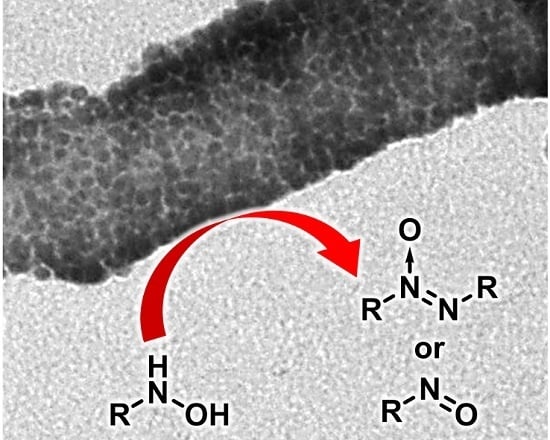
Supramolecular Assembly of Gold Nanoparticles on Carbon Nanotubes: Application to the Catalytic Oxidation of Hydroxylamines
Abstract
:1. Introduction
2. Results and Discussion
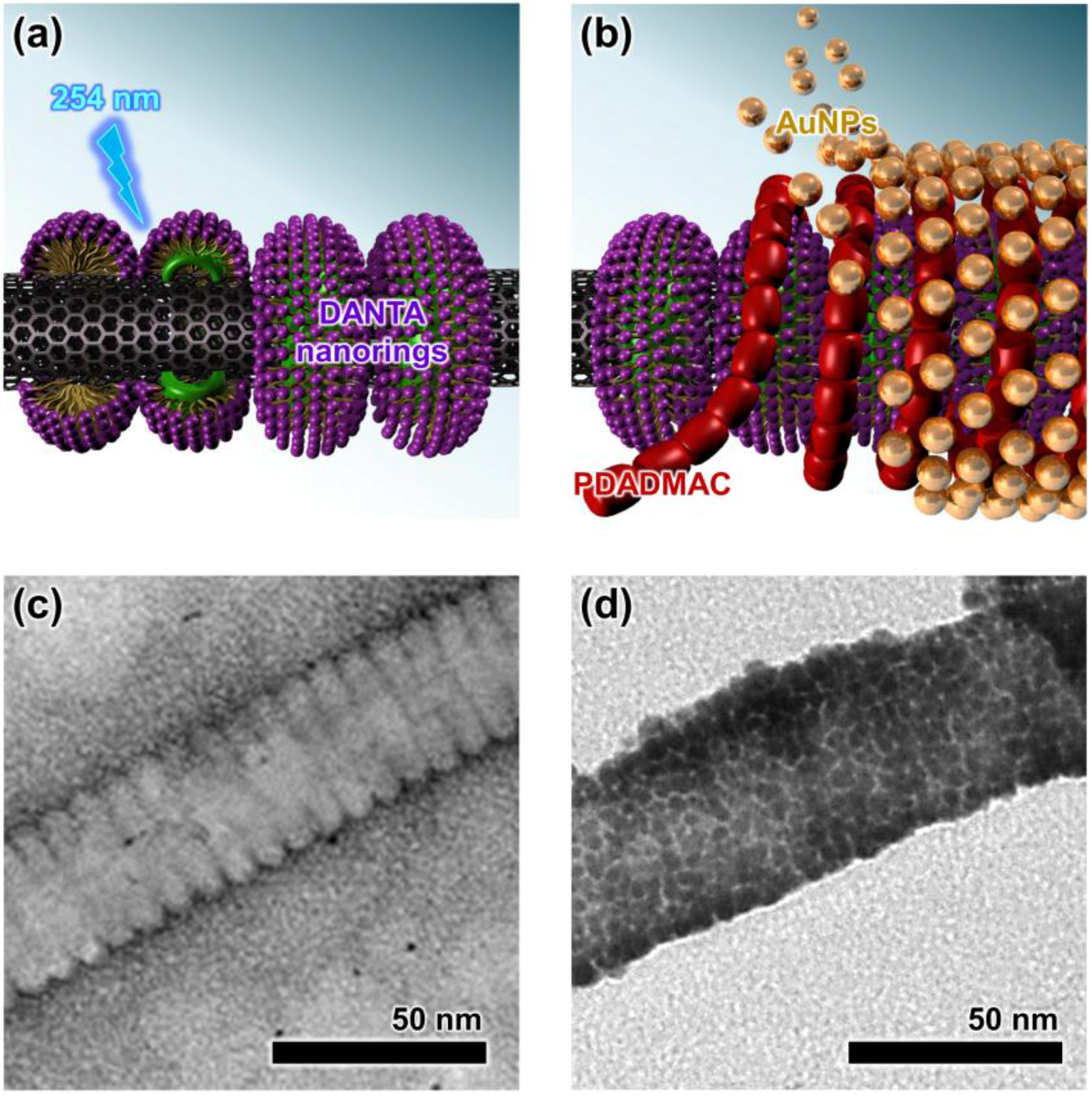
2.1. Assembly of the AuCNT Nanohybrid
2.2. Characterization of the AuCNT Nanohybrid
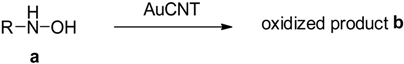
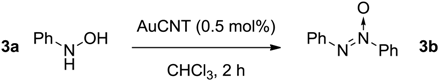
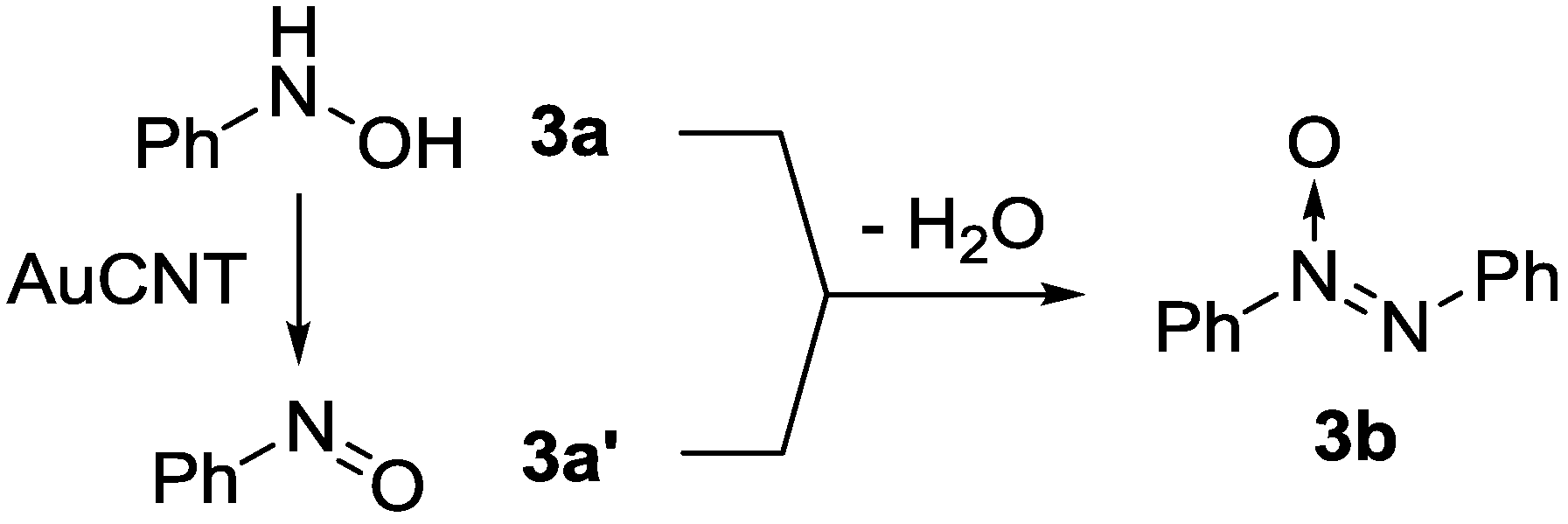
2.3. Oxidation of Hydroxylamines with the AuCNT Nanohybrid
2.3.1. Recycling of the AuCNT Nanohybrid
2.3.2. Is AuCNT a Heterogeneous Catalyst?
3. Experimental Section
3.1. Assembly of the Nanohybrid
3.2. Procedure for the Oxidation of Hydroxylamines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donohoe, T.J. Oxidation and Reduction in Organic Synthesis; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Hussain, H.; Green, I.R.; Ahmed, I. Journey describing applications of oxone in synthetic chemistry. Chem. Rev. 2013, 113, 3329–3371. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, R.A. Catalytic Oxidation: Principles and Applications; Sheldon, R.A., van Santen, R.A., Eds.; World Scientific: Singapore, 1995. [Google Scholar]
- Ali, M.E.; Shaheen, M.M.; Sarkar, M.; Hamid, S.B.A. Heterogeneous metal catalysts for oxidation reactions. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- John, J.; Gravel, E.; Namboothiri, I.N.N.; Doris, E. Advances in carbon nanotube-noble metal catalyzed organic transformations. Nanotechnol. Rev. 2012, 1, 515–539. [Google Scholar] [CrossRef]
- Singh, R.; Premkumar, T.; Shin, J.Y.; Geckeler, K.E. Carbon nanotube and gold-based materials: A symbiosis. Chem. Eur. J. 2010, 16, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef] [PubMed]
- Gravel, E.; Namboothiri, I.N.N.; Doris, E. Supramolecular assembly of gold nanoparticles on carbon nanotubes and catalysis of selected organic transformations. Synlett 2016, 27. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Gravel, E.; Hagège, A.; Li, H.; Gacoin, T.; Doris, E. Catalytic oxidation of silanes by carbon nanotube-gold nanohybrid. Angew. Chem. Int. Ed. 2011, 50, 7533–7536. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gravel, E.; Hagège, A.; Li, H.; Jawale, D.V.; Verma, D.; Namboothiri, I.N.N.; Doris, E. Carbon nanotube-gold nanohybrids for selective catalytic oxidation of alcohols. Nanoscale 2013, 5, 6491–6497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gravel, E.; Hagège, A.; Li, H.; Verma, D.; Namboothiri, I.N.N.; Doris, E. Direct reductive amination of aldehydes catalyzed by CNT-gold nanohybrids. ChemCatChem 2013, 5, 3571–3575. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Geertsen, V.; Li, H.; Shah, N.; Namboothiri, I.N.N.; Doris, E. Aerobic oxidation of phenols and related compounds using carbon nanotube-gold nanohybrid catalysts. ChemCatChem 2014, 6, 719–723. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Geersten, V.; Li, H.; Shah, N.; Namboothiri, I.N.N.; Doris, E. Size effect of gold nanoparticles supported on carbon nanotube as catalysts in selected organic reactions. Tetrahedron 2014, 70, 6140–6145. [Google Scholar] [CrossRef]
- Shah, N.; Gravel, E.; Jawale, D.V.; Doris, E.; Namboothiri, I.N.N. Carbon nanotube-gold nanohybrid catalyzed N-formylation of amines using aqueous formaldehyde. ChemCatChem 2014, 6, 2201–2205. [Google Scholar] [CrossRef]
- Shah, N.; Gravel, E.; Jawale, D.V.; Doris, E.; Namboothiri, I.N.N. Synthesis of quinoxalines via carbon nanotube-gold nanohybrid catalyzed cascade reaction of vicinal diols and ketoalcohols with diamines. ChemCatChem 2015, 7, 57–61. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Selective conversion of nitroarenes using a carbon nanotube-ruthenium nanohybrid. Chem. Commun. 2015, 51, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Room temperature Suzuki coupling of aryl iodides, bromides, and chlorides using a heterogeneous carbon nanotube-palladium nanohybrid catalyst. Catal. Sci. Technol. 2015, 5, 2388–2392. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Shah, N.; Dauvois, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Cooperative dehydrogenation of N-heterocycles using a carbon nanotube-rhodium nanohybrid. Chem. Eur. J. 2015, 21, 7039–7042. [Google Scholar]
- Donck, S.; Gravel, E.; Shah, N.; Jawale, D.V.; Doris, E.; Namboothiri, I.N.N. Tsuji-Wacker oxidation of terminal olefins using a palladium-carbon nanotube nanohybrid. ChemCatChem 2015, 7, 2318–2322. [Google Scholar] [CrossRef]
- Donck, S.; Gravel, E.; Shah, N.; Jawale, D.V.; Doris, E.; Namboothiri, I.N.N. Deoxygenation of amine N-oxides using gold nanoparticles supported on carbon nanotubes. RSC Adv. 2015, 5, 50865–50868. [Google Scholar] [CrossRef]
- Donck, S.; Gravel, E.; Li, A.; Prakash, P.; Shah, N.; Leroy, J.; Namboothiri, I.N.N.; Doris, E. Mild and selective catalytic oxidation of organic substrates by a carbon nanotube-rhodium nanohybrid. Catal. Sci. Technol. 2015, 5, 4542–4546. [Google Scholar] [CrossRef]
- Richard, C.; Balavoine, F.; Schultz, P.; Ebbesen, T.W.; Mioskowski, C. Supramolecular self-assembly of lipid derivatives on carbon nanotubes. Science 2003, 300, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Tamura, H.; Hatada, M.; Ishihara, T. Study of photoreaction processes of PDA Langmuir films. Langmuir 1988, 4, 903–906. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1443. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters. Formation and particle size variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Conte, M.; Hutchings, G.H. Modern Gold Catalyzed Synthesis; Hashmi, A.S.K., Toste, F.D., Eds.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Gowenlock, B.G.; Richter-Addo, G.B. Preparations of C-nitroso compounds. Chem. Rev. 2004, 104, 3315–3340. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]

| Entry | Substrate a | Product b | Yield (%) |
|---|---|---|---|
| 1 |  | 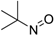 | 81 [b] |
| 2 |  | 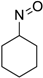 | 83 [b] |
| 3 |  | 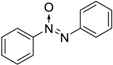 | 96 [c] |
| 4 | 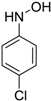 |  | 98 [c] |
| 5 | 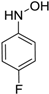 | 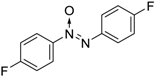 | 92 [c] |
| 6 | 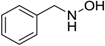 | 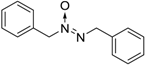 | 94 [c] |
| 7 |  | NR | -- [c] |
| Entry | AuCNT | Yield (%) |
|---|---|---|
| 1 | fresh | 96 |
| 2 | 1st reuse | 93 |
| 3 | 2nd reuse | 91 |
| 4 | 3rd reuse | 93 |
| 5 | 4th reuse | 94 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.; Basu, P.; Prakash, P.; Donck, S.; Gravel, E.; Namboothiri, I.N.N.; Doris, E. Supramolecular Assembly of Gold Nanoparticles on Carbon Nanotubes: Application to the Catalytic Oxidation of Hydroxylamines. Nanomaterials 2016, 6, 37. https://doi.org/10.3390/nano6030037
Shah N, Basu P, Prakash P, Donck S, Gravel E, Namboothiri INN, Doris E. Supramolecular Assembly of Gold Nanoparticles on Carbon Nanotubes: Application to the Catalytic Oxidation of Hydroxylamines. Nanomaterials. 2016; 6(3):37. https://doi.org/10.3390/nano6030037
Chicago/Turabian StyleShah, Nimesh, Pallabita Basu, Praveen Prakash, Simon Donck, Edmond Gravel, Irishi N. N. Namboothiri, and Eric Doris. 2016. "Supramolecular Assembly of Gold Nanoparticles on Carbon Nanotubes: Application to the Catalytic Oxidation of Hydroxylamines" Nanomaterials 6, no. 3: 37. https://doi.org/10.3390/nano6030037
APA StyleShah, N., Basu, P., Prakash, P., Donck, S., Gravel, E., Namboothiri, I. N. N., & Doris, E. (2016). Supramolecular Assembly of Gold Nanoparticles on Carbon Nanotubes: Application to the Catalytic Oxidation of Hydroxylamines. Nanomaterials, 6(3), 37. https://doi.org/10.3390/nano6030037