Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms
Abstract
:1. Introduction
2. Results
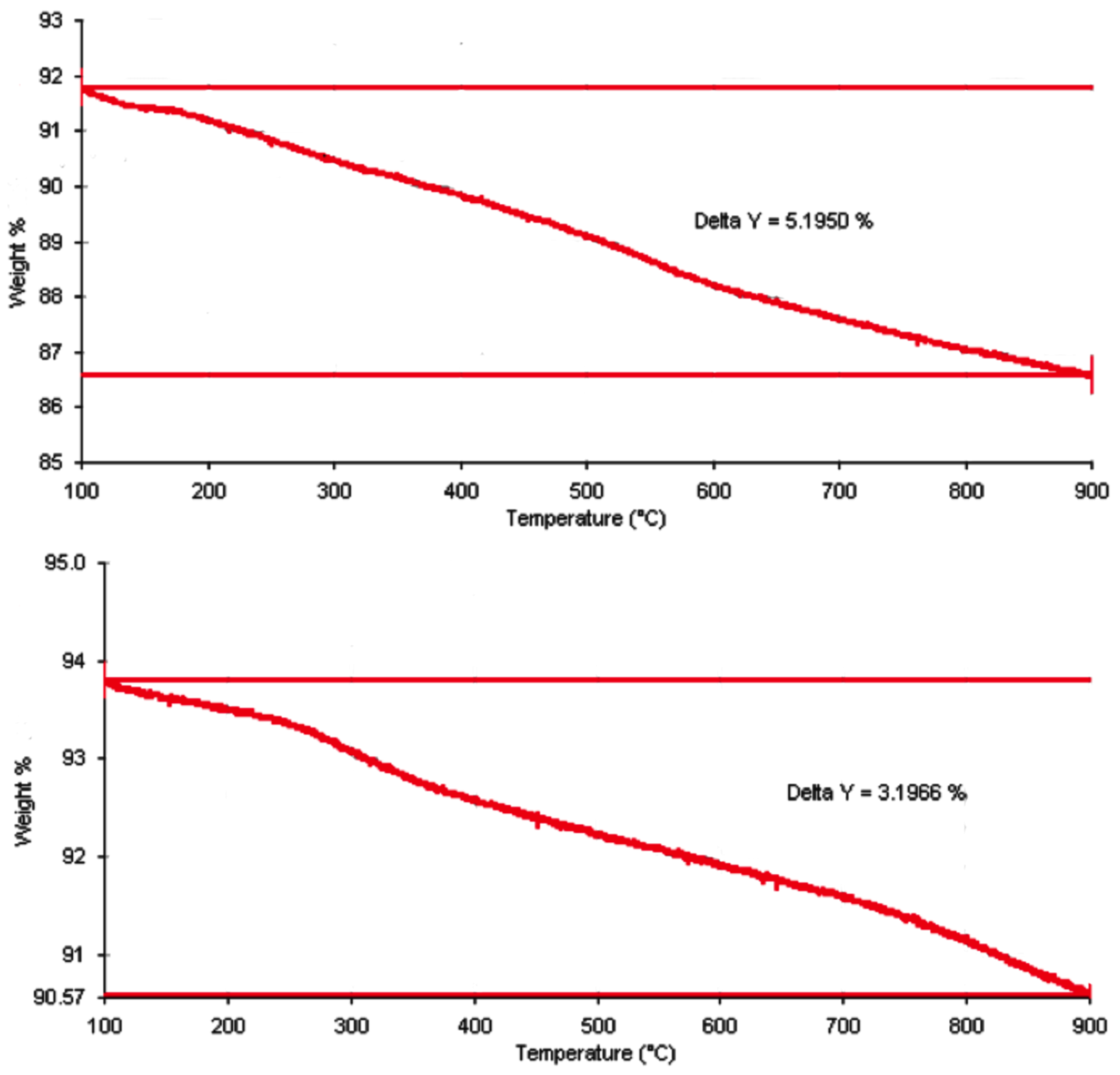
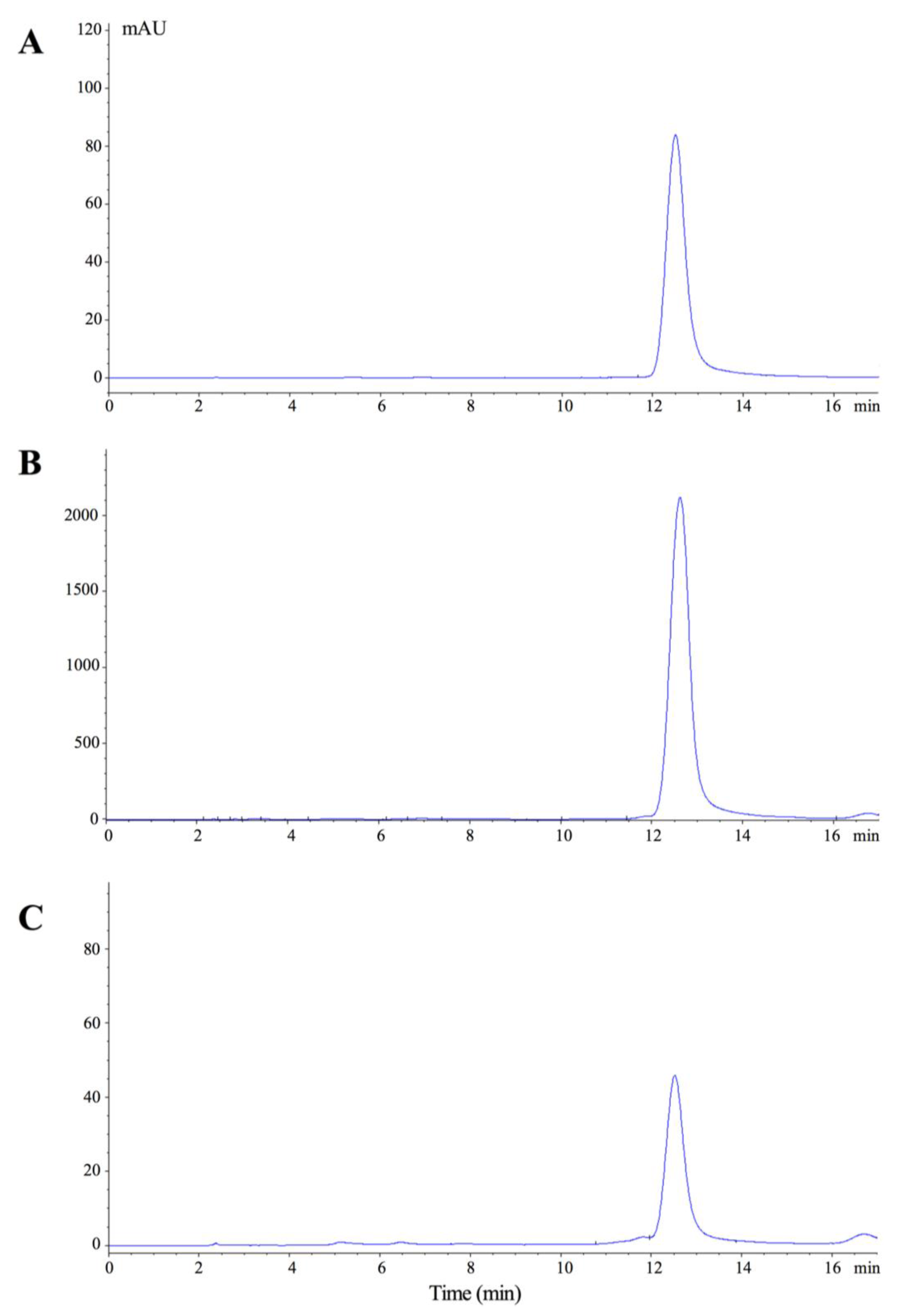
2.1. Loading and Releasing Profiles of Nano-SB
2.2. The Penetration Capacity of Nano-SB through Porcine Buccal Mucosa
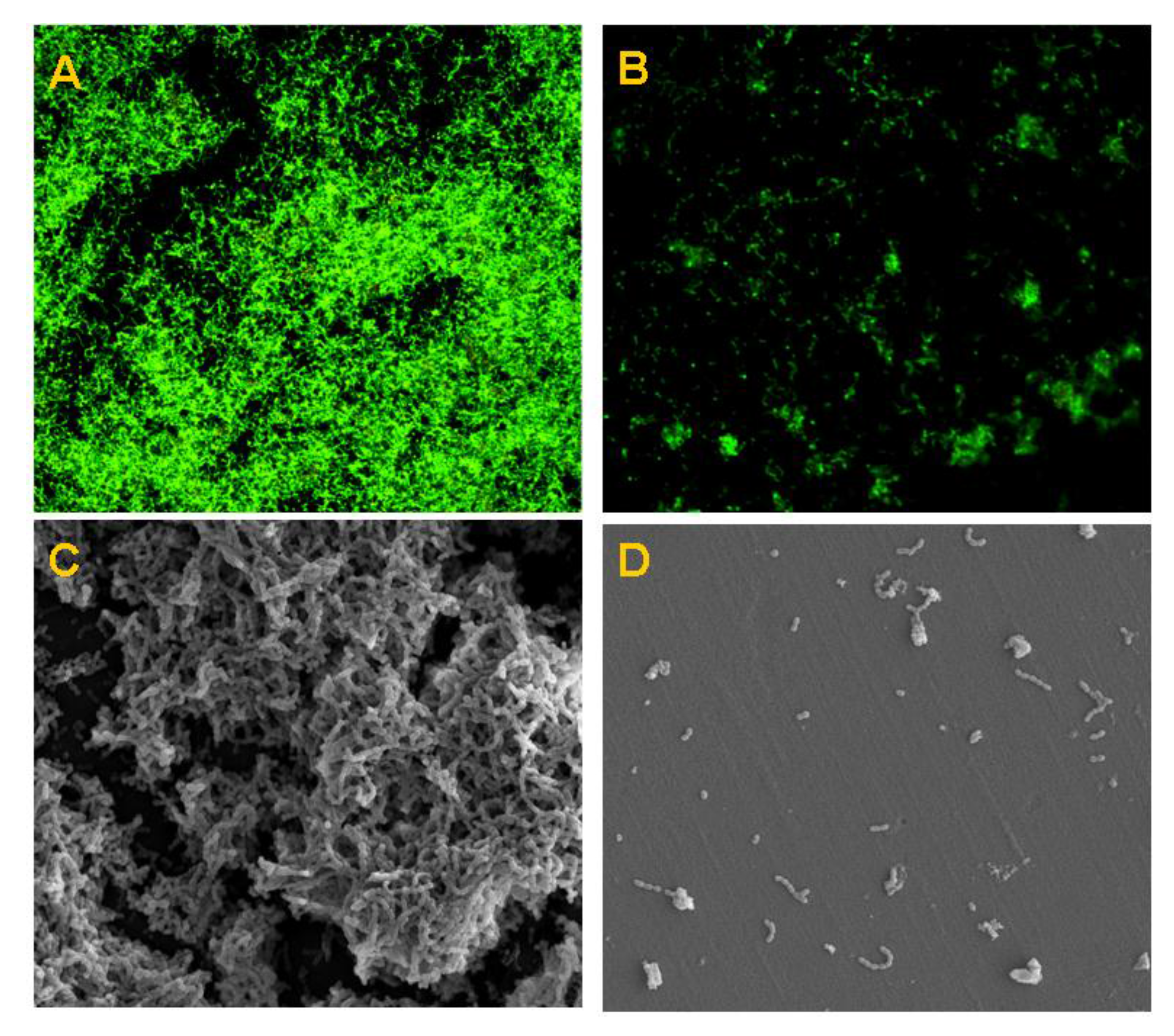
2.3. Antibacterial Effects of Nano-MIX on Planktonic Bacteria and Mono- and Multi-Species Oral Biofilms
3. Discussion
4. Materials and Methods
4.1. SB Extract and Nano-CHX
4.2. Thermogravimetric Analysis of SB Loading in MSN
4.3. High-Performance Liquid Chromatography (HPLC) Analyses
4.4. Preparation of Porcine Buccal Mucosal Membranes and Diffusion Assays
4.5. Anti-Biofilm Properties of the Nano-MIX (Nano-SB and Nano-CHX at 9:1 w/w)
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SB | Scutellaria baicalensis |
| Nano-CHX | Nanoparticle-encapsulated chlorhexidine |
| Nano-SB | Nanoparticle-encapsulated SB |
| HPLC | High-performance liquid chromatography |
| Nano-MIX | Nano-SB and Nano-CHX |
| SEM | Scanning electron microscopy |
| CSLM | Confocal scanning laser microscopy |
| MSN | Mesoporous silica nanoparticle |
| PBS | Phosphate buffered saline |
| MIC | Minimum inhibitory concentration |
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Jin, L.J.; Armitage, G.C.; Klinge, B.; Lang, N.P.; Tonetti, M.; Williams, R.C. Global oral health inequalities: Task group—Periodontal disease. Adv. Dent. Res. 2011, 23, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. Position paper of the american academy of periodontology: Periodontal disease as a potential risk factor for systemic diseases. J. Periodontol. 1998, 69, 841–850. [Google Scholar] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Chapple, I.L. Biological approaches to the development of novel periodontal therapies-consensus of the seventh european workshop on periodontology. J. Clin. Periodontol. 2011, 38, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Baehni, P.C.; Takeuchi, Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003, 9, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, M.N.; Gibson, J. Chlorhexidine and hypersensitivity reactions in dentistry. Br. Dent. J. 2012, 213, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.E.; Berchier, C.E.; Addy, M.; Van der Velden, U.; Van der Weijden, G.A. The efficacy of chlorhexidine dentifrice or gel on plaque, clinical parameters of gingival inflammation and tooth discoloration: A systematic review. Int. J. Dent. Hyg. 2014, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Aranha, A.M.; Nogueira, I.; Giro, E.M.; Hebling, J.; Costa, C.A. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S.; Sugimura, K.; Yoshida, N.; Yasumoto, R.; Wada, S.; Yamamoto, K.; Kishimoto, T. Antitumor effects of scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 2000, 55, 951–955. [Google Scholar] [CrossRef]
- Cao, C.F.; Sun, X.P. Herbal medicine for periodontal diseases. Int. Dent. J. 1998, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Cao, Z.G.; Yang, R.; Shang, Z.H.; Jin, L.J.; Cobert, E.F. Effects of baicalin on the expression of pro-MMP-1 and MMP-3 in human gingival fibroblasts and periodontal ligament cells. Chin. J. Stomatol. 2004, 39, 197–200. [Google Scholar]
- Luo, W.; Wang, C.Y.; Jin, L.J. Baicalin downregulates porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chiou, W.F.; Chou, Y.C.; Chen, C.F. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur. J. Pharmacol. 2003, 465, 171–181. [Google Scholar] [CrossRef]
- Wang, G.F.; Wu, Z.F.; Wan, L.; Wang, Q.T.; Chen, F.M. Influence of baicalin on the expression of receptor activator of nuclear factor-kappab ligand in cultured human periodontal ligament cells. Pharmacology 2006, 77, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Matsumura, S.; Kariya, N.; Nishimura, M.; Shimono, T. In vitro antibacterial activities of scutellaria baicalensis georgi against cariogenic bacterial. Pediatr. Dent. J. 2007, 17, 58–64. [Google Scholar] [CrossRef]
- Zhenhua, J.; Shuishan, S. Inhibition of Quorum Sensing Activity by Ethanol Extract of Scutellaria baicalensis Georgi. J. Plant Pathol. Microbiol. 2012, 7, 1–4. [Google Scholar]
- Jang, E.J.; Cha, S.M.; Choi, S.M.; Cha, J.D. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral Biol. 2014, 59, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Zhu, X.M.; Wang, Y.X.J.; Xuan, S.H.; You, Q.; Chan, W.H.; Wong, C.H.; Wang, F.; Yu, J.C.; Cheng, C.H. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Zhu, X.M.; Liang, Q.; Cheng, C.H.; Wang, W.; Leung, K.C.F. In vivo chemoembolization and magnetic resonance imaging of liver tumors by using iron oxide nanoshell/doxorubicin/poly(vinyl alcohol) hybrid composites. Angew. Chem. Int. Ed. 2014, 126, 4912–4915. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhu, X.M.; Lee, S.F.; Chan, H.M.; Li, H.W.; Kong, S.K.; Jimmy, C.Y.; Cheng, C.H.; Wang, Y.X.J.; Leung, K.C.F. Folate-conjugated Fe3O4@SiO2@gold nanorods@mesoporous SiO2 hybrid nanomaterial: A theranostic agent for magnetic resonance imaging and photothermal therapy. J. Mater. Chem. B 2013, 1, 2934–2942. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Leung, K.C.; Wong, C.H.; Lee, S.F.; Li, X.; Leung, P.C.; Lau, C.B.; Wat, E.; Jin, L.J. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.C.; Choi, W.Y.; Seo, Y.C.; Kim, J.S.; Yoon, C.S.; Lim, H.W.; Kim, H.S.; Ahn, J.; Lee, H.Y. Enhancement of the skin-protective activities of centella asiatica l. Urban by a nano-encapsulation process. J. Biotechnol. 2012, 157, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Galey, W.R.; Lonsdale, H.K.; Nacht, S. The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water. J. Investig. Dermatol. 1976, 67, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.; Brogden, K. Human Oral Mucosa: Development, Structure and Function; Wiley-Blackwell: West Sussex, UK, 2011. [Google Scholar]
- Nicolazzo, J.A.; Finnin, B.C. In vivo and in vitro models for assessing drug absorption across the buccal mucosa. In Drug Absorption Studies: In Situ, in Vitro and in Silico Models; Springer: New York, NY, USA, 2008; pp. 89–111. [Google Scholar]
- Kulkarni, U.; Mahalingam, R.; Pather, I.; Li, X.; Jasti, B. Porcine buccal mucosa as in vitro model: Effect of biological and experimental variables. J. Pharm. Sci. 2010, 99, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Squier, C.A. Cellular and molecular basis of barrier function in oral epithelium. Crit. Rev. Ther. Drug. Carr. Syst. 1991, 8, 237–269. [Google Scholar]
- Qing, L.S.; Xiong, J.; Xue, Y.; Liu, Y.M.; Guang, B.; Ding, L.S.; Liao, X. Using bacailin-functionalized magnetic nanoparticles for selectively extracting flavonoids from Rosa chinensis. J. Sep. Sci. 2011, 34, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Chen, B.; Xia, G.; Wang, S.; Cheng, J.; Shao, Z.; Gao, C.; Bao, W.; Tian, L.; et al. Effect of magnetic nanoparticles on apoptosis and cell cycle induced by wogonin in Raji cells. Int. J. Nanomed. 2012, 7, 789–798. [Google Scholar]
- Cheng, J.; Cheng, L.; Chen, B.; Xia, G.; Gao, C.; Song, H.; Bao, W.; Guo, Q.; Zhang, H.; Wang, X. Effect of magnetic nanoaparticles of Fe3O4 and wogonin on the reversal of multidrug resistance in K562/A02 cell line. Int. J. Nanomed. 2012, 7, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.N.; Kannan, S. Enhanced delivery of baicalein using cinnamaldehyde cross-linked chitosan nanoparticle inducing apotosis. Int. J. Biol. Macromol. 2012, 51, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Garapati, C.; Clarke, B.; Zadora, S.; Burney, C.; Cameron, B.D.; Fournier, R.; Baugh, R.F.; Boddu, S.H. Development and characterization of erythrosine nanoparticles with potential for treating sinusitis using photodynamic therapy. Photodiagn. Photodyn. 2015, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Ng, K.M.; Wibowo, C. Product design: A transdermal patch containing a traditional chinese medicinal tincture. Ind. Eng. Chem. Res. 2010, 49, 4904–4913. [Google Scholar] [CrossRef]
| Duration of Treatment | Sample | Original Amount of Baicalin (µg) | Baicalin Retained within Mucosa (%) | Baicalin Penetrated to Receiver Chamber (%) |
|---|---|---|---|---|
| 2 h | SB | 927 | 9.8% | 39.0% |
| Nano-SB | 20 | 14.6% | 0.0% | |
| 6 h | SB | 927 | 3.5% | 72.5% |
| Nano-SB | 20 | 5.8% | 46.7% |
| Stain | Planktonic Mode | Mono-Species Oral Biofilms |
|---|---|---|
| S. mutans | 50 | 50 |
| S. sobrinus | 50 | 50 |
| F. nucleatum | 25 | 50 |
| A. actinomycetemcomitans | 50 | 50 |
| E. faecalis | 50 | 200 * |
| Multi-Species Biofilms | 24 h | 48 h |
|---|---|---|
| S. mutans, F. nucleatum and P. gingivalis | 12.5 | 50 * |
| S. sobrinus, F. nucleatum and P. gingivalis | 12.5 | 50 * |
| S. mutans, F. nucleatum, A. actinomycetemcomitans and P. gingivalis | 12.5 | 50 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.C.-F.; Seneviratne, C.J.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wong, C.-H.; Pang, K.Y.; Wong, C.W.; Wat, E.; Jin, L. Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials 2016, 6, 61. https://doi.org/10.3390/nano6040061
Leung KC-F, Seneviratne CJ, Li X, Leung PC, Lau CBS, Wong C-H, Pang KY, Wong CW, Wat E, Jin L. Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials. 2016; 6(4):61. https://doi.org/10.3390/nano6040061
Chicago/Turabian StyleLeung, Ken Cham-Fai, Chaminda Jayampath Seneviratne, Xuan Li, Ping Chung Leung, Clara Bik San Lau, Chi-Hin Wong, Ka Yan Pang, Chun Wai Wong, Elaine Wat, and Lijian Jin. 2016. "Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms" Nanomaterials 6, no. 4: 61. https://doi.org/10.3390/nano6040061
APA StyleLeung, K. C.-F., Seneviratne, C. J., Li, X., Leung, P. C., Lau, C. B. S., Wong, C.-H., Pang, K. Y., Wong, C. W., Wat, E., & Jin, L. (2016). Synergistic Antibacterial Effects of Nanoparticles Encapsulated with Scutellaria baicalensis and Pure Chlorhexidine on Oral Bacterial Biofilms. Nanomaterials, 6(4), 61. https://doi.org/10.3390/nano6040061








