Nanomaterials for Tissue Engineering In Dentistry
Abstract
:1. Introduction
- prevention of main oral and dental biofilm-dependent diseases, like caries and periodontal diseases, with the addition of antibacterial and antidemineralizing particles in toothpastes, mouthwashes, and composite resins [4,9,10,11,12,13], or of active nanoparticles for remineralization in toothpastes [14,15], composite resins, and dental adhesives [16,17];
- help in diagnosis of malignant and pre-malignant oral diseases with some means, such as contrast particles for CT imaging, like gold nanoparticles (GNPs) [18,19,20]; ”quantum dots”, semi-conductor crystals of nanoscale inserted in diseased tissues that behave like fluorophores when exposed to luminescence NIR (near-infrared) [21]; the “oral fluid nanosensor tests” (OFNASET) for identification of tumoral salivary biomarkers [22]. These last ones are also used in periodontal disease diagnosis, for their capability to identify specific periodontopatogenic bacteria [23], such as “electronic microchip-assays” able to detect C-reactive protein (CRP), a biomarker of the inflammation connected to periodontal disease [24];
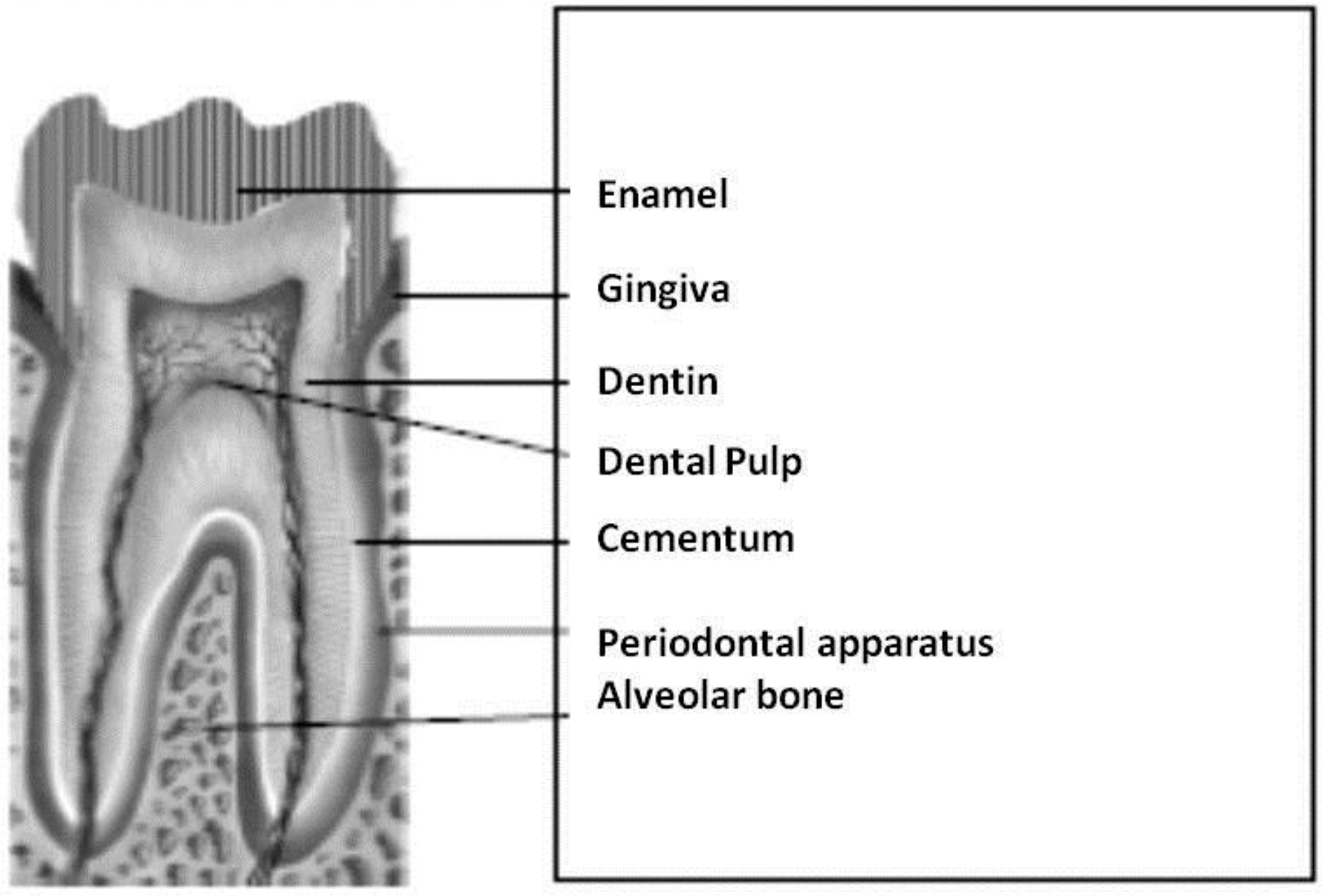
2. Structure and Diseases of Dental and Periodontal Tissues
- damage to the hard tissues of tooth like caries, fractures, cervical erosions, without loss of pulpal functionality [48];
- damage to periodontal complex and alveolar bone from periodontal disease and trauma;
- complete loss of one or more teeth in the most severe forms of these diseases or their absence for agenesis;
- small or medium bone losses by mandibular or maxillary cysts or odontogenic tumors.
3. Stem Cells in Dental and Periodontal Tissues Usable in Dentistry
- Pluripotent cells—called DPPSC (dental pulp pluripotent stem cells), isolated in third molars pulp [64], are potentially useful for regeneration of dental tissues both epithelial (enamel) that are mesenchymal;
- Mesenchymal cells, isolated from the adult pulp (dental pulp stem cells or DPSC) [65] and from the deciduous exfoliated teeth (SHED) [66]; from the apical part of dental papilla (stem cells from apical papilla, or SCAP) [67,68]; from the dental follicle (dental follicle stem cells, or DFSC [69]; or from the PDL (PDLSC) [70];
4. Main Usable Growth Factors and Signaling Molecules
5. The Main Biomaterials Usable to Build Scaffolds/Matrices
- -
- -
- periodontal tissue engineering, like “cell sheet technology”, which consists in a non-invasive approach, using a thermo responsive polymeric material, named poly N-isopropyacrylamide (PIPAAm). A continuous monolayer of cells and ECM components (plated on PIPAAm surface) can be obtained with a slight decrease of temperature [112,113].
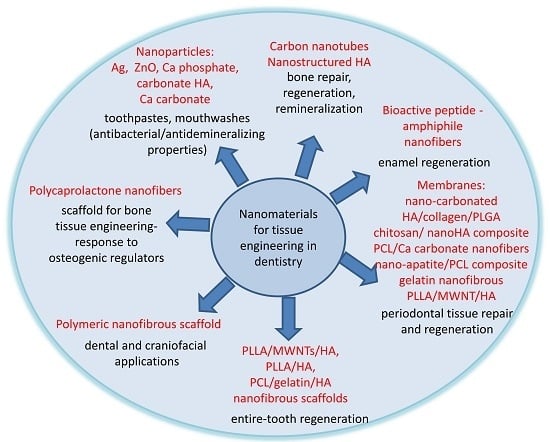
6. Nanostructured Materials in Use for Tissue Engineering in Dentistry
6.1. Nanoparticles that Offer to the Tissue the Use of Their Chemical Components and Their Bioactivity
6.2. Nanostructured Materials for Drug or Signaling Molecules Delivery
6.3. Nanostructured Materials for Build Scaffolds in Dentistry
7. Experimental Studies in Dentistry with the Use of Nanomaterials
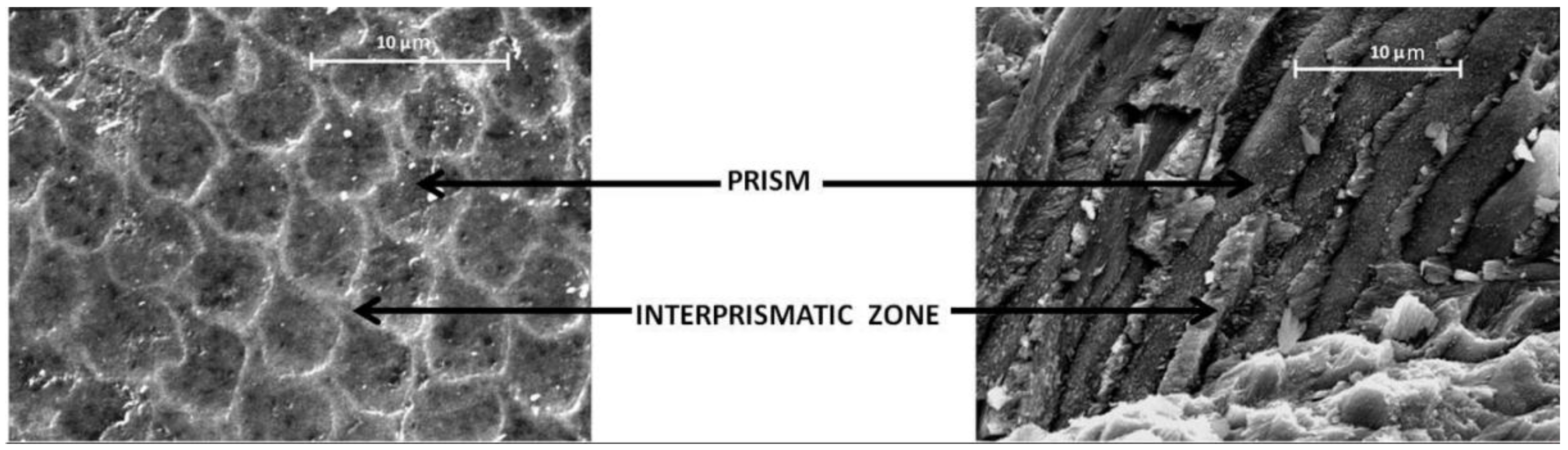
7.1. Enamel
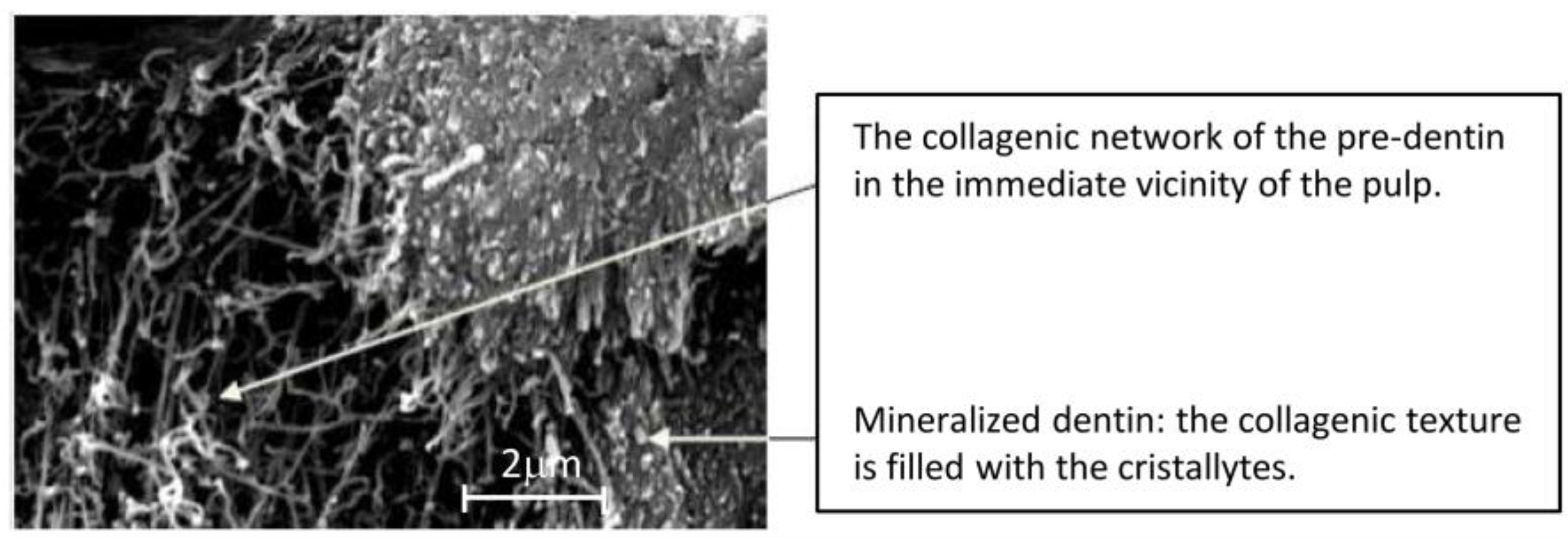
7.2. Pulpodentinal Complex
7.3. Periodontal Apparatus
7.4. Entire Tooth
8. Summary
9. Conclusions
Conflicts of Interest
Abbreviations
| TE | Tissue Engineering |
| NM | Nanomaterials |
| NIR | Near-infrared |
| GNPs | gold nanoparticles |
| OFNASET | oral fluid nanosensor tests |
| CRP | C reactive protein |
| GTR | tissue guided regeneration |
| EDJ | Enamel-Dentin Junction |
| ERM | Epithelial Rests of Malassez |
| DEJ | dentin-enamel junction |
| HA | hydroxyapatite |
| PDL | periodontal ligament |
| DPSC | dental pulp stem cells |
| SCAP | Stem cells from apical papilla |
| DFSC | dental follicle stem cells |
| BFGF | basic Fibroblast growth factor |
| PDGF | Platelet derived growth factor |
| SCF | stem cells factor |
| G-CSF | Granulocyte colony-stimulating factor |
| CaP | calcium phosphate |
| ACP | amorphous CaP |
| PLA | Polyactic acid |
| PGA | Polyglycolic acid |
| PLGA | PolylactideCo-glycolide |
| PCL | Poly-caprolactone |
| PEG | polyethylene glycol |
| nano-HA | nanostructured hydroxyapatite |
| GTR | Guided Tissue Regeneration |
| GBR | Guided Bone Regeneration |
| FGMs | functionally graded periodontal membranes |
| MWNTs | multi-walled carbon nanotubes |
| MET | metronidazole |
| PDL | Periodontal ligament |
| PLCL | polyactidecaprolactone |
| FDM | Fused Deposition Modelling |
References
- Mitziadis, T.A.; Woloszyk, A.; Jimenez-Rojo, L. Nanodentistry: combining nanostructured materials and stem cells for dental tissue regeneration. Nanomedicine 2012, 7, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Ozak, S.T.; Ozkan, P. Nanotechnology and dentistry. Eur. J. Dent. 2013, 7, 145–151. [Google Scholar] [PubMed]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of Nanomaterials in Dentistry: Interactions with the Oral Microenvironment, Clinical Applications, Hazards, and Benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.A. Nanodentistry. J. Am. Dent. Assoc. 2000, 131, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, T.L. Nanodentistry: Fact or fiction? J. Am. Dent. Assoc. 2000, 131, 1567–1568. [Google Scholar] [CrossRef] [PubMed]
- Bhavikatti, S.K.; Bhardwaj, S.; Prabhuji, M.L.V. Current applications of nanotechnology in dentistry: A review. General Dent. 2014, 62, 72–77. [Google Scholar]
- Neel, E.A.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; Alikhani, M.Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.; Xu, H.H. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, P.B.; Agnelli, J.A.; Kurachi, C.; de Souza, C.W. Addition of silver nanoparticles to composite resin: effect on physical and bactericidal properties in vitro. Braz. Dent. J. 2014, 25, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Roveri, N.; Foresti, E.; Lelli, M.; Foresti, E.; Iafisco, M.; Lelli, M.; Palazzo, B.; Rimondini, L. Synthetic biomimetic carbonate hydroxyapatite nanocrystals for enamel remineralization. Adv. Mater. Res. 2008, 4, 821–824. [Google Scholar] [CrossRef]
- Nakashima, S.; Yoshie, M.; Sano, H.; Bahar, A. Effect of a test dentifrice containing nano-sized calcium carbonate on remineralization of enamel lesions in vitro. J. Oral. Sci. 2009, 51, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Weir, M.D.; Sun, L.; Takagi, S.; Chow, L.C. Effects of calcium phosphate nanoparticles on CaPO4 composite. J. Dent. Res. 2007, 86, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Weir, M.D.; Sun, L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent. Mater. 2009, 25, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, T.; Motiei, M.; Romman, Z.; Popovtzer, A.; Popovtzer, R. Targeted gold nanoparticles enable molecular CT imaging of cancer: An in vivo study. Int. J. Nanomed. 2011, 6, 2859–2864. [Google Scholar]
- Chalmers, N.I.; Palmer, R.J.; Du-Thumm, L.; Sullivan, R.; Shi, W.; Kolenbrander, P.E. Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Appl. Environ. Microbiol. 2007, 73, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gau, V.; Wong, D. Oral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platform. Ann. N. Y. Acad. Sci. 2007, 1098, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Yakob, M.; Wong, D.T.W. Emerging horizons of salivary diagnostics for periodontal disease. Br. Dent. J. 2014, 217, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.; Mohanty, S.; Miller, C.S.; Langub, M.C.; Floriano, P.N.; Dharshan, P.; Ali, M.F.; Bernard, B.; Romanovicz, D.; Anslyn, E.; et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab. Chip. 2005, 5, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Sennerby, L.; Wennerberg, A. State of the art of oral implants. Periodontol 2008, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Louarn, G.; Layrolle, P. Nanotechnology and dental implants. Int. J. Biomater. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Biofunctionalization of titanium for dental implant. Jpn. Dent. Sci. Rev. 2010, 46, 93–101. [Google Scholar] [CrossRef]
- Ballo, A.; Agheli, H.; Lausmaa, J.; Thomsen, P.; Petronis, S. Nanostructured model implants for in vivo studies: influence of well-defined nanotopography on de novo bone formation on titanium implants. Int. J. Nanomed. 2011, 6, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S.; et al. Nanostructured surfaces of dental implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Thakral, G.; Thakral, R.; Sharma, N.; Seth, J.; Vashisht, P. Nanosurface—The future of implants. J. Clin. Diagn. Res. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S.; Gottlow, J.; Karring, T.; Lindhe, J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J. Clin. Periodontol. 1982, 9, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mat. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Sam, G.; Pillai, B.R.M. Evolution of Barrier Membranes in Periodontal Regeneration—“Are the third Generation Membranes really here?”. J. Clin. Diagn. Res. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Matras, H. The use of fibrin sealant in oral and maxillofacial surgery. J. Oral. Maxillofac. Surg. 1982, 40, 617–622. [Google Scholar] [CrossRef]
- Roselló-Camps, À.; Monje, A.; Lin, G.; Khoshkam, V.; Chávez-Gatty, M.; Wang, H.L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. Platelet-rich plasma for periodontal regeneration in the treatment of intrabony defects: A meta-analysis on prospective clinical trials. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2015, 120, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.J.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Della Bona, A.; Cavalcanti, B.N.; Nör, J.E. Tissue engineering: from research to dental clinics. Dent. Mater. 2012, 28, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology. In Development, Structure, and Function, 8th ed.; Elsevier-Mosby: St. Louis, MO, USA, 2012. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Robinson, C.; Kirkham, J.; Brookes, S.J.; Shore, R.C. Chemistry of mature enamel. In Dental Enamel: From Formation to Destruction; Robinson, C., Kirkham, J., Shore, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 167–191. [Google Scholar]
- Piesco, N.P.; Simmelink, J. Histology of Enamel. In Oral Development and Histology; Avery, J.K., Ed.; Georg Thieme Verlag: New York, NY, USA, 2002. [Google Scholar]
- Mjör, I.; Fejerskov, O. Human Oral Embryology and Histology; Munksgaard: Copenhagen, Denmark, 1986. [Google Scholar]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine; Karger: Basel, Switzerland, 1991. [Google Scholar]
- Moreno, E.C.; Aoba, T. Solubility of human enamel mineral. J. Biol. Buccale 1990, 18, 195–203. [Google Scholar] [PubMed]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25 and 37 °C. J. Res. Natl. Bur. Stds. 1977, 81, 273–278. [Google Scholar] [CrossRef]
- Patel, P.; Brown, W. Thermodynamic solubility product of human tooth enamel powdered samples. J. Dent. Res. 1975, 54, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and its Clinical Management, 3rd ed.; Wiley-Blackwell: Oxford, UK, 2015. [Google Scholar]
- Conversini, A.; Eramo, S.; Manna, M.G.; Negri, P. Dental pulp diseases. Ultramicroscopic studies. Minerva Stomatol. 1999, 47, 631–648. [Google Scholar]
- Eramo, S.; Baldi, M.; Marci, M.C.; Monaco, A. Histopathological and therapeutical aspects of cervical lesions. Minerva Stomatol. 2002, 52, 69–74. [Google Scholar]
- Gong, T.; Heng, B.C.; Lo, E.C.M.; Zhang, C. Current Advance and Future Prospects of Tissue Engineering Approach to Dentin/Pulp Regenerative Therapy. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.N.; Mar, K.; Chang, H.C.; Chiang, Y.L.; Hu, H.Y.; Lai, C.C.; Chu, R.M.; Ma, C.M. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 2011, 32, 6995–7005. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Robey, P.G.; Gronthos, S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplant. 2015, 24, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induced pluripotent stem cells: past, present, and future. Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hynes, K.; Gronthos, S.; Bartold, P.M. iPSC for dental tissue regeneration. Curr. Oral Health Rep. 2014, 1, 9–15. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T.J. iPS cells reprogrammed from human mesenchymal like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Kishigami, R.; Oikawa-Sasaki, A.; Fukumoto, S.; Yamada, A.; Fujiwara, N.; Ishizeki, K.; Harada, H. Differentiation of induced pluripotent stem cells into dental mesenchymal cells. Stem Cells Dev. 2012, 21, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, F.; Zhang, W.; Li, Y.; Yu, M.; Nan, X.; Chen, L.; Yue, W.; Xu, X.; Pei, X. Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Eng. Part A 2012, 18, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Dziubińska, P.; Jaskólska, M.; Przyborowska, P.; Adamiak, Z. Stem cells in dentistry—Review of literature. Polish J. Vet. Sci. 2013, 16, 135–140. [Google Scholar] [CrossRef]
- Silva, L. Stem Cells in the Oral Cavity. Glob. J. Stem Cell Biol. Transplant. 2015, 1, 12–16. [Google Scholar]
- Atari, M.; Gil-Recio, C.; Fabregat, M.; García-Fernández, D.; Barajas, M.; Carrasco, M.A.; Jung, H.S.; Alfaro, F.H.; Casals, N.; Prosper, F.; et al. Dental pulp of third molar: a new source of pluripotent-like stem cells. J. Cell. Sci. 2012, 125, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.; Shi, S. Postnatal human dental pulp stem cell (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.; Robey, P. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J.; et al. Characterization of the apical papillla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Saito, M.; Yamauchi, M.; Kiyono, T.; Sato, S.; Teranaka, T.; Narayanan, A.S. Cementum matrix formation in vivo by cultured dental follicle cells. Bone 2002, 31, 606–611. [Google Scholar] [CrossRef]
- Seo, B.; Miura, M.; Sonoyama, W.; Coppe, C.; Stanyon, R.; Shi, S. Recovery of stem cells from cryopreserved periodontal ligament. J. Dent. Res. 2005, 84, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.J.; Tsuchiya, S.; Sumita, Y.; Sagara, H.; Ueda, M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials 2007, 28, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Lee, G. Identification of novel epithelial stem cell-like cells in human deciduous dental pulp. Biochem. Biophys. Res. Comm. 2009, 386, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, Y.; Tsuchiya, S.; Hata, K.; Honda, M.J. Quiescent epithelial cell rests of Malassez can differenziate into ameloblast-like cells. J. Cell. Physiol. 2008, 217, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, J.; Park, J.; Park, J.C.; Kim, J.W.; Seo, B.M.; Lee, J.C.; Lee, G. Expression profile of the stem cell markers in human Hertwig's epithelial root sheath/Epithelial rests of Malassez cells. Mol. Cells 2011, 31, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Murakami, S.; Beirne, O.R. The use of biologic mediators and tissue engineering in dentistry. Periodontology 2009, 50, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.B.; Morris, K.H. A systematic review of the use of growth factors in human periodontal regeneration. J. Periodontol. 2013, 84, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Bartolommei, L.; Galosi, C.; Renga, G.; Oikonomou, V.; Zamparini, F.; Ricci, G.; Borghi, M.; Puccetti, M.; Piobbico, D.; et al. Fine-tuning of Th17 Cytokines in Periodontal Disease by IL-10. J. Dent. Res. 2015, 94, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodont. Res. 2016, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Kim, S.G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative endodontics: barriers and strategies for clinical translation. Dent. Clin. N. Am. 2012, 56, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, G.; Chen, Z. Pulp Regeneration: Current Approaches and Future Challenges. Front Physiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Archs. Oral. Biol. 1994, 39, 1085–1089. [Google Scholar] [CrossRef]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nor, J.E. Dentin-derived BMP-2 and odontoblast differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Mizutani, S.; Nakano, M.; Hirukawa, K.; Togari, A. FGF-2 regulates enamel and dentine formation in mouse tooth germ. Calcif. Tissue Int. 2003, 73, 496–501. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yu, J.; Liu, Y.; Lu, S.; Liu, H.; Shi, J.; Jin, Y. Effects of FGF2 and TGFβ1 on the differentiation of human dental pulp stem cells in vitro. Cell. Biol. Int. 2008, 32, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Mullane, E.M.; Dong, Z.; Sedgley, C.M.; Hu, J.C.; Botero, T.M.; Holland, G.R.; Nör, J.E. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J. Dent. Res. 2008, 87, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic properties of human dental pulp stem cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.S.; Merchant, Y.; Nagarajan, P. Tissue Engineering of Craniofacial Tissues—A Review. J. Reg. Med. Tissue Eng. 2013, 2. [Google Scholar] [CrossRef]
- Sharma, S.; Srivastava, D.; Grover, S.; Sharma, V. Biomaterials in Tooth Tissue Engineering: A Review. J. Clin. Diag. Res. 2014, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Leong, N.L.; Jiang, J.; Lu, H.H. Polymer-ceramic composite scaffold induces osteogenic differentiation of human mesenchymal stem cells. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 1, 2651–2654. [Google Scholar] [PubMed]
- Chakkalakal, D.A.; Strates, B.S.; Garvin, K.L.; Novak, J.R.; Fritz, E.D.; Mollner, T.J.; McGuire, M.H. Demineralized bone matrix as a biological scaffold for bone repair. Tissue Eng. 2001, 7, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin. Orthop. Relat. Res. 1998, 355, S239–S246. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Ribeiro, A.S.; Malafaya, P.B.; Reis, R.L.; Cunha, A.M. A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: Morphology, mechanical and degradation behaviour. Biomaterials 2001, 22, 883–889. [Google Scholar] [CrossRef]
- Sartoris, D.J.; Gershuni, D.H.; Akeson, W.H.; Holmes, R.E.; Resnick, D. Coralline hydroxyapatite bone graft substitutes: Preliminary report of radiographic evaluation. Radiology 1986, 159, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Goshima, J.; Goldberg, V.M.; Caplan, A.I. The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin. Orthop. Relat. Res. 1991, 262, 298–311. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cell. Mater. 2010, 20, 1–12. [Google Scholar] [PubMed]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta. Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Sariibrahimoglu, K.; Wolke, J.G.; Leeuwenburgh, S.C.; Yubao, L.; Jansen, J.A. Injectable biphasic calcium phosphate cements as a potential bonesubstitute. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Wright, A.J.; Gbureck, U.; Bolarinwa, A.; Song, J.; Liu, Y.; Farrar, D.F.; Howling, G.; Rose, J.; Barralet, J.E. The effect of amorphous pyrophosphate on calcium phosphate cement resorption and bone generation. Biomaterials 2013, 34, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanicalproperties. Acta. Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun Fibers for Dental and Craniofacial Applications. Curr. Stem Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Period. Implant. Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C 2014, 45, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Park, S.H.; Park, J.H.; Lee, B.K.; Yun, J.H.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. In Vivo Osteogenic Differentiation of Human Dental Pulp Stem Cells Embedded in an Injectable In Vivo-Forming Hydrogel. Macromol. Biosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.W. Core–shell designed scaffolds for drug delivery and tissue engineering. Acta. Biomate. 2015, 21, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 8, 590–595. [Google Scholar] [CrossRef]
- Syed-Picard, F.N.; Ray, H.L., Jr.; Kumta, P.N.; Sfeir, C. Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J. Dent. Res. 2014, 93, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Int. Med. 2010, 267, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Utoh, R.; Nagase, K.; Okano, T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 2014, 190, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, H.J.; Ko, J.S.; Choi, Y.S.; Ahn, M.W.; Kim, S.; Do, S.H. Comparative characteristics of porous bioceramics for an osteogenic response in vitro and in vivo. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kandori, K.; Kuroda, T.; Togashi, S.; Katayama, E. Preparation of calcium hydroxyapatite nanoparticles using microreactor and their characteristics of protein adsorption. J. Phys. Chem. B. 2011, 115, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta. Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Webster, T.J. Nanotechnology and biomaterials for orthopedic medical applications. Nanomedicine (Lond.) 2006, 1, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Deng, C.; Chen, X.; Zhao, X.; Chen, Y.; Fan, Y.; Zhang, X. Mechanical and biological properties of the micro-/nano-grain functionally graded hydroxyapatite bioceramics for bone tissue engineering. J. Mechan. Behav. Biomed. Mater. 2015, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Bracagalia, L.; Thompson, J.A.; Fisher, J.P. Hydroxyapatite doped alginate beads as scaffolds for the osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2016. [Google Scholar] [CrossRef] [PubMed]
- Talal, A.; McKay, I.J.; Tanner, K.E.; Hughes, F.J. Effects of hydroxyapatite and PDGF concentrations on osteoblast growth in a nanohydroxyapatite-polylactic acid composite for guided tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, R.; Mukudai, Y.; Yoshizawa, Y.; Nagasaki, M.; Shiogama, S.; Suzuki, M.; Kondo, S.; Shintani, S.; Shirota, T. A Combination of Low-Intensity Pulsed Ultrasound and Nanohydroxyapatite Concordantly Enhances Osteogenesis of Adipose-Derived Stem Cells From Buccal Fat Pad. Cell Med. 2015, 7, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Martin, C. Smart nanotubes for biomedical and biotechnological applications. Drug News Perspect. 2003, 16, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Martins-Júnior, P.A.; Alcântara, C.E.; Resende, R.R.; Ferreira, A.J. Carbon nanotubes: Directions and perspectives in oral regenerative medicine. J. Dent. Res. 2013, 92, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.J.; Ma, P.X. Nanofibrous Scaffolds for Dental and Craniofacial Applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Binulal, N.; Deepthy, M.; Selvamurugan, N.; Shalumon, K.; Suja, S.; Mony, U. Role of nanofibrous poly(caprolactone) scaffolds in human mesenchymal stem cell attachment and spreading for in vitro bone tissue engineering-response to osteogenic regulators. Tissue Eng. Part A 2010, 16, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug. Del. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, L.; Hu, J.; Ma, P. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.J.; Hata, K. Enamel Tissue Engineering; Eberli, D., Ed.; INTECH Publisher: Rijeka, Croatia, 2010. [Google Scholar]
- Jayasudha, B.; Baswara, J.; Navin, H.K.; Prasanna, K.B. Enamel Regeneration–Current Progress and Challenges. J. Clin. Diag. Res. 2014, 8, E06–E09. [Google Scholar]
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interf. Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yun, S.; Fang, J.; Chen, H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem. Commun. 2009, 39, 5892–5894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, D.; Zhang, J.; Lin, Q.; Huang, Z. Synthesis of Dental Enamel-like Hydroxyapaptite through Solution Mediated Solid-State Conversion. Langmuir 2010, 26, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sargeant, T.D.; Hulvat, J.F.; Mata, A.; Bringas, P., Jr.; Ko, C.Y. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J. Bone Miner. Res. 2008, 23, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Newcomb, C.J.; Zhou, Y.; Lei, Y.P.; Bringas, P., Jr.; Stupp, S.I.; Snead, M.L. The role of bioactive nanofibers in enamel regeneration mediated through integrin signals acting upon C/EBPα and c-Jun. Biomaterials 2013, 34, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A.J. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit. Rev. Oral. Biol. 2004, 15, 13–27. [Google Scholar] [CrossRef]
- Yu, J.H.; Deng, Z.H.; Shi, J.N.; Zhai, H.; Nie, X.; Zhuang, H.; Li, Y.; Jin, Y. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006, 12, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.S.; Alsanea, R.; Tayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; John, A.S.; George, A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, dentin matrix protein 1 transplantation in mice. J. Endodont. 2008, 34, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.H.; He, Y.; Zhang, X.J.; Lu, W.; Wang, C.; Yu, H.; Liu, Y.; Li, Y.; Zhou, Y.; Zhou, J.; et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009, 30, 6708–6723. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, H.; Kitamura, C.; Morotomi, T.; Tabata, Y.; Nishihara, T.; Chen, K.K.; Terashita, M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatine hydrogels. J. Endodont. 2009, 35, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, W.H.; Yang, B.; Guo, L.J.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F. Stem Cells and Regeneration of the Pulpodentin Complex; Quintessence Publishing Co Inc.: Hanover Park, IL, USA, 2012. [Google Scholar]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Liu, Y.; Huang, G.T.J.; Zhang, C. Immunohistochemical and histochemical analysis of newly formed tissues in root canal space transplanted with dental pulp stem cells plus platelet-rich plasma. J. Endod. 2014, 40, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Dejou, J.; About, I. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J. Endodod. 2014, 40 (Suppl. 4), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Jing, J.; Ren, Y.; Ma, C.; Feng, J.Q.; Yu, Q.; Liu, X. Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix. Acta. Biomater. 2015, 16, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells andendothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [PubMed]
- El-Sayed, F.; Jakusz, K.M.; Jochens, K.; Dörfer, A.; Schwendicke, F. Stem Cell transplantation for pulpal regeneration: A systematic review. Tissue Eng. Part B 2015, 21, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zhang, Y.F.; Wan, C.Y.; Sun, Z.Y.; Nie, S.; Jian, S.J.; Zhang, L.; Song, G.T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative Endodontics: Barriers and Strategies for Clinical Translation. Dent. Clin. N. Am. 2012, 56, 639–664. [Google Scholar]
- Lin, Z.; Rios, H.F.; Cochran, D.L. Emerging Regenerative Approaches for Periodontal Reconstruction: A Systematic Review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. 2), S134–S152. [Google Scholar] [CrossRef] [PubMed]
- Aaboe, M.; Pinholt, E.M.; Hjorting-Hansen, E. Healing of experimentally created defects: A review. Br. J. Oral. Maxillofac. Surg. 1995, 33, 312–318. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; MacNeil, R.L. Guided tissue regeneration. Absorbable barriers. Dent. Clin. N. Am. 1998, 42, 505–522. [Google Scholar] [PubMed]
- Schmidmaier, G.; Baehr, K.; Mohr, S.; Kretschmar, M.; Beck, S.; Wildemann, B. Biodegradable polylactide membranes for bone defect coverage: Biocompatibility testing, radiological and histological evaluation in a sheep model. Clin. Oral. Implants Res. 2006, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.X.; Peng, Z.; Li, S.D.; Bartold, P.M. Nanotechnology and its role in the management of periodontal diseases. Periodontology 2006, 40, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Kaur, H.; Jain, S.; Kapoor, D.; Nanda, T.; Jain, M. Comparison of nano-sized hydroxyapatite and b tricalcium phosphate in the treatment of human periodontal intrabony defects. J. Clin. Diag. Res. 2014, 8, ZC74–ZC78. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Watari, F.; Zhu, Y.; Uo, M.; Akasaka, T.; Wang, W.; Xu, G.; Cui, F. The degradation of the three layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane in vitro. Dent. Mater. 2007, 23, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wang, W.; Uo, M.; Ohkawa, S.; Akasaka, T.; Tamura, K.; Cui, F.; Watari, F. A three-layered nano-carbonated hydroxyapatite–collagen–PLGA composite membrane for guided tissue regeneration. Biomaterials 2005, 26, 7564–7571. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, Y.; Cheng, X.; Yang, W.; Wang, H.; Li, Y. Preparation and characterization of nano hydroxyapatite/polyamide 66 composite GBR membrane with asymmetric porous structure. J. Mater. Sci. Mater. Med. 2009, 20, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Lee, E.J.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Oh, J.S. Chitosan/nano-hydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J. Biomed. Mater. Res. A 2009, 88, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate compositenano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta. Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Inanc, B.; Arslan, Y.; Seker, S.; Elcin, A.; Elcin, Y. Periodontal ligament cellular structures engineered with electrospun poly(DL-lactide-coglycolide) nanofibrous membrane scaffolds. J. Biomed. Mater. Res. A 2009, 90, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Yang, F.; Cheng, X.; Walboomers, X.F.; Jansen, J.A. The effect of electrospun fiber alignment on the behavior of rat periodontal ligament cells. Eur. Cells Mater. 2010, 19, 180–192. [Google Scholar]
- Zhang, S.; Huang, Y.; Yang, X.; Mei, F.; Ma, Q.; Chen, G. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatine solution for guided tissue regeneration. J. Biomed. Mater. Res. A 2009, 90, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Li, P. Electrospun PLLA/MWNT/HA Membrane for Guided Tissue Regeneration. Available online: http://acs.omnibooksonline.com/data/papers/2007_D147.pdf (accessed on 19 July 2016).
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta. Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/p eriodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.H.; Sharpe, P.T. Regeneration of teeth using stem cell based tissue engineering. Exp. Opin. Biol. Ther. 2006, 6, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Ide, Y. Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum. Cell. 2007, 20, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, J.; Deng, Z.; Zhuang, H.; Nie, X.; Wang, R.N.; Jin, Y. Cell pellets from dental papillae can reexhibit dental morphogenesis and dentinogenesis. Biochem. Biophys. Res. Commun. 2006, 346, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6419. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.; Mason, J. Gene-enhanced tissue engineering for dental hard tissue regeneration: (2) dentin-pulp and periodontal regeneration. Head Face Med. 2006, 2, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mui, B.; Mehrabzadeh, M. Regeneration of Tissues of the Oral Complex: Current Clinical Trends and Research Advances. J. Can. Dent. Assoc. 2013, 79, d1–d9. [Google Scholar] [PubMed]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Bartlett, J.D.; Yelick, P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Kim, S.W.; Qin, C.; Baba, O.; Butler, W.T.; Taylor, R.R.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Developmental analysis and computer modelling of bioengineered teeth. Arch. Oral. Biol. 2005, 50, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.F.; Huang, A.T.; Chang, H.H.; Lin, F.H.; Chen, S.T.; Chen, R.S.; Chou, C.H.; Lin, H.C.; Chiang, H.; Chen, M.H. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J. Biomed. Mater. Res. A 2008, 86A, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.F.; Lin, H.C.; Yang, K.C.; Lin, F.H.; Chen, M.H.; Wu, C.C.; Chang, H.H. Bone Marrow Combined With Dental Bud Cells Promotes Tooth Regeneration in Miniature Pig Model. Artif. Orgs. 2011, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Mei, F.; Li, D.; Yang, X.P.; Sui, G.; Deng, X.L.; Hu, X.Y. Electrospun poly(l-lacticacid)/nanohydroxyapatite hybrid nanofibers and their potential in dental tissue engineering. Key Eng. Mater. Bioceram. 2007, 19, 377–380. [Google Scholar] [CrossRef]
- Deng, X.L.; Xu, M.M.; Li, D.; Sui, G.; Hu, X.Y.; Yang, X.P. Electrospun PLLA/MWNTs/HA hybrid nanofiber scaffolds and their potential in dental tissue engineering. Key Eng. Mater. Bioceram. 2007, 19, 393–396. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Walboomers, X.; Bian, Z.; Fan, M.; Jansen, J. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. A 2010, 93, 247–257. [Google Scholar] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA. 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Modino, S.A.; Miletich, I.; Sharpe, P.T. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.F.; Magini, R.S.; Sharpe, P.T. Biological tooth replacement and repair. J. Oral. Rehabil. 2007, 34, 933–939. [Google Scholar] [CrossRef] [PubMed]
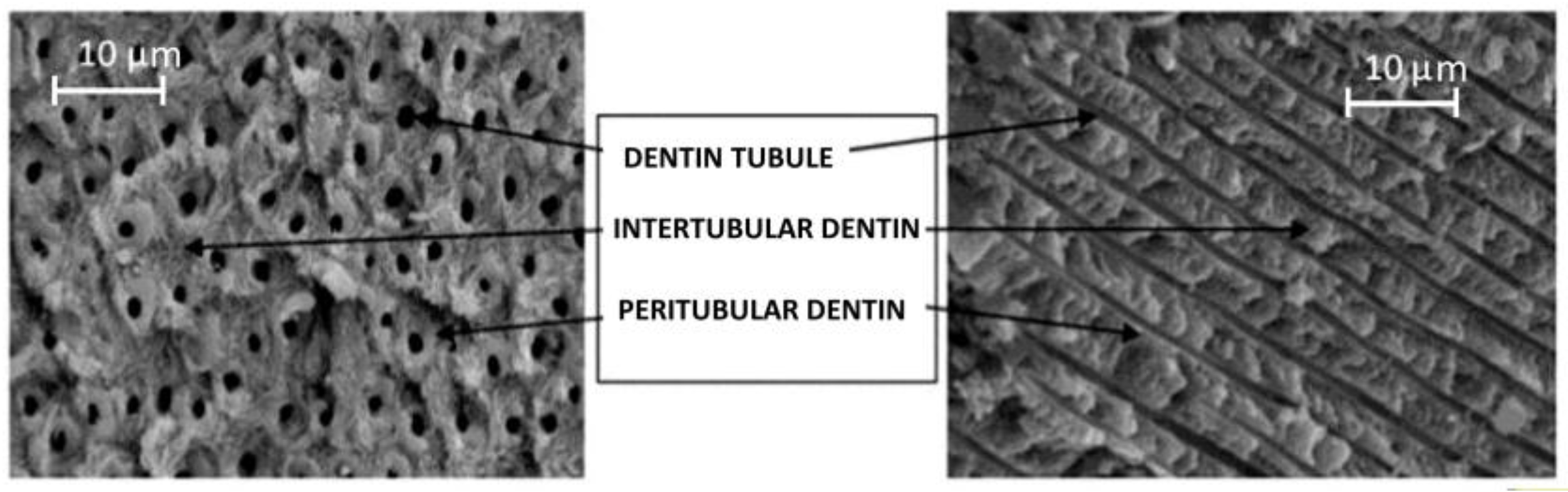
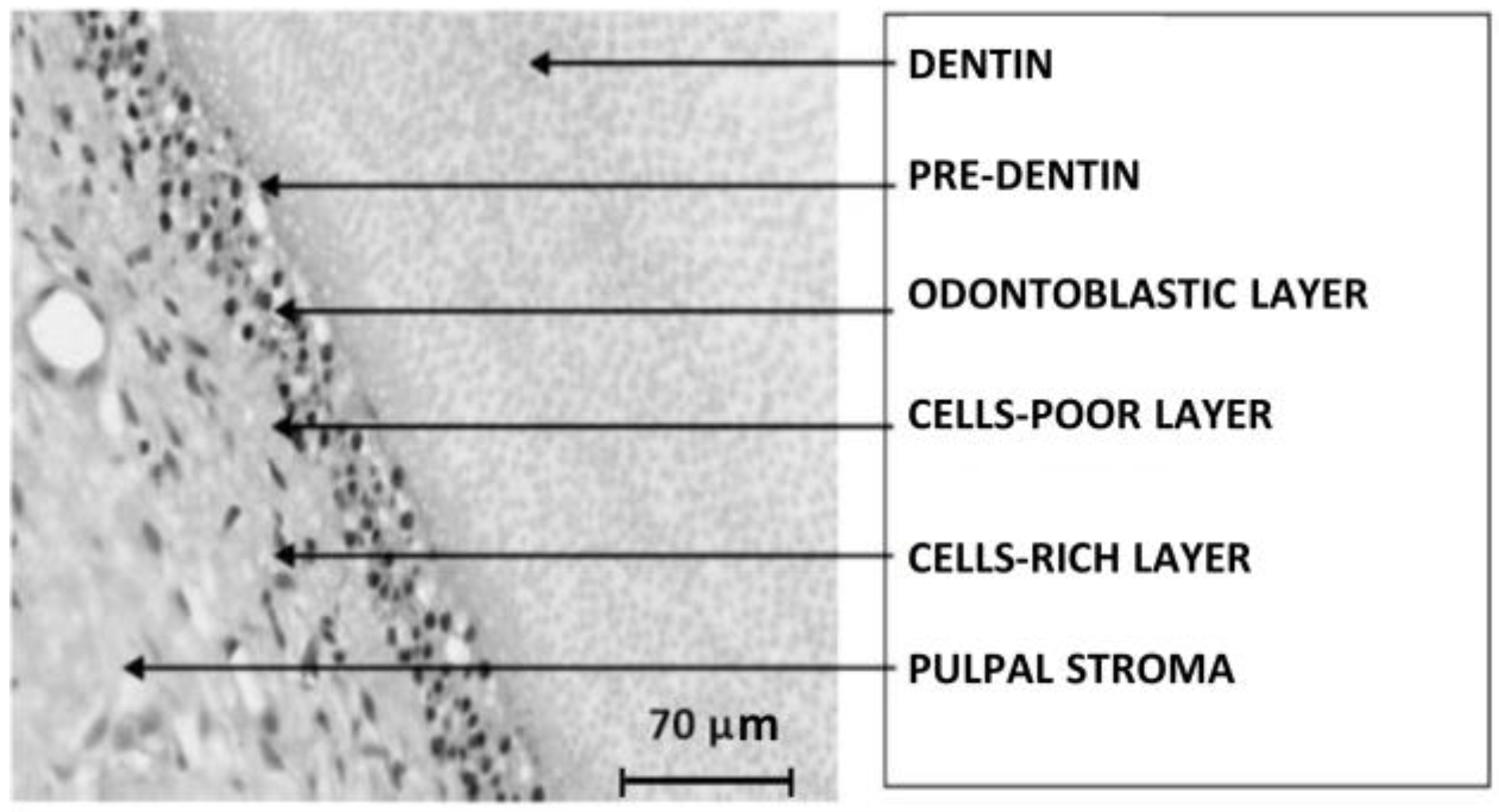
| Composition | Enamel | Dentin | Bone | Hidroxyapatite |
|---|---|---|---|---|
| Calcium (wt. %) | 36.5 | 35.1 | 34.8 | 39.6 |
| Phosphorus (wt. %) | 17.7 | 16.9 | 15.2 | 18.5 |
| Ca/P (molar ratio) | 1.63 | 1.61 | 1.71 | 1.67 |
| Carbonate (CO32−) (wt. %) | 3.5 | 5.6 | 7.4 | - |
| Sodium (wt. %) | 0.5 | 0.6 | 0.9 | - |
| Magnesium (wt. %) | 0.44 | 1.23 | 0.72 | - |
| Potassium (wt. %) | 0.08 | 0.05 | 0.03 | - |
| Fluoride (wt. %) | 0.01 | 0.06 | 0.03 | - |
| Chloride (wt. %) | 0.30 | 0.01 | 0.13 | - |
| Pyrophosphate (P2O74−) (wt. %) | 0.022 | 0.1 | 0.07 | - |
| Totale inorganic (wt. %) | 97 | 70 | 65 | 100 |
| Total organic (wt. %) | 1.5 | 20 | 25 | - |
| Water | 1.5 | 10 | 10 | - |
| a axis (nm) | 0.9441 | 0.9421 | 0.941 | 0.9430 |
| c axis (nm) | 0.6880 | 0.6887 | 0.689 | 0.6891 |
| Cristallinity Index (HA = 100) | 70–75 | 33–37 | 33–37 | 100 |
| Cristalline size (nm) | 100 × 90 × 30 | 35 × 25 × 4 | 50 × 25 × 4 | 200–600 |
| Ignition products (800 °C) | β-TCP + HA | β-TCP + HA | HA + CaO | HA |
| Elasticity modulus (GPa) | 80 | 15 | 0.34–13.8 | 10 |
| Compressive strenght (MPa) | 10 | 100 | 150 | 100 |
| Nanomaterials | Applications | References |
|---|---|---|
| silver and zinc oxide nanoparticles | toothpastes, mouthwashes and composite resins for prevention of caries and periodontal diseases (antibacterial and antidemineralizing properties) | [10,11,13] |
| amorphous calcium phosphate nanoparticles | [12] | |
| carbonate hydroxyapatite nanocrystal | [14] | |
| calcium carbonate nanoparticles | [15] | |
| calcium phosphate nanoparticles | toothpastes, composite resins and dental adhesives for remineralization of tooth lesions | [16,17] |
| gold nanoparticles | diagnosis of malignant and pre-malignant oral diseases | [18,19,20] |
| semi-conductor nanocrystals | [21] | |
| nano-textured surfaces | surface modifications of dental implants | [25,26,27,28,29,30,31] |
| nanostructured hydroxyapatite | promotion of bone remineralization | [97,116,117,119,120,121,122] |
| carbon nanotubes | bone repair/regeneration | [125] |
| polymeric nanofibrous scaffold | dental and craniofacial applications | [126] |
| polycaprolactone nanofibers | scaffold for bone tissue engineering-response to osteogenic regulators | [127] |
| peptide-amphiphile nanofibers | scaffold for bone tissue repair | [128] |
| bioactive peptide -amphiphile nanofibers | enamel regeneration | [137,138] |
| nanohydroxyapatite | periodontal tissue repair and regeneration | [164] |
| nano-carbonated hydroxyapatite/collagen/PLGA membrane | [165,166] | |
| nano hydroxyapatite/polyamide 66 GBR membrane | [167] | |
| chitosan/nanohydroxyapatite composite membrane | [168] | |
| polycaprolactone/calcium carbonate composite nanofibers membrane | [169] | |
| nano-apatite/PCL composite membrane | [170] | |
| poly(DL-lactide-coglycolide) nanofibrous membrane | [171] | |
| gelatin nanofibrous membrane | [173] | |
| PLLA/MWNT/HA membrane | [174] | |
| PLLA/MWNTs/HA, PLLA/HA, PCL/gelatin/HA nanofibrous scaffolds | entire-tooth regeneration | [187,188,189] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials 2016, 6, 134. https://doi.org/10.3390/nano6070134
Chieruzzi M, Pagano S, Moretti S, Pinna R, Milia E, Torre L, Eramo S. Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials. 2016; 6(7):134. https://doi.org/10.3390/nano6070134
Chicago/Turabian StyleChieruzzi, Manila, Stefano Pagano, Silvia Moretti, Roberto Pinna, Egle Milia, Luigi Torre, and Stefano Eramo. 2016. "Nanomaterials for Tissue Engineering In Dentistry" Nanomaterials 6, no. 7: 134. https://doi.org/10.3390/nano6070134
APA StyleChieruzzi, M., Pagano, S., Moretti, S., Pinna, R., Milia, E., Torre, L., & Eramo, S. (2016). Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials, 6(7), 134. https://doi.org/10.3390/nano6070134