.; Mélinon, P.; Perez, A.; Hou, Q.; Hou, M. Deposition of AuN clusters on Au(111) surfaces. Experimental results and comparison with simulations. Phys. Rev. B 2000, 62, 2835. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Kreibig, U. Electronic properties of small silver particles: The optical constants and their temperature dependence. J. Phys. F Met. Phys. 1974, 4, 999. [Google Scholar] [CrossRef]
- Derkachova, A.; Kolwas, K.; Demchenko, I. Dielectric Function for Gold in Plasmonics Applications: Size Dependence of Plasmon Resonance Frequencies and Damping Rates for Nanospheres. Plasmonics 2016, 11, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, H.; Martinson, A.B.F.; Rosenmann, D.; Khorashad, L.K.; Besteiro, L.V.; Govorov, A.O.; Wiederrecht, G.P. Anomalous ultrafast dynamics of hot plasmonic electrons in nanostructures with hot spots. Nat. Nanotechnol. Lett. 2015, 10, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Chumanov, G.; Evanoff, D.D. Synthesis and properties of silver nanoparticles. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef] [PubMed]
- Toccafondi, C.; Uttiya, S.; Cavalleri, O.; Gemme, G.; Barborini, E.; Bisio, F.; Canepa, M. Optical properties of nanogranular and highly porous TiO2 thin films. J. Phys. D Appl. Phys. 2014, 47, 485301. [Google Scholar] [CrossRef]
- Auld, B.A. Acoustic Fields and Waves in Solids; John Wiley and Sons: New York, NY, USA, 1973; Volume 1. [Google Scholar]
- Mineralogy Database of Hudson Mineralogy Institute. Available online: https://www.mindat.org/min-213.html (accessed on 28 September 2017).
- Joo, S.; Baldwin, D.F. Adhesion mechanisms of nanoparticle silver to substrate materials: Identification. Nanotechnology 2010, 21, 055204. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaliere, E.; Benetti, G.; Van Bael, M.; Winckelmans, N.; Bals, S.; Gavioli, L. Exploring the Optical and Morphological Properties of Ag and Ag/TiO2 Nanocomposites Grown by Supersonic Cluster Beam Deposition. Nanomaterials 2017, 7, 442. https://doi.org/10.3390/nano7120442
Cavaliere E, Benetti G, Van Bael M, Winckelmans N, Bals S, Gavioli L. Exploring the Optical and Morphological Properties of Ag and Ag/TiO2 Nanocomposites Grown by Supersonic Cluster Beam Deposition. Nanomaterials. 2017; 7(12):442. https://doi.org/10.3390/nano7120442
Chicago/Turabian StyleCavaliere, Emanuele, Giulio Benetti, Margriet Van Bael, Naomi Winckelmans, Sara Bals, and Luca Gavioli. 2017. "Exploring the Optical and Morphological Properties of Ag and Ag/TiO2 Nanocomposites Grown by Supersonic Cluster Beam Deposition" Nanomaterials 7, no. 12: 442. https://doi.org/10.3390/nano7120442
APA StyleCavaliere, E., Benetti, G., Van Bael, M., Winckelmans, N., Bals, S., & Gavioli, L. (2017). Exploring the Optical and Morphological Properties of Ag and Ag/TiO2 Nanocomposites Grown by Supersonic Cluster Beam Deposition. Nanomaterials, 7(12), 442. https://doi.org/10.3390/nano7120442






