Shape Evolution of Hierarchical W18O49 Nanostructures: A Systematic Investigation of the Growth Mechanism, Properties and Morphology-Dependent Photocatalytic Activities
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Samples
2.2. Material Characterization
2.3. Photocatalytic Evaluation
2.4. Photoelectrochemical Measurements
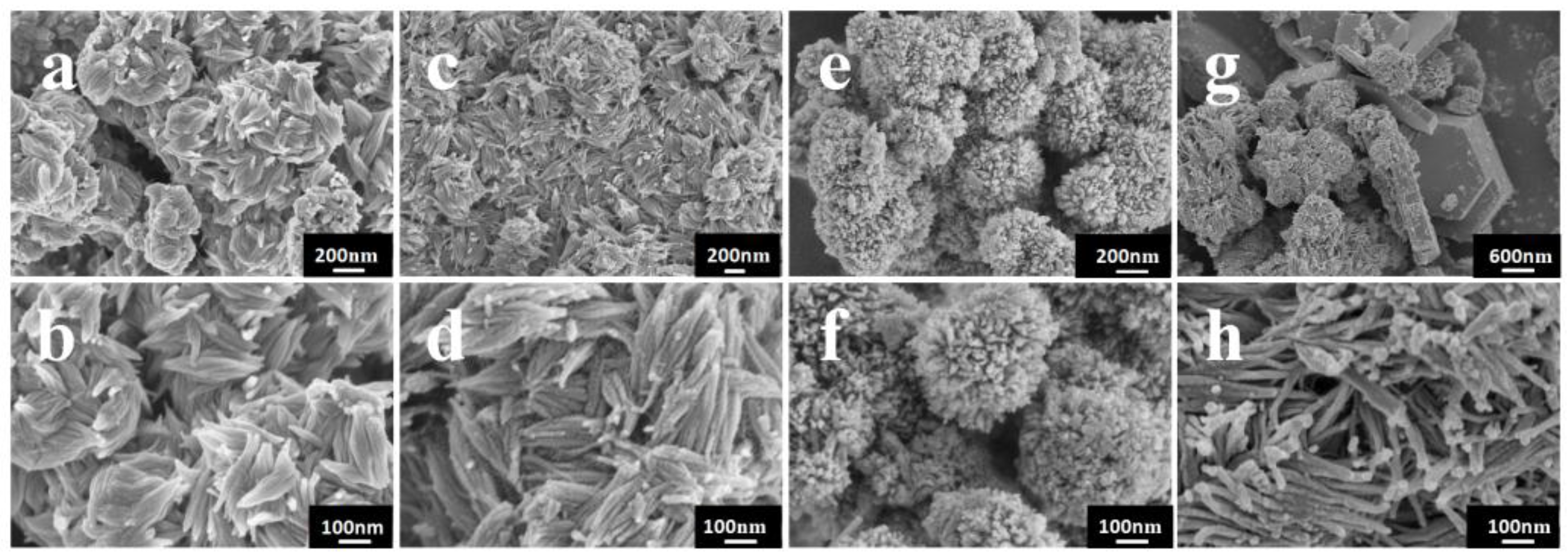
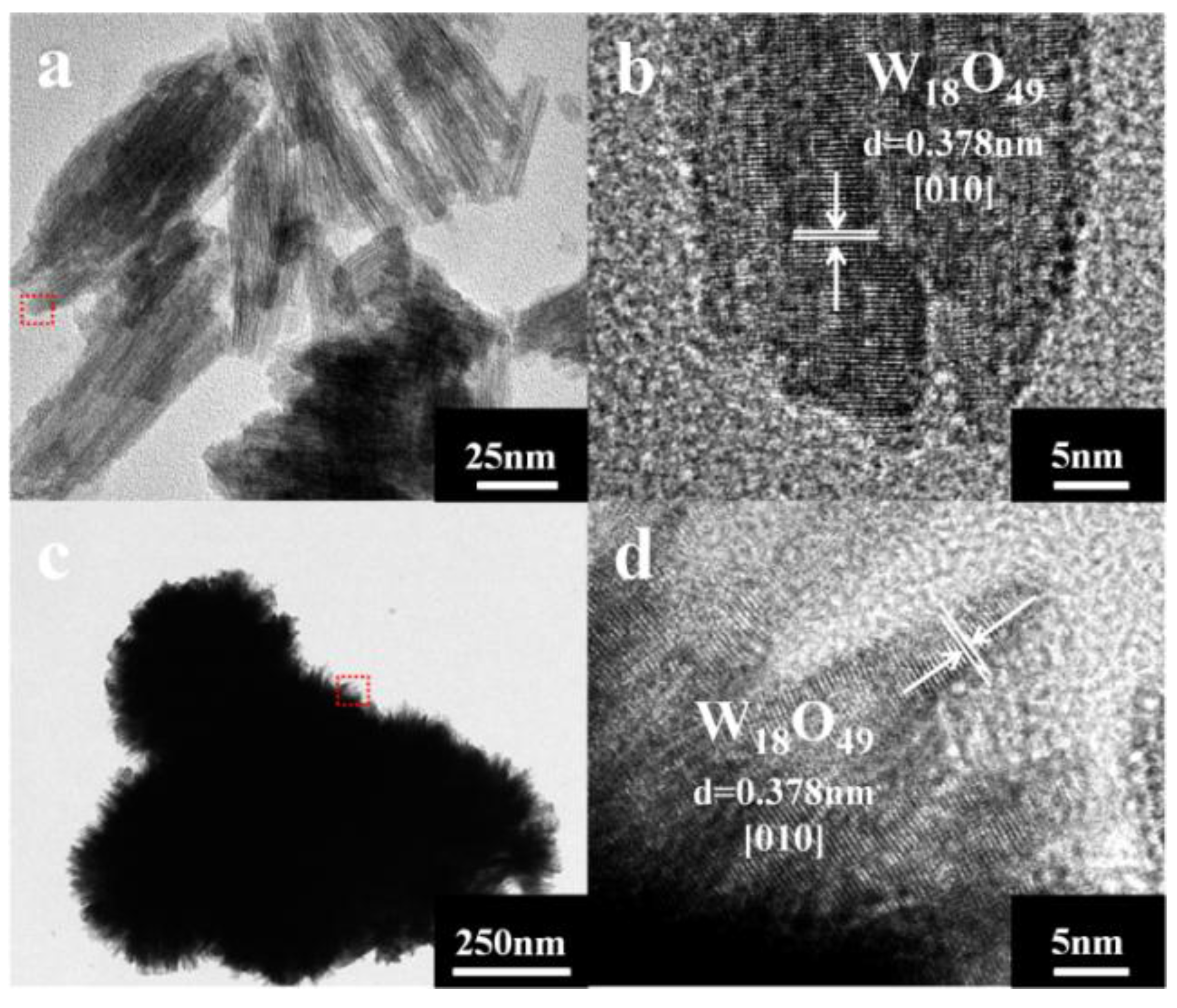
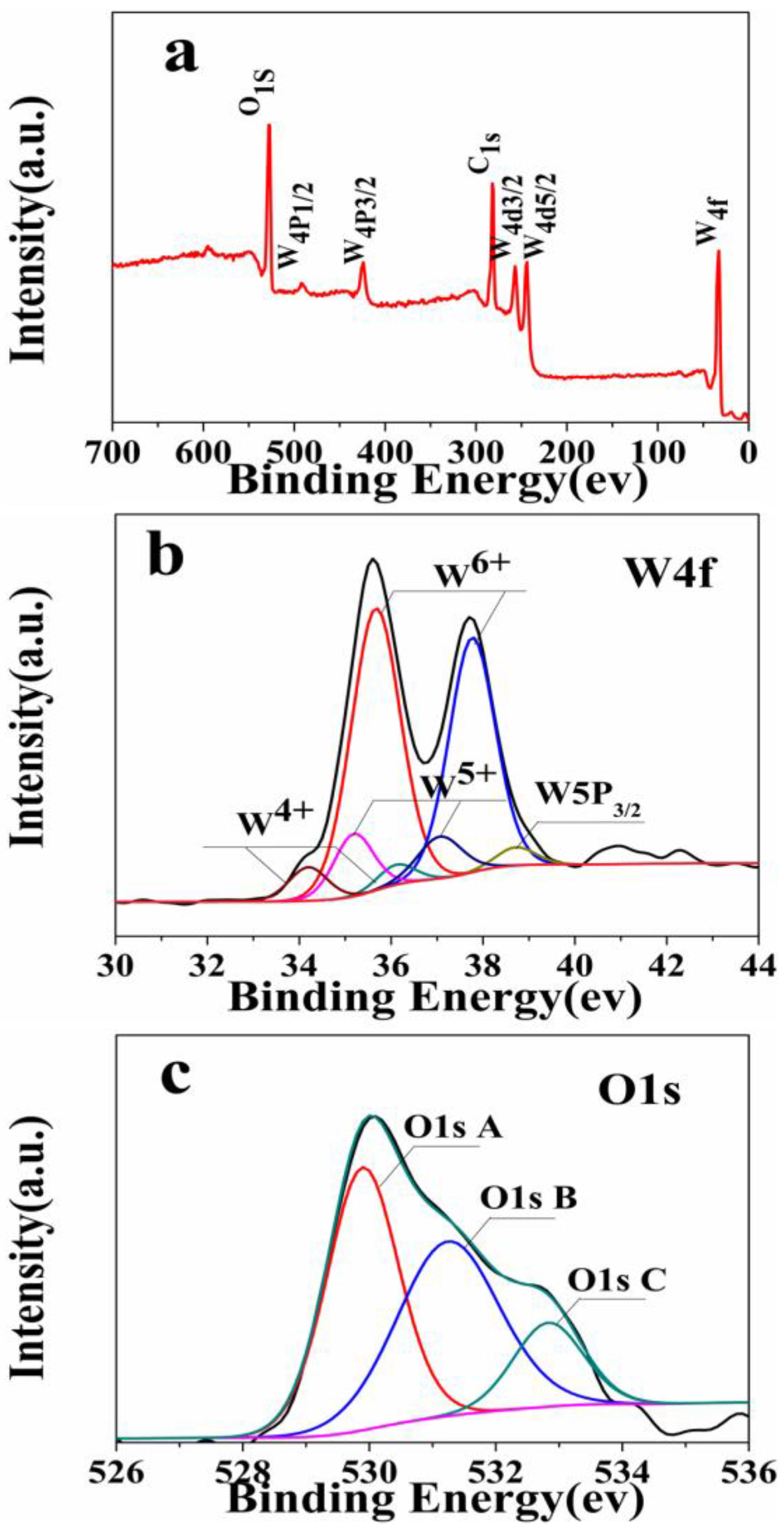
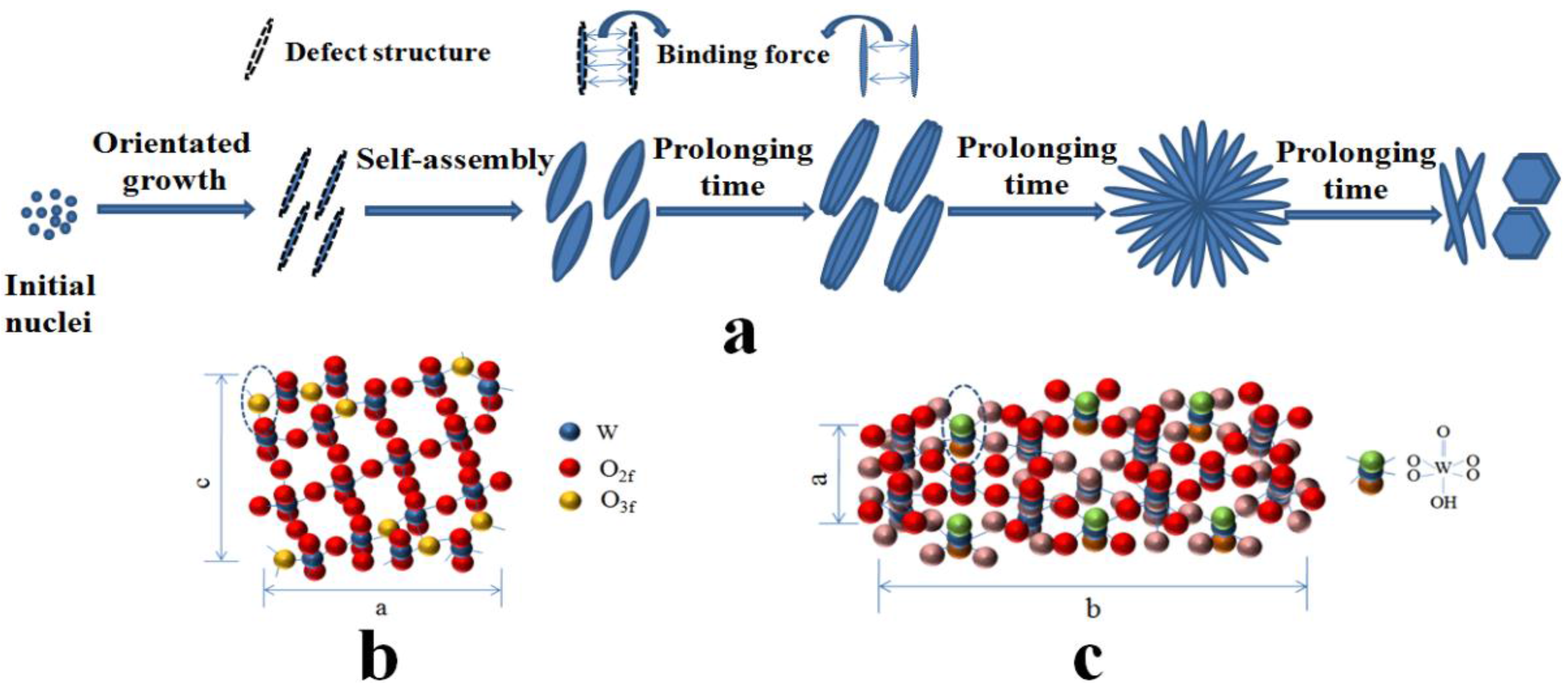
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Choi, W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]
- Artioli, G.A.; Mancini, A.; Barbieri, V.R.; Quattrini, M.C.; Quartarone, E.; Mozzati, M.C.; Drera, G.; Sangaletti, L.; Gombac, V.; Fornasiero, P.; et al. Correlation between Deposition Parameters and Hydrogen Production in CuO Nanostructured Thin Films. Langmuir 2016, 32, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhou, J.; Zhu, H.; Yang, W.; Liu, J.; Wang, Y.; Zou, Z. Constructing a High-Efficiency MoO3/Polyimide Hybrid Photocatalyst Based on Strong Interfacial Interaction. ACS Appl. Mater. Interf. 2015, 7, 14628–14637. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Müller, R.; Singh, J.; Mathur, S. Tailoring surface states in WO3 photoanodes for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2015, 347, 448–453. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Lee, H.C.; Bard, A.J. Rapid Screening by Scanning Electrochemical Microscopy (SECM) of Dopants for Bi2WO6 Improved Photocatalytic Water Oxidation with Zn Doping. J. Phys. Chem. C 2013, 117, 9633–9640. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile fabrication of highly efficient g-C3N4/Ag2O heterostructured photocatalysts with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interf. 2013, 5, 12533–12540. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Q.; Wang, H.; Zhang, L.; Song, G.; Song, L.; Hu, J.; Wang, H.; Liu, J.; Zhu, M.; Zhao, D. Ultrathin PEGylated W18O49 nanowires as a new 980 nm-laser-driven photothermal agent for efficient ablation of cancer cells in vivo. Adv. Mater. 2013, 25, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Zhang, S.H.; Guo, C.S.; Liu, S.Q. Absorption and electrochromic modulation of near-infrared light: Realized by tungsten suboxide. Nanoscale 2016, 8, 9861–9868. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.J.; Zheng, J.; Wang, J.L.; Xu, J.; Li, H.H.; Yu, S.H. Ultrathin W18O49 nanowire assemblies for electrochromic devices. Nano Lett. 2013, 13, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xiao, F.; Yang, C.; Wang, J.; Su, X. Hydrothermal fabrication of W18O49 nanowire networks with superior performance for water treatment. J. Mater. Chem. A 2013, 1, 5831. [Google Scholar] [CrossRef]
- Liu, F.; Mo, F.Y.; Jin, S.Y.; Li, L.; Chen, Z.S.; Sun, R.; Chen, J.; Deng, S.Z.; Xu, N.S. A novel lift-off method for fabricating patterned and vertically-aligned W18O49 nanowire arrays with good field emission performance. Nanoscale 2011, 3, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yin, S.; Yan, M.; Kobayashi, M.; Kakihana, M.; Sato, T. Morphology-controlled synthesis of W18O49 nanostructures and their near-infrared absorption properties. Inorg. Chem. 2012, 51, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Hua, C.Z. An inorganic route for controlled synthesis of W18O49 nanorods and nanofibers in solution. Inorg. Chem. 2003, 42, 6169–6171. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, H.; Li, G.; Lu, Z.; Xiao, S.; Chen, R. Citrate/Urea/Solvent Mediated Self-Assembly of (BiO)2CO3 Hierarchical Nanostructures and Their Associated Photocatalytic Performance. Ind. Eng. Chem. Res. 2013, 52, 12604–12612. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X. Enhanced fluorescence imaging performance of hydrophobic colloidal ZnO nanoparticles by a facile method. J. Alloys Comp. 2015, 619, 98–101. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Li, C. Chemical and structural analysis of solvothermal synthesized tungsten oxide nanotube without template and its hydrogen sensitive property. J. Alloys Comp. 2014, 584, 546–552. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, H.; Zhu, X.; Li, D.; Zhang, S.; Chen, X.; Song, Y. Electropolymerization of Aniline onto Anodic WO3 Film: An Approach to Extend Polyaniline Electroactivity Beyond pH 7. J. Phys. Chem. C 2014, 118, 27449–27458. [Google Scholar] [CrossRef]
- Bai, H.; Su, N.; Li, W.; Zhang, X.; Yan, Y.; Li, P.; Ouyang, S.; Ye, J.; Xi, G. W18O49 nanowire networks for catalyzed dehydration of isopropyl alcohol to propylene under visible light. J. Mater. Chem. A 2013, 1, 6125–6129. [Google Scholar] [CrossRef]
- Jeon, S.; Yong, K. Direct synthesis of W18O49 nanorods from W2N film by thermal annealing. Nanotechnology 2007, 18, 245602. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, H.Y.; Yang, X.N.; Guo, X. NO sensing by single crystalline WO3 nanowires. Sens. Actuators B 2015, 219, 346–353. [Google Scholar] [CrossRef]
- Saleem, M.; Al-Kuhaili, M.F.; Durrani, S.M.A.; Hendi, A.H.Y.; Bakhtiari, I.A.; Ali, S. Influence of hydrogen annealing on the optoelectronic properties of WO3 thin films. Int. J. Hydrog. Energy 2015, 40, 12343–12351. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Guo, C.S.; Yan, M.; Liu, S.Q. CsxWO3 nanorods: Realization of full-spectrum-responsive photocatalytic activities from UV, visible to near-infrared region. Appl. Catal. B 2016, 183, 142–148. [Google Scholar] [CrossRef]
- Yadav, K.; Jaggi, N. Aging effect on the structural and optical properties of ZnSe nanostructures. J. Mater. Sci. Mater. Electr. 2015, 27, 393–398. [Google Scholar] [CrossRef]
- Yan, M.; Li, G.L.; Guo, C.S.; Guo, W.; Ding, D.D.; Zhang, S.H.; Liu, S. WO3−x sensitized TiO2 spheres with full-spectrum-driven photocatalytic activities from UV to near infrared. Nanoscale 2016, 8, 17828–17835. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.K.; Basu, J.; Kashyap, S.; Chawake, N.; Yadav, D.; Murty, B.S. Crystallographic-shear-phase-driven W18O49 nanowires growth on nanocrystalline W surfaces. Scr. Mater. 2016, 115, 28–32. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Y.; Dong, Q.; Lin, J.; Wang, A.; Ma, T. Surface Oxygen Vacancy-Dependent Electrocatalytic Activity of W18O49 Nanowires. J. Phys. Chem. C 2014, 118, 20100–20106. [Google Scholar] [CrossRef]
- Lu, D.Y.; C, J.; Deng, S.Z.; Xu, N.S.; Zhang, W.H. The most powerful tool for the structural analysis of tungsten suboxide nanowires: Raman spectroscopy. J. Mater. Res. 2008, 23, 402–408. [Google Scholar] [CrossRef]
- Qin, Y.X.; Shen, W.J.; Li, X.; Hu, M. Effect of annealing on microstructure and NO2− sensing properties of tungsten oxide nanowires synthesized by solvothermal method. Sens. Actuators B 2011, 155, 646–652. [Google Scholar] [CrossRef]
- Xiao, B.X.; Zhao, Q.; Xiao, C.H.; Yang, T.Y.; Wang, P.; Wang, F.; Chen, X.D.; Zhang, M.Z. Low-temperature solvothermal synthesis of hierarchical flowerlike WO3 nanostructures and their sensing properties for H2S. Crystengcomm 2015, 17, 5710–5716. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, J.; Yu, M.M.; Lu, P.; Wei, J.; Qian, Y.Q.; Wang, Y.; Yu, C. Green synthesis of hexagonal-shaped WO3·0.33H2O nanodiscs composed of nanosheets. Cryst. Growth Des. 2008, 8, 3993–3998. [Google Scholar] [CrossRef]
- Gerand, B.; Nowogrocki, G.; Figlarz, M. A new tungsten trioxide hydrate, WO3·0.33H2O: Preparation, characterization, and crystallographic study. J. Solid State Chem. 1981, 38, 312–320. [Google Scholar] [CrossRef]
- Chen, G.; Sun, M.; Wei, Q.; Zhang, Y.; Zhu, B.; Du, B. Ag3PO4/graphene-oxide composite with remarkably enhanced visible-light-driven photocatalytic activity toward dyes in water. J. Hazard. Mater. 2013, 244–245, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xing, Z.; Yu, X.; Cheng, X. One-step hydrothermal synthesis of C-N-S-tridoped TiO2-based nanosheets photoelectrode for enhanced photoelectrocatalytic performance and mechanism. Electrochim. Acta 2015, 170, 182–190. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, J.; Dong, F.; Chu, P.K.; Zhang, T.; Zhang, Y. Anionic Group Self-Doping as a Promising Strategy: Band-Gap Engineering and Multi-Functional Applications of High-Performance CO32−-Doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, Y.; Zhu, Y. Enhanced Photocatalytic Performance for the BiPO4−xNanorod Induced by Surface Oxygen Vacancy. J. Phys. Chem. C 2013, 117, 18520–18528. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Feng, B.; Wang, X.; Wan, B. Synthesis and photocatalytic property of ZnSe flowerlike hierarchical structure. Appl. Surf. Sci. 2011, 257, 10679–10685. [Google Scholar] [CrossRef]
- Bai, X.; Lv, L.; Zhang, X.; Hua, Z. Synthesis and photocatalytic properties of Palladium-loaded three dimensional flower-like anatase TiO2 with dominant {001} facets. J. Colloid Interf. Sci. 2016, 467, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Yu, T.; Zhang, L.Z.; Chen, X.Y.; Zou, Z.G. Photocatalytic Degradation of Acetaldehyde on Mesoporous TiO2: Effects of Surface Area and Crystallinity on the Photocatalytic Activity. Chin. J. Chem. Phys. 2007, 20, 733–738. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, G.; Huang, J.; Cao, L.; Jie, Y.; Li, J.; Wang, X. Shape Evolution of Hierarchical W18O49 Nanostructures: A Systematic Investigation of the Growth Mechanism, Properties and Morphology-Dependent Photocatalytic Activities. Nanomaterials 2016, 6, 240. https://doi.org/10.3390/nano6120240
Hai G, Huang J, Cao L, Jie Y, Li J, Wang X. Shape Evolution of Hierarchical W18O49 Nanostructures: A Systematic Investigation of the Growth Mechanism, Properties and Morphology-Dependent Photocatalytic Activities. Nanomaterials. 2016; 6(12):240. https://doi.org/10.3390/nano6120240
Chicago/Turabian StyleHai, Guojuan, Jianfeng Huang, Liyun Cao, Yanni Jie, Jiayin Li, and Xing Wang. 2016. "Shape Evolution of Hierarchical W18O49 Nanostructures: A Systematic Investigation of the Growth Mechanism, Properties and Morphology-Dependent Photocatalytic Activities" Nanomaterials 6, no. 12: 240. https://doi.org/10.3390/nano6120240
APA StyleHai, G., Huang, J., Cao, L., Jie, Y., Li, J., & Wang, X. (2016). Shape Evolution of Hierarchical W18O49 Nanostructures: A Systematic Investigation of the Growth Mechanism, Properties and Morphology-Dependent Photocatalytic Activities. Nanomaterials, 6(12), 240. https://doi.org/10.3390/nano6120240




