Constructing Asymmetric Polyion Complex Vesicles via Template Assembling Strategy: Formulation Control and Tunable Permeability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
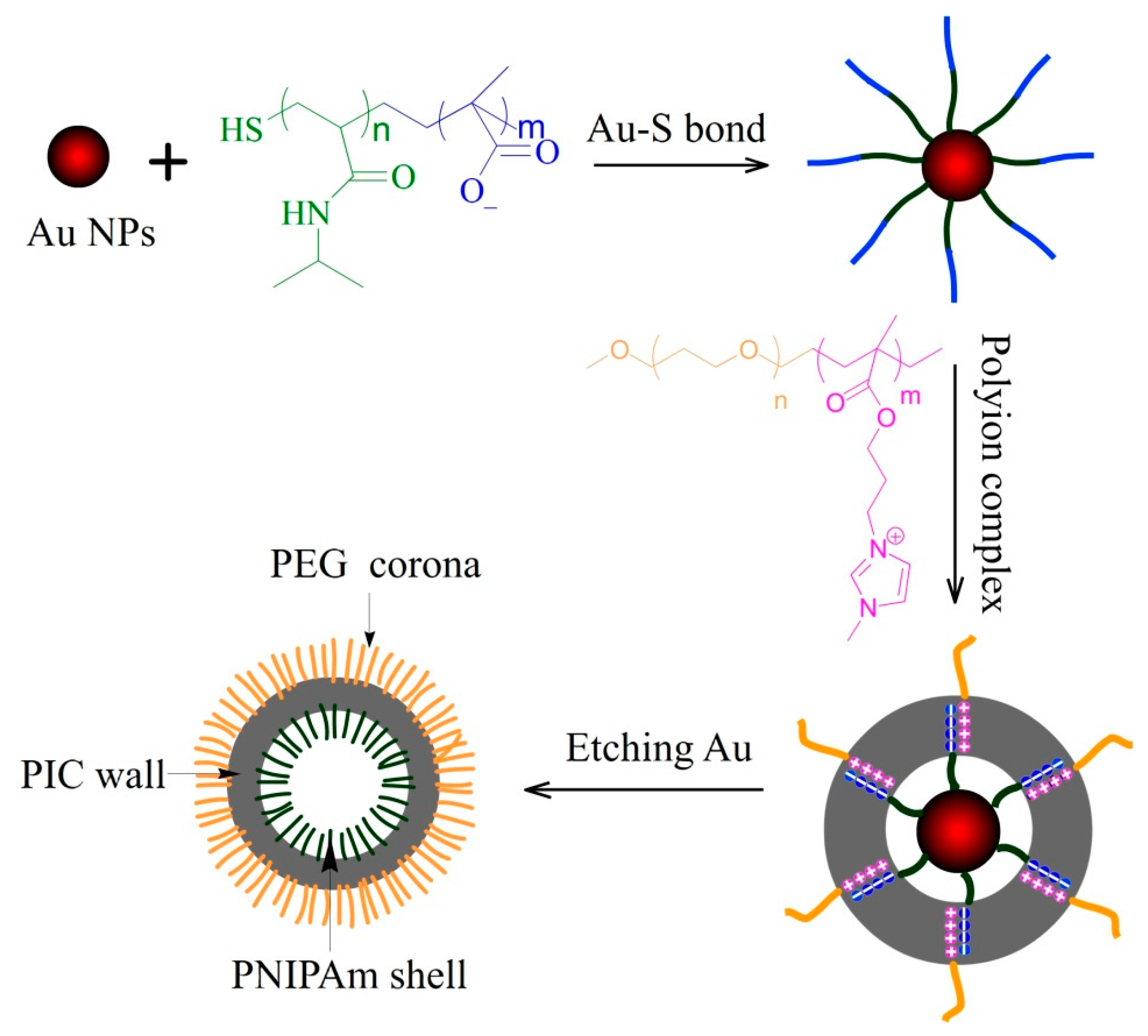
2.2. Preparation of PMAA-b-PNIPAm Capped Au NPs
2.3. Polyion Complex PMAA-b-PNIPAm-@-Au NPs with PEG-b-PMMPImB
2.4. Preparation of Asymmetric PICsomes
2.5. Characterization
3. Results and Discussion
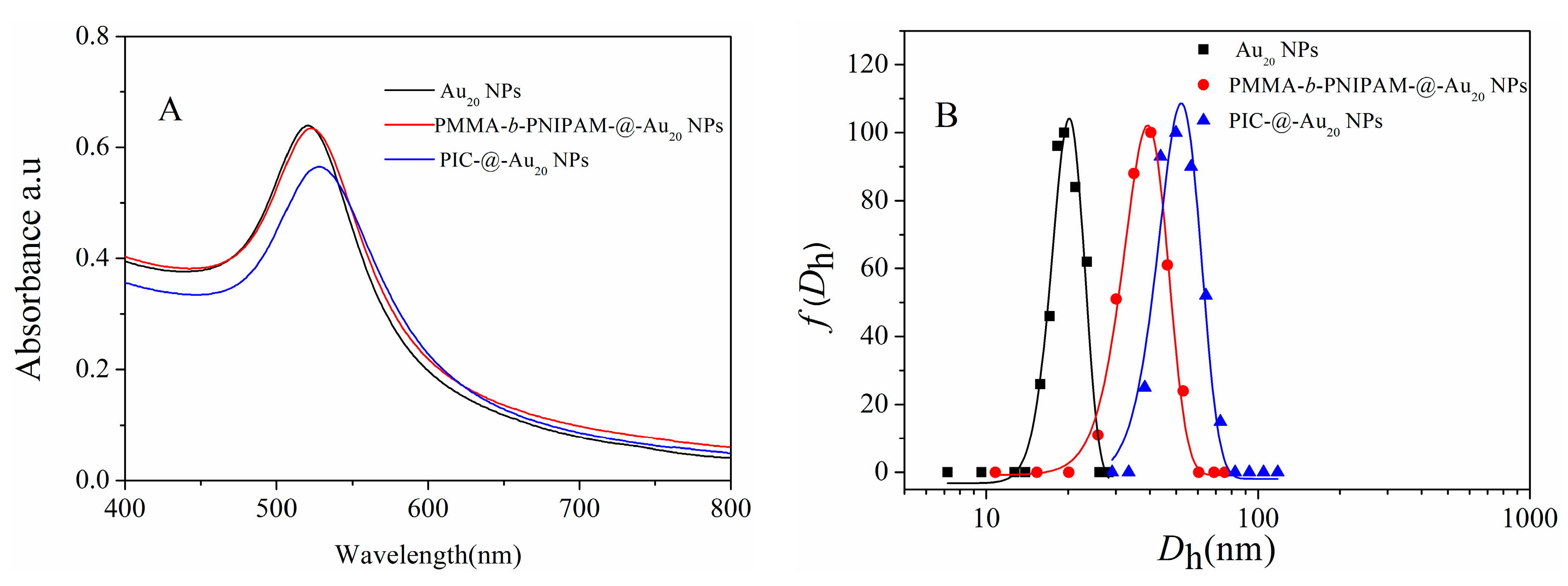
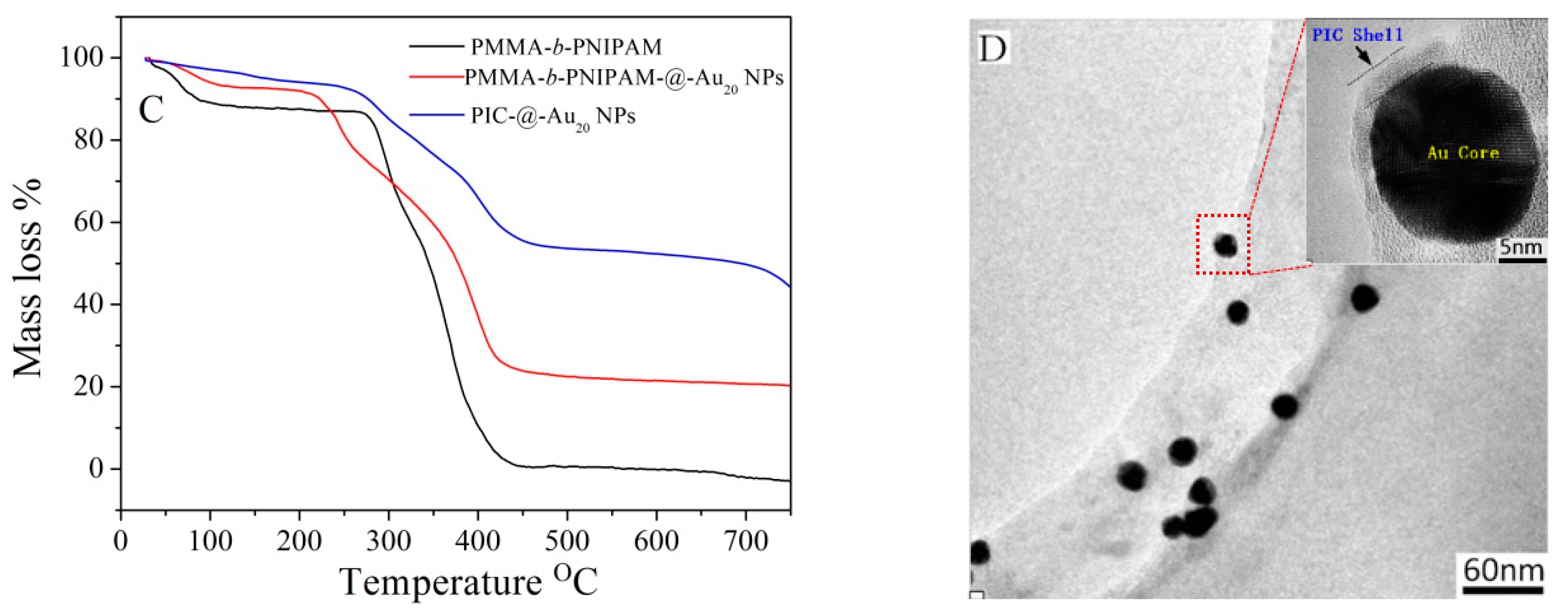
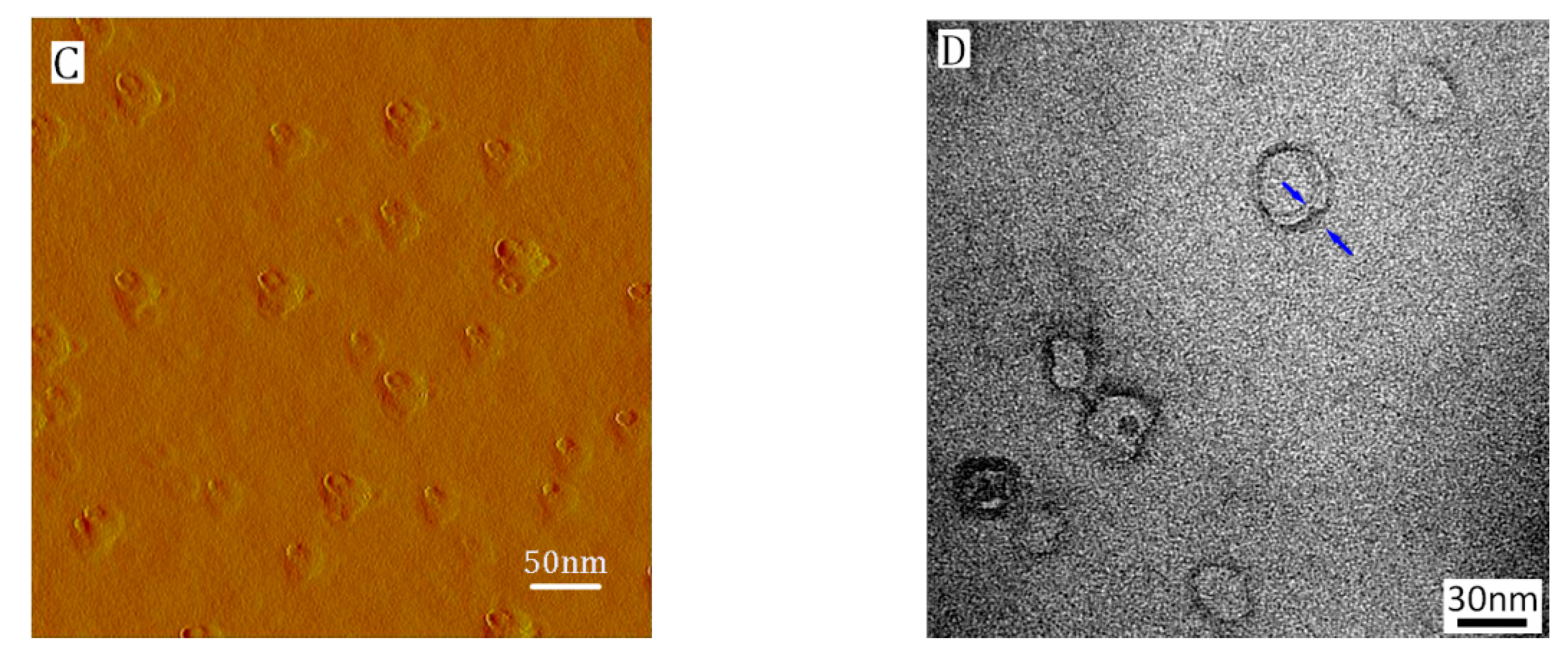
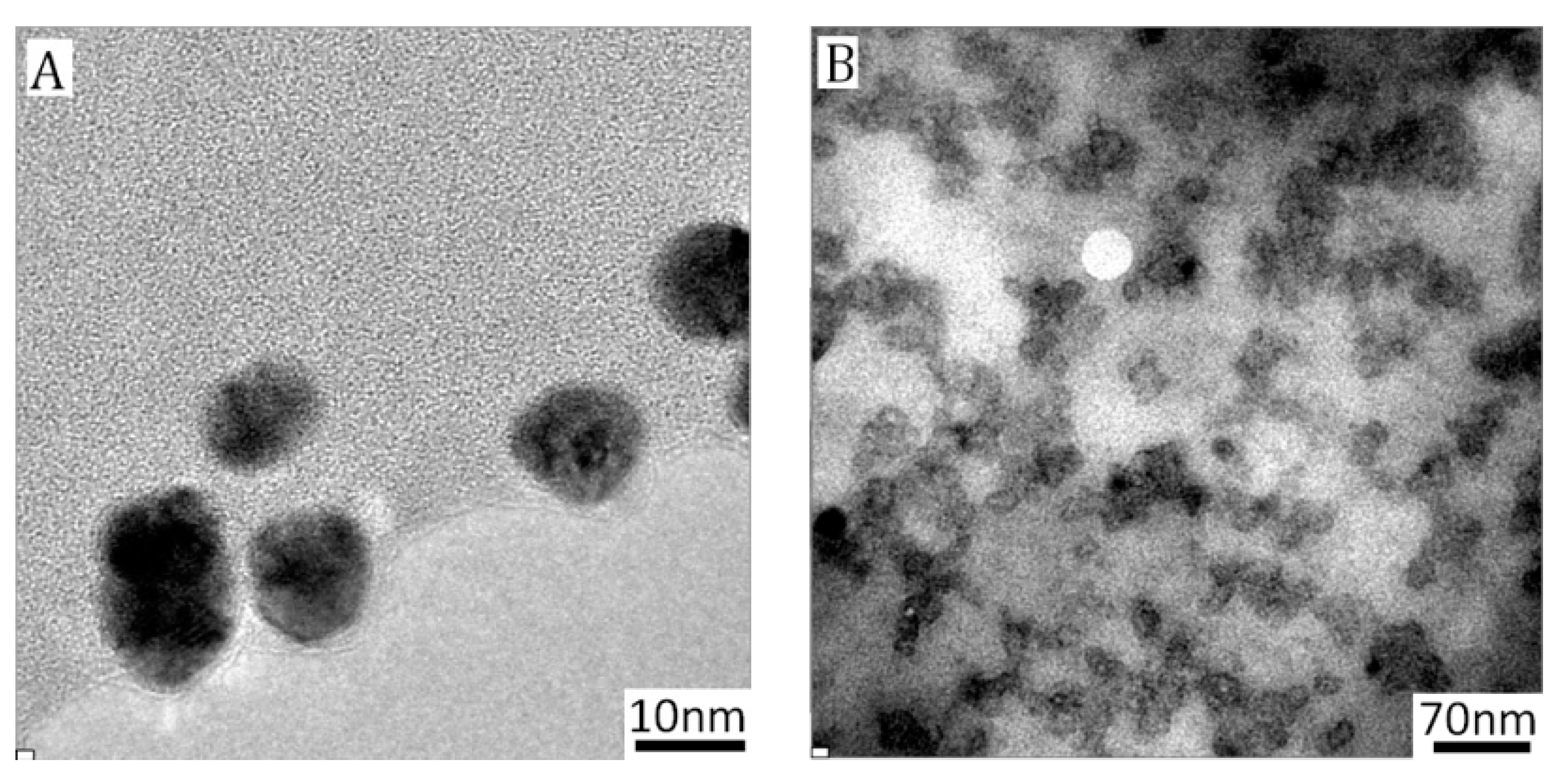
3.1. Characterization of PICs-@-Au NPs
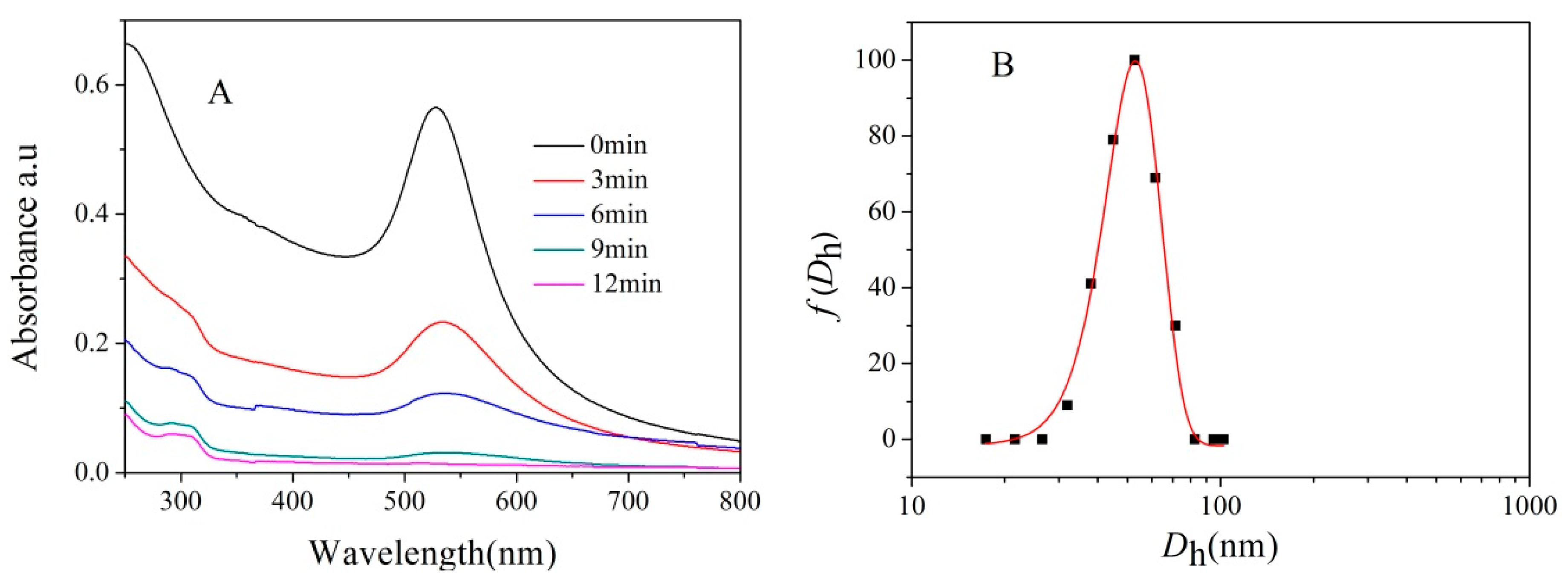
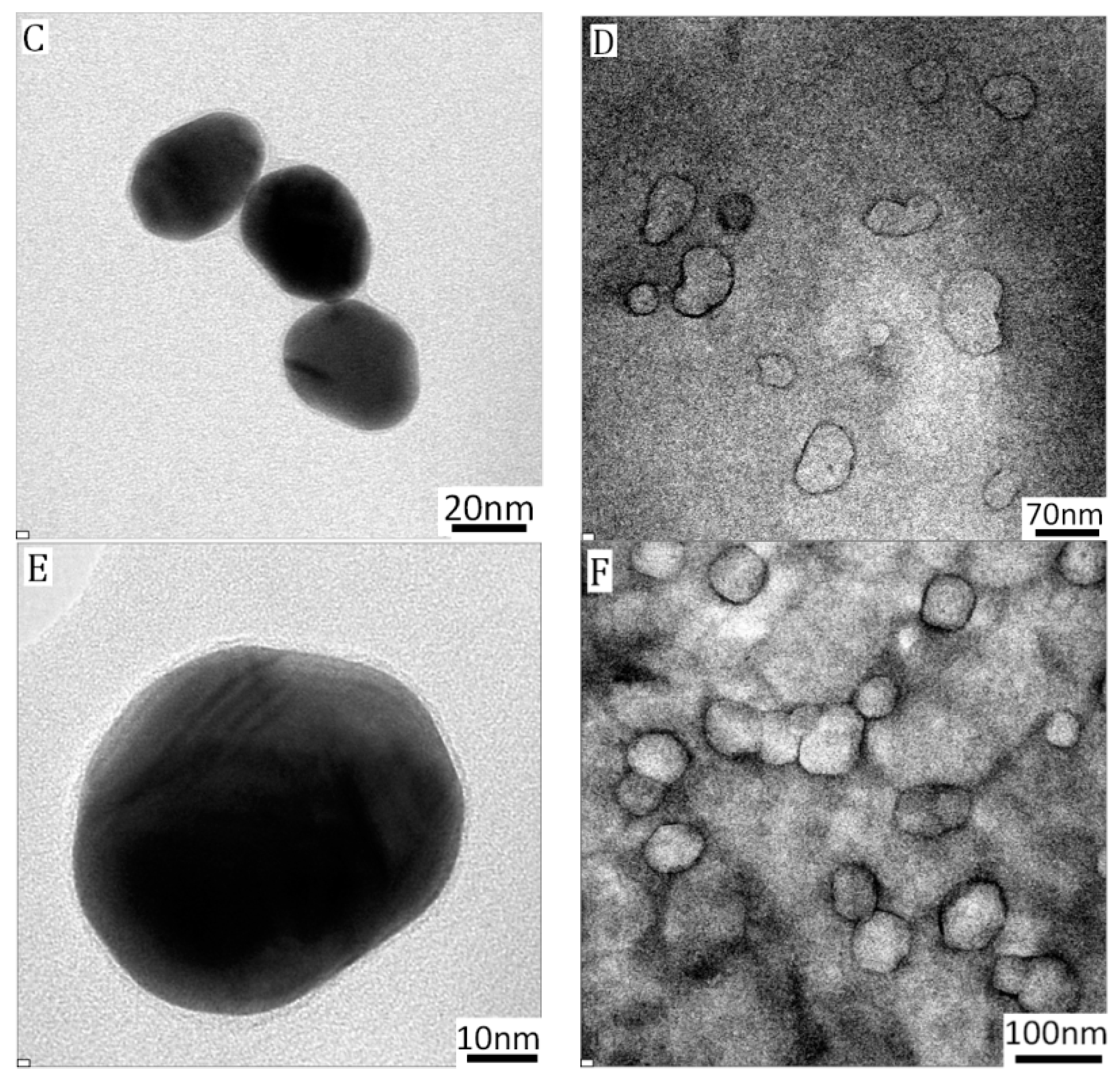
3.2. Formation of Asymmetric PICsomes
3.2.1. Size Control of Asymmetric PICsomes
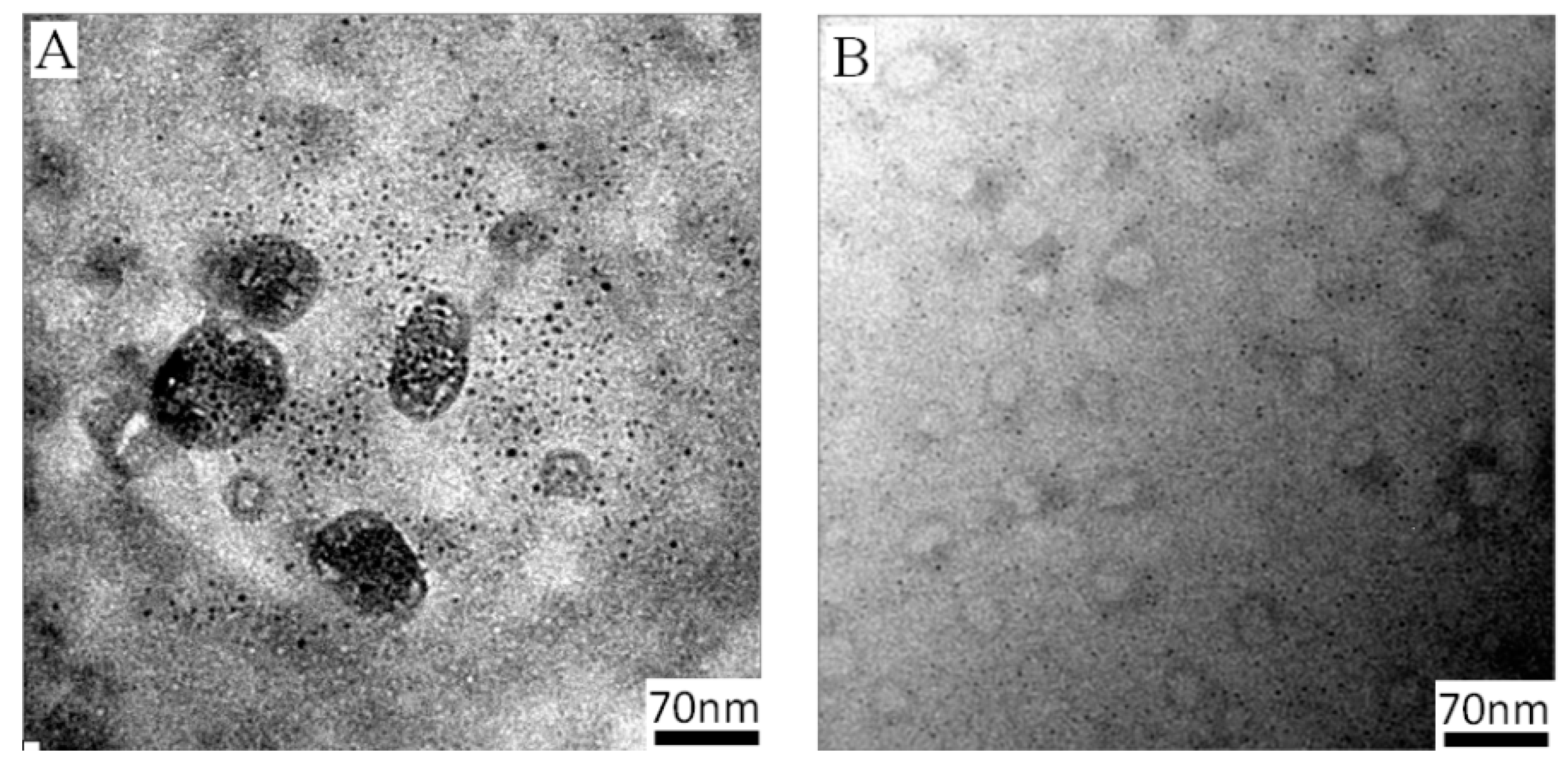
3.2.2. Thermoresponsive Permeability of Asymmetric PICsomes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamiya, K.; Kawano, R.; Osaki, T.; Akiyoshi, K.; Takeuchi, S. Cell-Sized Asymmetric Lipid Vesicles Facilitate the Investigation of Asymmetric Membranes. Nat. Chem. 2016, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kim, S.H.; Weitz, D.A. Photo- and Thermoresponsive Polymersomes for Triggered Release. Angew. Chem. Int. Ed. 2012, 124, 12667–12671. [Google Scholar] [CrossRef]
- Li, B.; Qi, Y.; He, S.S.; Wang, Y.; Xie, Z.; Jing, X.; Huang, Y. Asymmetric Copolymer Vesicles to Serve as a Hemoglobin Vector for Ischemia Therapy. Biomater. Sci. 2014, 2, 1254–1261. [Google Scholar] [CrossRef]
- Cui, J.; Han, Y.; Jiang, W. Asymmetric Vesicle Constructed by AB/CB Diblock Copolymer Mixture and Its Behavior: A Monte Carlo Study. Langmuir 2014, 30, 9219–9227. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Han, Y.; Jiang, W. Structural transformation of vesicles formed by a polystyrene-b-poly(acrylic acid)/polystyrene-b-poly(4-vinyl pyridine) mixture: from symmetric to asymmetric membranes. Soft Matter 2017, 13, 2634. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A. Development of polyion complex vesicles (PICsomes) from block copolymers for biomedical applications. Polym. J. 2013, 45, 892–897. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, S.; Chen, J.; Du, J. An Asymmetrical Polymer Vesicle Strategy for Significantly Improving T1 MRI Sensitivity and Cancer-Targeted Drug Delivery. Nanomed. Nanotechnol. 2016, 12, 483. [Google Scholar] [CrossRef]
- Massignani, M.; Lomas, H.; Battaglia, G. Polymersomes: A Synthetic Biological Approach to Encapsulation and Delivery. Adv. Polym. Sci. 2010, 229, 115–154. [Google Scholar]
- Du, Y.; Chen, W.; Zheng, M.; Meng, F.; Zhong, Z. pH-Sensitive Degradable Chimaeric Polymersomes for the Intracellular Release of Doxorubicin Hydrochloride. Biomaterials 2012, 33, 7291–7299. [Google Scholar] [CrossRef] [PubMed]
- Njikang, G.; Han, D.; Wang, J.; Liu, G. ABC Triblock Copolymer Micelle-Like Aggregates in Selective Solvents for A and C. Macromolecules 2008, 41, 9727–9735. [Google Scholar] [CrossRef]
- Liu, F.; Eisenberg, A. Preparation and pH Triggered Inversion of Vesicles from Poly(acrylic Acid)-block-Polystyrene-block-Poly(4-vinyl Pyridine). J. Am. Chem. Soc. 2003, 125, 15059. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; He, X.; Gao, C.; Khan, H.; Shi, P.; Zhang, W. Asymmetrical vesicles: convenient in situ RAFT synthesis and controllable structure determination. Polym. Chem. 2015, 6, 6563–6572. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Kamiya, M.; Tanaka, S.; Nomoto, T.; Toh, K.; Matsumoto, Y.; Fukushima, S.; Sueyoshi, D.; Kano, M.R. Systemically Injectable Enzyme-Loaded Polyion Complex Vesicles as in Vivo Nanoreactors Functioning in Tumors. Angew. Chem. Int. Ed. 2016, 55, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Asano, I.; So, S.; Lodge, T.P. Oil-in-Oil Emulsions Stabilized by Asymmetric Polymersomes Formed by AC + BC Block Polymer Co-Assembly. J. Am. Chem.Soc. 2016, 138, 4714–4717. [Google Scholar] [CrossRef] [PubMed]
- Gittins, D.I.; Caruso, F. Multilayered Polymer Nanocapsules Derived from Gold Nanoparticle Templates. Adv. Mater. 2000, 12, 1947–1949. [Google Scholar] [CrossRef]
- Kékicheff, P.; Schneider, G.F.; Decher, G. Size-Controlled Polyelectrolyte Complexes: Direct Measurement of the Balance of Forces Involved in the Triggered Collapse of Layer-by-Layer Assembled Nanocapsules. Langmuir 2013, 29, 10713–10726. [Google Scholar] [CrossRef] [PubMed]
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric Multilayer Capsules in Drug Delivery. Angew. Chem. Int. Ed. 2010, 49, 6954. [Google Scholar]
- Choi, I.; Malak, S.T.; Xu, W.; Heller, W.T.; Tsitsilianis, C.; Tsukruk, V.V. Multicompartmental Microcapsules from Star Copolymer Micelles. Macromolecules 2013, 46, 1425–1436. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, L.; Anraku, Y.; Mi, P.; Liu, X.; Cabral, H.; Inoue, A.; Nomoto, T.; Kishimura, A.; Nishiyama, N. Polyion complex vesicles for photo-induced intracellular delivery of amphiphilic photosensitizer. J. Am. Chem. Soc. 2014, 136, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Yen, H.C.; Anraku, Y.; Fukushima, S.; Lai, P.S.; Kato, M.; Kishimura, A.; Kataoka, K. Facile Preparation of Delivery Platform of Water-Soluble Low-Molecular-Weight Drugs Based on Polyion Complex Vesicle (PICsome) Encapsulating Mesoporous Silica Nanoparticle. ACS Biomater. Sci. Eng. 2017, 3, 807–815. [Google Scholar] [CrossRef]
- Koide, A.; Kishimura, A.; Osada, K.; Jang, W.D.; Yamasaki, Y.; Kataoka, K. Semipermeable Polymer Vesicle (PICsome) Self-Assembled in Aqueous Medium from a Pair of Oppositely Charged Block Copolymers: Physiologically Stable Micro-/Nanocontainers of Water-Soluble Macromolecules. J. Am. Chem.Soc. 2006, 128, 5988–5989. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of Myoglobin in PEGylated Polyion Complex Vesicles Made from a Pair of Oppositely Charged Block Ionomers: A Physiologically Available Oxygen Carrier. Angew. Chem. Int. Ed. 2007, 46, 6085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, W.; Han, C.; Zhang, S.; Zhou, H.; Guo, J. Aggregation Behavior of pH- and Thermo-Responsive Block Copolymer Protected Gold Nanoparticles. Colloid Polym. Sci. 2014, 292, 1657–1664. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Wu, W.; Zhang, S.; Zhang, K.; Zhou, H. AuCl4−-Responsive Self-Assembly of Ionic Liquid Block Copolymers for Obtaining Composite Gold Nanoparticles and Polymeric Micelles with Controlled Morphologies. New J. Chem. 2014, 38, 2508–2513. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and Characterization of Au Colloid Monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Luo, S.; Xu, J.; Zhang, Y.; Liu, S.; Wu, C. Double Hydrophilic Block Copolymer Monolayer Protected Hybrid Gold Nanoparticles and Their Shell Cross-Linking. J. Phys. Chem. B 2005, 109, 22159–22166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Zhang, S.J.; Liang, J.; Wu, W.L.; Guo, J.W.; Zhou, H.Y. One-Dimensional Assembly of Polymeric Ionic Liquid Capped Gold Nanoparticles Driven by Electrostatic Dipole Interaction. RSC Adv. 2015, 5, 7994–8001. [Google Scholar] [CrossRef]
- Li, F.; De Haan, L.H.J.; Marcelis, A.T.M.; Leermakers, F.A.M.; Cohen Stuart, M.A.; Sudhölter, E.J.R. Pluronic Polymersomes Stabilized by Core Cross-Linked Polymer Micelles. Soft Matter 2009, 5, 4042–4046. [Google Scholar] [CrossRef]
- Wu, M.; O’Neill, S.A.; Brousseau, L.C.; Mcconnell, W.P.; Shultz, D.A.; Linderman, R.J.; Feldheim, D.L. Synthesis of Nanometer-Sized Hollow Polymer Capsulesfrom Alkanethiol-Coated Gold Particles. Chem. Commun. 2000, 9, 775–776. [Google Scholar] [CrossRef]
- Smart, T.P.; Fernyhough, C.; Ryan, A.J.; Battaglia, G. Controlling Fusion and Aggregation in Polymersome Dispersions. Macromol. Rapid Commun. 2008, 29, 1855–1860. [Google Scholar] [CrossRef]
- Wu, T.; Ge, Z.; Liu, S. Fabrication of Thermoresponsive Cross-Linked Poly(N-isopropylacrylamide) Nanocapsules and Silver Nanoparticle-Embedded Hybrid Capsules with Controlled Shell Thickness. Chem. Mater. 2011, 23, 2370–2380. [Google Scholar] [CrossRef]
- Li, G.; Qi, M.; Yu, N.N.; Liu, X. Hybrid Vesicles Co-Assembled from Anionic Graft Copolymer and Metal Ions for Controlled Drug Release. Chem. Eng. J. 2015, 262, 710–715. [Google Scholar] [CrossRef]
- Dong, W.F.; Kishimura, A.; Anraku, Y.; Chuanoi, S.; Kataoka, K. Monodispersed Polymeric Nanocapsules: Spontaneous Evolution and Morphology Transition from Reducible Hetero-PEG PICmicelles by Controlled Degradation. J. Am. Chem. Soc. 2009, 131, 3804–3805. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; An, Y.; Lü, J.; Li, J.; Xiong, D.; Shi, L. Novel Structured Composites Formed from Gold Nanoparticles and Diblock Copolymers. Macromol. Rapid Commun. 2010, 28, 1350–1355. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liang, L.; Liang, J.; Wu, W.; Zhou, H.; Guo, J. Constructing Asymmetric Polyion Complex Vesicles via Template Assembling Strategy: Formulation Control and Tunable Permeability. Nanomaterials 2017, 7, 387. https://doi.org/10.3390/nano7110387
Li J, Liang L, Liang J, Wu W, Zhou H, Guo J. Constructing Asymmetric Polyion Complex Vesicles via Template Assembling Strategy: Formulation Control and Tunable Permeability. Nanomaterials. 2017; 7(11):387. https://doi.org/10.3390/nano7110387
Chicago/Turabian StyleLi, Junbo, Lijuan Liang, Ju Liang, Wenlan Wu, Huiyun Zhou, and Jinwu Guo. 2017. "Constructing Asymmetric Polyion Complex Vesicles via Template Assembling Strategy: Formulation Control and Tunable Permeability" Nanomaterials 7, no. 11: 387. https://doi.org/10.3390/nano7110387
APA StyleLi, J., Liang, L., Liang, J., Wu, W., Zhou, H., & Guo, J. (2017). Constructing Asymmetric Polyion Complex Vesicles via Template Assembling Strategy: Formulation Control and Tunable Permeability. Nanomaterials, 7(11), 387. https://doi.org/10.3390/nano7110387




