Lipid Nanovectors to Deliver RNA Oligonucleotides in Cancer
Abstract
:1. Introduction
1.1. RNA Interference and Cancer
1.2. RNA Biopharmaceutical Issues and Therapies
2. Lipid Vesicles
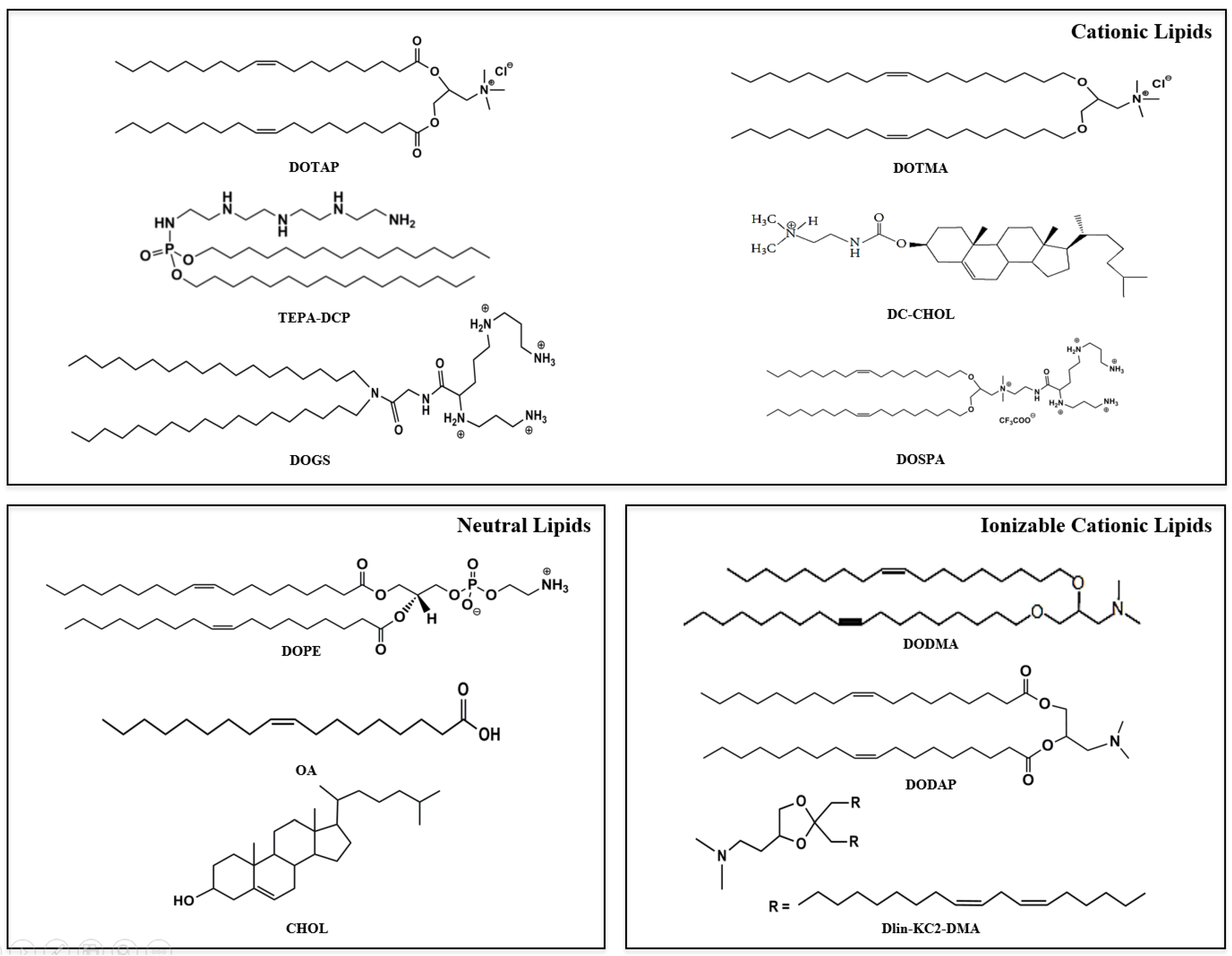
2.1. Cationic Lipids
2.2. Intracellular Delivery of ncRNAs
2.3. Local and Systemic Delivery of ncRNA
2.4. Stable Nucleic acid Lipid Particles
3. Self-Assembled Core/Shell Lipid Nanoparticles
4. Solid Lipid Nanoparticles
5. Lipid Micelles
6. Lipid Nanovectors for ncRNA Delivery: The Clinical Trials
7. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
References
- Sioud, M. RNA Interference: Challenges and Therapeutic Opportunities, 1st ed.; Humana Press: New York, NY, USA, 2015; pp. 1–17. [Google Scholar]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Sandoghchian Shotorbani, S.; Baradaran, B. RNA Interference and Its Role in Cancer Therapy. Adv. Pharm. Bull. 2014, 4, 313–321. [Google Scholar] [PubMed]
- Bora, R.S.; Dikshi, G.; Mukkur, T.K.; Saini, K.S. RNA Interference Therapeutics for Cancer: Challenges and Opportunities (review). Mol. Med. Rep. 2012, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Anders, W.; Lieberman, J. Knocking Down Disease: A Progress Report on SiRNA Therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar]
- Layzer, J.M.; McCaffrey, A.P.; Tanner, A.K.; Huang, Z.; Kay, M.A.; Sullenger, B.A. In Vivo Activity of Nuclease-resistant SiRNAs. RNA 2004, 10, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Sorim, C.; Kim, Y.J.; Kim, S.; Park, H.O.; Choi, Y.C. Chemical Modification of SiRNAs to Improve Serum Stability without Loss of Efficacy. Biochem. Biophys. Res. Commun. 2006, 342, 919–927. [Google Scholar]
- Jesper, C.; Litherland, K.; Faller, T.; van de Kerkhof, E.; Natt, F.; Hunziker, J.; Krauser, J.; Swart, P. Metabolism Studies of Unformulated Internally [3H]-labeled Short Interfering RNAs in Mice. Drug Metab. Dispos. 2013, 41, 1211–1219. [Google Scholar]
- Van de Water, F.M.; Boerman, O.C.; Wouterse, A.C.; Peters, J.G.; Russel, F.G.; Masereeuw, R. Intravenously Administered Short Interfering RNA Accumulates in the Kidney and Selectively Suppresses Gene Function in Renal Proximal Tubules. Drug Metab. Dispos. 2006, 34, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Tomanin, R.; Scarpa, M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr. Gene Ther. 2004, 4, 357–372. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomedicine 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.G.; Kierstead, P.H.; Venditto, V.J.; Walsh, C.L.; Szoka, F.C. Designer lipids for drug delivery: From heads to tails. J. Control Release 2014, 190, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, L. Cationic liposomes-mediate gene transfer. Gene Ther. 1995, 2, 710–722. [Google Scholar] [PubMed]
- Xiong, F.; Mi, Z.; Gu, N. Cationic liposomes as gene delivery systems: Transfection efficiency and new application. Pharmazie 2011, 66, 158–164. [Google Scholar] [PubMed]
- Weisman, S.; Hirsch-Lerner, D.; Barenholz, Y.; Talmon, Y. Nanostructure of Cationic Lipid-Oligonucleotide Complexes. Biophys. J. 2004, 87, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Guo, P.; Wen, C.W.; Wong, H.L. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; De Stefano, D.; Galeone, A. Oligonucleotide delivery in cancer therapy. Expert Opin. Drug Deliv. 2010, 7, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.; Benimetskaya, L.; Stein, C.A.; Vilenchik, M. Cellular delivery of antisense oligonucleotides. Eur. J. Pharm. Biopharm. 2000, 50, 101–119. [Google Scholar] [CrossRef]
- Enlund, E.; Fischer, S.; Handrick, R.; Otte, K.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. Establishment of Lipofection for Studying miRNA Function in Human Adipocytes. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felegner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Sun, J.; Gao, J.; Liu, W.; Li, B.; Guo, Y.; Chen, J. DC-Chol/DOPE cationic liposomes: A comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int. J. Pharm. 2010, 390, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Felgner, J.H.; Kumar, R.; Sridhar, C.N.; Wheeler, C.J.; Tsai, Y.J.; Border, R.; Ramsey, P.; Martin, M.; Felgner, P.L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulation. J. Biol. Chem. 1994, 269, 2550–2561. [Google Scholar] [PubMed]
- Zelphati, O.; Szoka, F.C. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 11493–11498. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Baradia, D.; Vhora, I.; Rathi, M.; Misra, A. Development and characterization of siRNA lipoplexes: Effect of different lipids, in vitro evaluation in cancerous cell lines and in vivo toxicity study. AAPS PharmSciTech 2014, 15, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Hafez, I.M.; Maurer, N.; Cullis, P.R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001, 8, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 30, 280–285. [Google Scholar] [CrossRef]
- Bennett, M.J.; Nantz, M.H.; Balasubramaniam, R.P.; Gruenert, D.C.; Malone, R.W. Cholesterol enhances cationic liposome-mediated DNA transfection of human respiratory epithelial cells. Biosci. Rep. 1995, 15, 47–53. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; De Stefano, D.; Laguardia, V.; Arpicco, S.; Simeon, V.; Carnuccio, R.; Fattal, E. Novel cationic liposome formulation for the delivery of an oligonucleotide decoy to NF-kappaB into activated macrophages. Eur. J. Pharm. Biopharm. 2008, 70, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nakamura, A.; Arai, S.; Kawano, K.; Maitani, Y.; Yonemochi, E. siRNA delivery to lung-metastasized tumor by systemic injection with cationic liposomes. J. Liposome Res. 2015, 25, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Litzinger, D.C.; Huang, L. Biodistribution and immunotargetability of ganglioside-stabilized dioleoylphosphatidylethanolamine liposomes. Biochim. Biophys. Acta 1992, 1104, 179–187. [Google Scholar] [CrossRef]
- Wasungu, L.; Hoekstra, D. Cationic lipids, lipoplexes and intracellular delivery. J. Control Release 2006, 116, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Rathi, M.; Baradia, D.; Trehan, S.; Misra, A. In vivo delivery aspects of miRNA, shRNA and siRNA. Crit. Rev. Ther. Drug Carrier Syst. 2012, 6, 487–527. [Google Scholar] [CrossRef]
- Wang, X.; Yu, B.; Ren, W.; Mo, X.; Zhou, C.; He, H.; Jia, H.L.; Wang, L.; Robert, J.S.T.; Lee, R.J.; et al. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J. Control Release 2013, 172, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Asai, T.; Koide, H.; Okamoto, A.; Maeda, N.; Tomita, K.; Dewa, T.; Minamino, T.; Oku, N. Polycation liposomes as a vector for potential intracellular delivery of microRNA. J. Gene Med. 2013, 15, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Okamoto, A.; Tsuchida, H.; Ando, H.; Ariizumi, S.; Kiyokawa, C.; Hashimoto, M.; Asai, T.; Dewa, T.; Oku, N. One-step encapsulation of siRNA between lipid-layers of multi-layer polycation liposomes by lipoplex freeze-thawing. J. Control Release 2016, 228, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Kuo, A.; Barnathan, E.S.; Langer, D.J. Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochim. Biophys. Acta 1998, 1369, 320–334. [Google Scholar] [CrossRef]
- Barichello, J.M.; Kizuki, S.; Tagami, T.; Soares, L.A.; Ishida, T.; Kikuchi, H.; Kiwada, H. Agitation during lipoplex formation harmonizes the interaction of siRNA to cationic liposomes. Int. J. Pharm. 2012, 430, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N.; de Lima, M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Rel. 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, I.; Visser, H.W.; ter Beest, M.B.; Wagenaar, A.; Ruiters, M.H.; Engberts, J.B.; Hoekstra, D. Parameters influencing the introduction of plasmid DNA into cells by the use of synthetic amphiphiles as a carrier system. Biochim. Biophys. Acta 1995, 1240, 34–40. [Google Scholar] [CrossRef]
- Wu, J.; Lizarzaburu, M.E.; Kurth, M.J.; Liu, L.; Wege, H.; Zern, M.A.; Nantz, M.H. Cationic lipid polymerization as a novel approach for constructing new DNA delivery agents. Bioconjug. Chem. 2001, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bottega, R.; Epand, R.M. Inhibition of protein kinase C by cationic amphiphiles. Biochemistry 1992, 31, 9025–9030. [Google Scholar] [CrossRef] [PubMed]
- Ilies, M.A.; Seitz, W.A.; Johnson, B.H.; Ezell, E.L.; Miller, A.L.; Thompson, E.B.; Balaban, A.T. Lipophilic Pyrylium Salts in the Synthesis of Efficient Pyridinium-Based Cationic Lipids, Gemini Surfactants, and Lipophilic Oligomers for Gene Delivery. J. Med. Chem. 2006, 49, 3872–3887. [Google Scholar] [CrossRef] [PubMed]
- Pinnaduwage, P.; Schmitt, L.; Huang, L. Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim. Biophys. Acta 1989, 985, 33–37. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Saad, M.; Betigeri, S.; Zhang, M.; Vetcher, A.A.; Soldatenkov, V.A.; Reimer, D.C.; Pozharov, V.P.; Minko, T. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm. Res. 2009, 26, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Dorrani, M.; Garbuzenko, O.B.; Minko, T.; Michniak-Kohn, B. Development of edge-activated liposomes for siRNA delivery to human basal epidermis for melanoma therapy. J. Control Release 2016, 228, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim. Biophys. Acta 2001, 1514, 191–205. [Google Scholar] [CrossRef]
- Zelphati, O.; Uyechi, L.S.; Barron, L.G.; Szoka, F.C., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim. Biophys. Acta 1998, 1390, 119–133. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Brown, J.M.; Wala, I.; Kaufman, S.A.; Van, G.Y.; Farrell, C.L.; Collins, D. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim. Biophys. Acta 1996, 1281, 139–149. [Google Scholar] [CrossRef]
- Wolf, H.K.; Snel, C.J.; Verbaan, F.J.; Schiffelers, R.M.; Hennink, W.E.; Storm, G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int. J. Pharm. 2007, 331, 167–175. [Google Scholar] [PubMed]
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Hollins, A.J.; Omidi, Y.; Benter, I.F.; Akhtar, S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J. Drug Target. 2007, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Dams, E.T.; Laverman, P.; Oyen, W.J.; Storm, G.; Scherphof, G.L.; van Der Meer, J.W.; Corstens, F.H.; Boerman, O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.Y.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Yu, B.; Mao, Y.; Nana-Sinkam, S.P.; Lee, L.J. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol. Pharm. 2011, 8, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Mao, Y.; Lee, R.J.; Davis, I.C.; Elton, T.S.; Lee, L.J.; Nana-Sinkam, S.P. Therapeutic Delivery of MicroRNA-29b by Cationic Lipoplexes for Lung Cancer. Mol. Ther. Nucl. Acids. 2013, 16. [Google Scholar] [CrossRef]
- Lee, H.Y.; Mohammed, K.A.; Kaye, F.; Sharma, P.; Moudgil, B.M.; Clapp, W.L.; Nasreen, N. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. Int. J. Nanomed. 2013, 8, 4481–4494. [Google Scholar]
- Passadouro, M.; Pedroso de Lima, M.C.; Faneca, E. MicroRNAmodulation combined with sunitinib as a novel therapeutic strategy for pancreatic cancer. Int. J. Nanomed. 2014, 9, 3203–3217. [Google Scholar]
- Meissner, J.M.; Toporkiewicz, M.; Czogalla, A.; Matusewicz, L.; Kuliczkowski, K.; Sikorski, A.F. Novel antisense therapeutics delivery systems: In vitro and in vivo studies of liposomes targeted with anti-CD20 antibody. J. Control Release 2015, 220, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control Release 2005, 107, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.S.; Lee, A.C.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Robbins, M.; Tavakoli, I.; Levi, J.; Hu, L.; Fronda, A.; Ambegia, E.; McClintock, K.; MacLachlan, I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Investig. 2009, 119, 661–673. [Google Scholar] [CrossRef] [PubMed]
- De Antonellis, P.; Liguori, L.; Falanga, A.; Carotenuto, M.; Ferrucci, V.; Andolfo, I.; Marinaro, F.; Scognamiglio, I.; Virgilio, A.; De Rosa, G.; et al. MicroRNA 199b-5p delivery through stable nucleic acid lipid particles (SNALPs) in tumorigenic cell lines. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, C.; Biroccio, A.; Benassi, B.; Stringaro, A.; Stoppacciaro, A.; Semple, S.C.; Zupi, G. Encapsulation of c-myc antisense oligodeoxynucleotides in lipid particles improves antitumoral efficacy in vivo in a human melanoma line. Cancer Gene Ther. 2001, 8, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, I.; Di Martino, M.T.; Campani, V.; Virgilio, A.; Galeone, A.; Gullà, A.; Cantafio, M.E.; Misso, G.; Tagliaferri, P.; Tassone, P.; et al. Transferrin-conjugated SNALPs encapsulating 2’-O-methylated miR-34a for the treatment of multiple myeloma. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Cardoso, A.L.; Custódia, C.; Cunha, P.; de Almeida, L.P.; de Lima, M.C. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. J. Control Release 2015, 207, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.C.; Tseng, Y.C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Chono, S.; Huang, L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol. Ther. 2008, 16, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.J.; Huang, L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol. Ther. 2010, 18, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Chono, S.; Li, S.D.; Conwell, C.C.; Huang, L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J. Control Release 2008, 131, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sen, J.; Bathula, S.R.; Yang, Q.; Fittipaldi, R.; Huang, L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol. Pharm. 2009, 6, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta 2009, 788, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Mortenson, E.D.; Radkevich-Brown, O.; Wang, Y.; Fu, Y.X. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol. Ther. 2013, 21, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Wang, Y.; Li, J.; Liu, F.; Huang, L. Nanoparticle delivery of pooled siRNA for effective treatment of non-small cell lung cancer. Mol. Pharm. 2012, 9, 2280–2289. [Google Scholar] [PubMed]
- Tagalakis, A.D.; Lee do, H.D.; Bienemann, A.S.; Zhou, H.; Munye, M.M.; Saraiva, L.; McCarthy, D.; Du, Z.; Vink, C.A.; Maeshima, R.; et al. Multifunctional, selfassembling anionic peptide-lipid nanocomplexes for targeted siRNA delivery. Biomaterials 2014, 35, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Landesman-Milo, D.; Goldsmith, M.; Leviatan Ben-Arye, S.; Witenberg, B.; Brown, E.; Leibovitch, S.; Azriel, S.; Tabak, S.; Morad, V.; Peer, D. Hyaluronan grafted lipid-based nanoparticles as RNAi carriers for cancer cells. Cancer Lett. 2013, 334, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar]
- Kim, H.R.; Kim, I.K.; Bae, K.H.; Lee, S.H.; Lee, Y.; Park, T.G. Cationic solid lipid nanoparticles reconstituted from low density lipoprotein components for delivery of siRNA. Mol Pharm. 2008, 5, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Bae, K.H.; Yang, H.; Lee, S.J.; Kim, H.; Kim, Y.; Joo, K.M.; Seo, S.W.; Park, T.G.; Nam, D.H. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug. Chem. 2011, 22, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Lobovkina, T.; Jacobson, G.B.; Gonzalez-Gonzalez, E.; Hickerson, R.P.; Leake, D.; Kaspar, R.L.; Contag, C.H.; Zare, R.N. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano 2011, 5, 9977–9983. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, E.; Park, D.E.; Shim, G.; Lee, S.; Kim, Y.B.; Kim, C.W.; Oh, Y.K. Cationic solid lipid nanoparticles for co-delivery of paclitaxel and siRNA. Eur. J. Pharm. Biopharm. 2012, 80, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Riehle, R.; Navarro, G.; Perche, F.; De Rosa, G.; Torchilin, V.P. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: A promising strategy to overcome drug resistance in cancer. Cancer Lett. 2014, 343, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Navarro, G.; Trivedi, M.S.; De Rosa, G.; Torchilin, V.P. Multifunctional Polymeric Micelles Co-loaded with Anti-Survivin siRNA and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeong, J.H.; Lee, S.H.; Kim, S.W.; Park, T.G. PEG conjugated VEGF siRNA for anti-angiogenic gene therapy. J. Control Release 2006, 116, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Sawant, R.R.; Biswas, S.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine 2012, 7, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Essex, S.; Navarro, G.; Sabhachandani, P.; Chordia, A.; Trivedi, M.; Movassaghian, S.; Torchilin, V.P. Phospholipid-modified PEI-based nanocarriers for in vivo siRNA therapeutics against multidrug-resistant tumors. Gene Ther. 2015, 22, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Biswas, S.; Wang, T.; Zhu, L.; Torchilin, V.P. Hypoxia-targeted siRNA delivery. Angew. Chem. Int. Ed. Engl. 2014, 53, 3362–3366. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, T.; Vaze, O.; D’Souza, G.; Torchilin, V.P. Effective stabilization and delivery of siRNA: Reversible siRNA-phospholipid conjugate in nanosized mixed polymeric micelles. Bioconjug. Chem. 2010, 21, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Costa, D.F.; Torchilin, V.P. siRNA Delivery by Stimuli-Sensitive Nanocarriers. Curr. Pharm. Des. 2015, 21, 4566–4573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- The Homepage of ClinicalTrials.gov. Available online: http//clinicaltrials.gov (accessed on 2 May 2016).
- Li, B.; Hu, Y.; Ye, F.; Li, Y.; Lv, W.; Xie, X. Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int. J. Gynecol. Cancer 2010, 20, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Daige, C.L.; Kelnar, K.; Priddy, L.; Dysart, S.; Wiggins, J.; Zhao, J.; Leatherbury, N.; Omotola, M.; Stoudemire, J.; et al. Preclinical data of a microRNA-based therapy for hepatocellular carcinoma. In Proceedings of the Annual AACR Conference, Chicago, IL, USA, 31 March–4 April 2012.
- Daige, C.; Priddy, L.; Kelnar, K.; Zhao, J.; Dysart, S.; Bader, A.; Brown, D. The development of a miRNA-based therapeutic candidate for hepatocellular carcinoma. In Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, San Francisco, CA, USA, 12–16 November 2011. Abstract No. C142.
| Nanocarrier Composition | Findings | Reference |
|---|---|---|
| DOTMA/DOPE-CLs | Lipid/RNA weight ratio of 2.5 showed the highest RNA transfection (70% of the transfected cells) | [24] |
| DC-chol/DOPE-CLs | The highest siRNA transfection efficiency were found at the DC-chol/RNA weight ratio of 5 or 10 and at a DC-chol/DOPE molar ratio of 1 | [25] |
| DOTAP/DMPG-CLs | DMPG was found to neutralize the net surface charge of CLs reducing the cytotoxicity and the RNA complexation efficiency | [27] |
| DOTAP/HSPC-CLs | HSPC increased the complexation strength between DOTAP liposomes and siRNA | [28] |
| TEPA-PCL-CLs | The proton sponge effect of the polycation lipid TEPA-PCL enhanced the cellular-uptake and endosomal escape of miR-92a | [38] |
| DOPE/chol/DCP-DETA-CLs | The inclusion of polycationic lipid DCP-DETA in liposomes increased the biological stability of encapsulated siRNA compared to conventional CLs | [38] |
| DOTMA/DOPE-CLs | Pre-incubation of CLs with phosphate buffer reduced the time for RNA transfection | [40] |
| DOPE-CLs | Agitation during siRNA/CLs complex formation increased the complexation efficiency and gene knockdown | [41] |
| DOTMA-CLs | DOTMA is more cytotoxic than DOTAP due to the more stable ether linker | [42] |
| NaChol/DOTAP-CLs | DOTAP/NaChol at the weight ratio of 8:1 and siRNA at a RNA/CL weight ratio of 16:1 allowed to achieve the highest permeation through the and highest siRNA internalization into melanoma UACC-903 cells | [51] |
| DOTMA/chol/D-Alpha-tocopheryl-PEG succinate-CLs | The use of chol as helper lipid increased the RNA delivery into the lungs, and reduced the RNA delivery in other organs, e.g., into the liver | [60] |
| DOTMA/OA/PEGylated chol-CLs | The use of OA as helper lipid changed lipoplex biodistribution improving miR-122 level in liver in an experimental model of liver cancer, reducing toxicity in non-target organs | [61] |
| DODAP-SNALPs | The ionizable lipid DODAP improved RNA encapsulation efficiency; the possibility to neutralize the charge after RNA encapsulation enhanced the vesicle stability in biological fluids | [66] |
| DLinDMA-SNALPs | The highest number of double bonds of DLinDMA reduced the phase transition temperature with a significant improvement of the transfection efficiency | [66] |
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Clinical Trials Identifier | Lipid Nanovector | ncRNA | Condition | Administration Route | Companies |
| NCT01591356 | Neutral liposome | siRNA | Advanced Cancers | intravenous | M.D. Anderson Cancer Center |
| NCT01262235 | SNALP | RNA | Neuroendocrine Tumors; Adrenocortical Carcinoma | intravenous | Arbutus Biopharma Corporation |
| NCT02410733 | SNALP | RNA | Melanoma | intravenous | Biontech RNA Pharmaceuticals GmbH |
| NCT01829971 | SMARTICLES® | mRNA | Primary Liver Cancer; Lymphoma; Melanoma; Multiple Myeloma; Renal Cell Carcinoma | intravenous | Mirna Therapeutics, Inc. |
| NCT02110563 | Stable lipid particles * | siRNA | Solid Tumors; Multiple Myeloma; Non-Hodgkins Lymphoma; Pancreatic Neuroendocrine Tumors | intravenous infusion | Dicerna Pharmaceuticals, Inc. |
| NCT02314052 | Stable lipid particles * | siRNA | Hepatocellular Carcinoma | intravenous infusion | Dicerna Pharmaceuticals, Inc. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campani, V.; Salzano, G.; Lusa, S.; De Rosa, G. Lipid Nanovectors to Deliver RNA Oligonucleotides in Cancer. Nanomaterials 2016, 6, 131. https://doi.org/10.3390/nano6070131
Campani V, Salzano G, Lusa S, De Rosa G. Lipid Nanovectors to Deliver RNA Oligonucleotides in Cancer. Nanomaterials. 2016; 6(7):131. https://doi.org/10.3390/nano6070131
Chicago/Turabian StyleCampani, Virginia, Giuseppina Salzano, Sara Lusa, and Giuseppe De Rosa. 2016. "Lipid Nanovectors to Deliver RNA Oligonucleotides in Cancer" Nanomaterials 6, no. 7: 131. https://doi.org/10.3390/nano6070131
APA StyleCampani, V., Salzano, G., Lusa, S., & De Rosa, G. (2016). Lipid Nanovectors to Deliver RNA Oligonucleotides in Cancer. Nanomaterials, 6(7), 131. https://doi.org/10.3390/nano6070131








