A Facile Method to In-Situ Synthesize Porous Ni2GeO4 Nano-Sheets on Nickel Foam as Advanced Anode Electrodes for Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
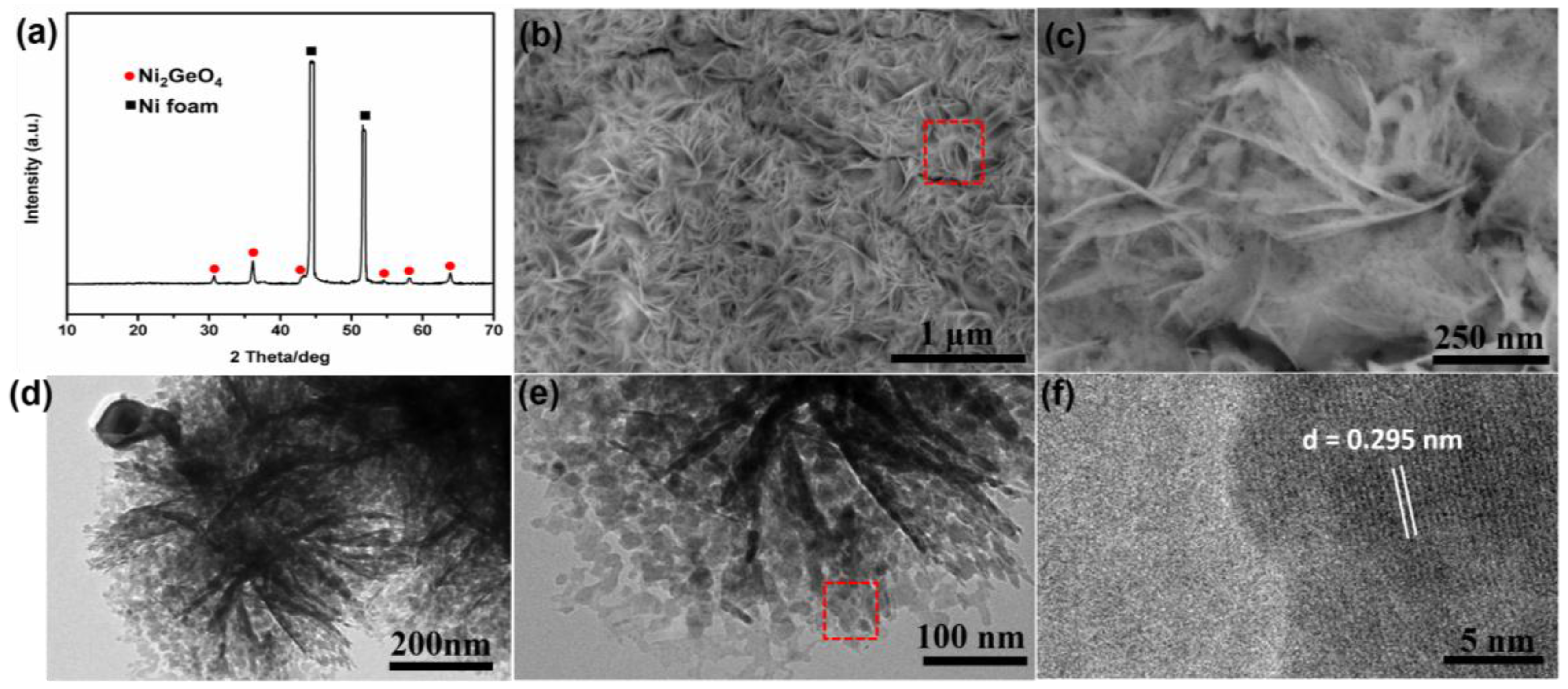
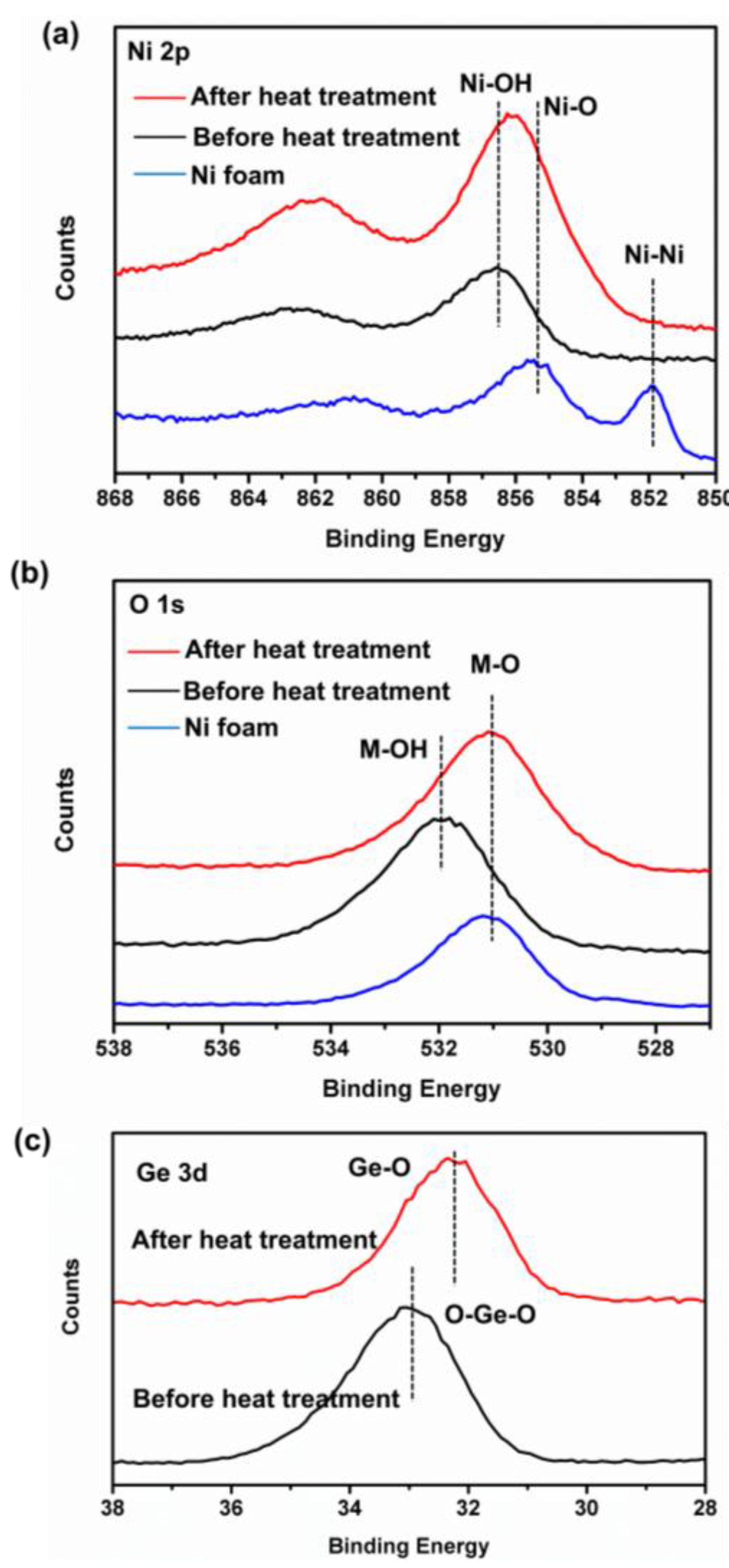
3.1. Electrode Synthesis and Microstructure Characterization
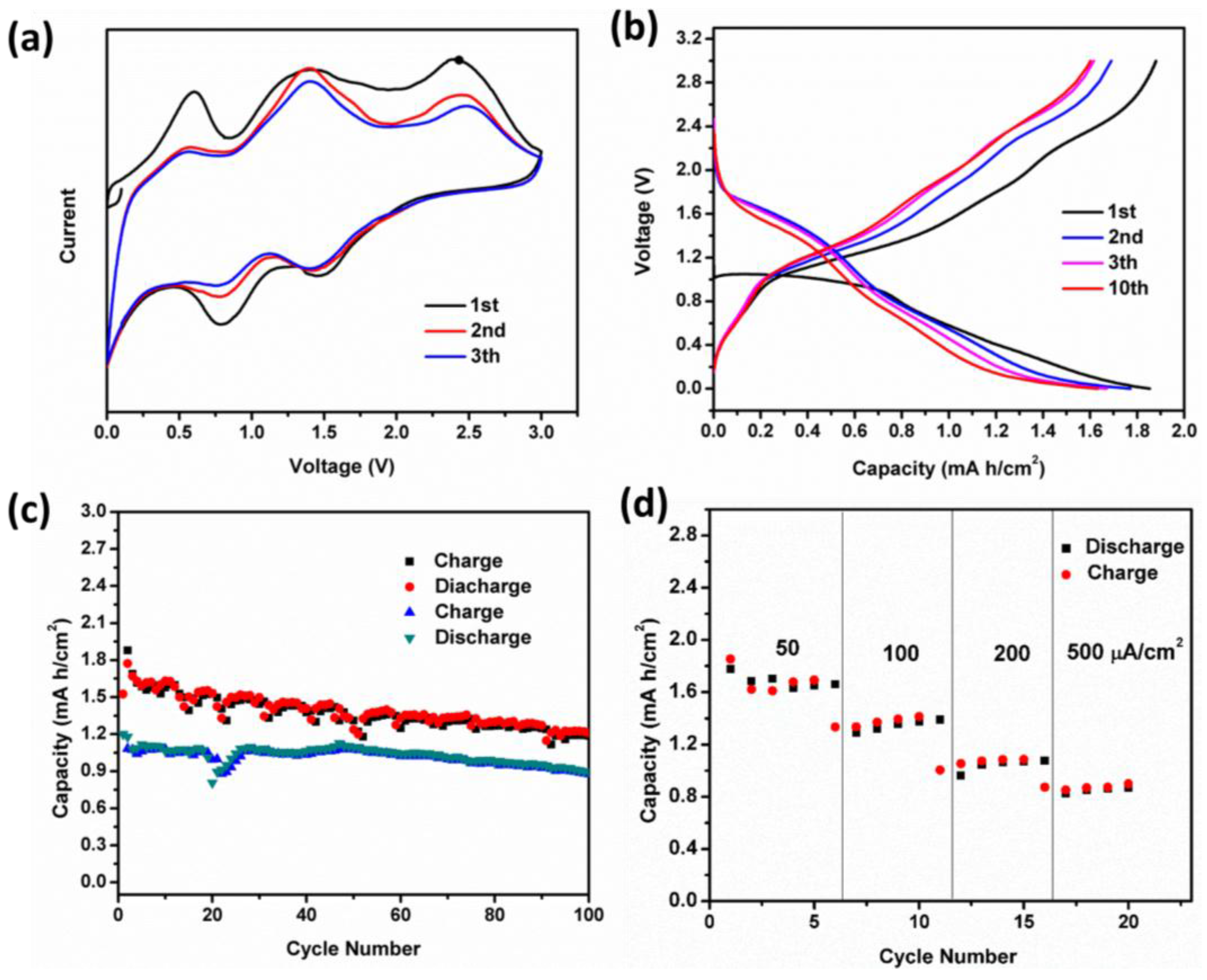
3.2. Cell Assembly and Electrochemical Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, S.; Wang, R.; Wang, Z.; Lin, Z. CuGeO3 nanowires covered with graphene as anode materials of lithium ion batteries with enhanced reversible capacity and cyclic performance. Nanoscale 2014, 6, 8350–8358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, X.; Chen, X.; Xue, Z. Fast preparation and growth mechanism of erythrocyte-like Cd2Ge2O6 superstructures via a microwave-hydrothermal process. CrystEngComm. 2011, 13, 2464–2471. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, B.; Chen, G.; Yu, G.; Xie, Z.; Gao, L.; Chen, D.; Shen, G. Contact printing of horizontally aligned Zn2GeO4 and In2Ge2O7 nanowire arrays for multi-channel field-effect transistors and their photoresponse performances. J. Mater. Chem. C 2013, 1, 131–137. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Qie, L.; Jiang, Y.; Xiong, X.; Qiao, Y.; Huang, Y. Facile synthesis of sandwiched Zn2GeO4–graphene oxide nanocomposite as a stable and high-capacity anode for lithium-ion batteries. Nanoscale 2014, 6, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-Yield Synthesis of Ultralong and Ultrathin Zn2GeO4 Nanoribbons Toward Improved Photocatalytic Reduction of CO2 into Renewable Hydrocarbon Fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qi, Y.; Jia, C.J.; Jin, Z.; Fan, W. Enhanced visible-light photocatalytic activity of g-C3N4/Zn2GeO4 heterojunctions with effective interfaces based on band match. Nanoscale 2014, 6, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Song, S.; Zhang, H. Co2GeO4 nanoplates and nano-octahedrons from low-temperature controlled synthesis and their magnetic properties. CrystEngComm 2012, 14, 7306–7311. [Google Scholar] [CrossRef]
- Ge, X.; Wang, X.Z.; Yao, S.; Feng, J.; Liu, D.; Zhang, H. Strongly Coupled Pt–Ni2GeO4 Hybrid Nanostructures as Potential Nanocatalysts for CO Oxidation. Chem. Eur. J. 2015, 21, 14768–14771. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, Y.X.; Xin, S.; Song, W.G.; Guo, Y.G. Low-cost and large-scale synthesis of alkaline earth metal germanate nanowires as a new class of lithium ion battery anode material. Energy Environ. Sci. 2012, 5, 8007–8013. [Google Scholar] [CrossRef]
- Ma, D.L.; Wang, H.G.; Li, Y.; Xu, D.; Yuan, S.; Huang, X.L.; Zhang, Y. In situ generated FeF3 in homogeneous iron matrix toward high-performance cathode material for sodium-ion batteries. Nano Energy 2014, 10, 95–304. [Google Scholar] [CrossRef]
- Ma, D.L.; Cao, Z.Y.; Hu, A.M. Si-Based Anode Materials for Li-Ion Batteries: A Mini Review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W.D. Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Gillot, F.; Boyanov, S.; Dupont, L.; Doublet, M.L.; Morcrette, M.; Monconduit, L.; Tarascon, J.M. Electrochemical Reactivity and Design of NiP2 Negative Electrodes for Secondary Li-Ion Batteries. Chem. Mater. 2005, 17, 6327–6337. [Google Scholar] [CrossRef]
- Boyanov, S.; Bernardi, J.; Bekaert, E.; Menetrier, M.; Doublet, M.L.; Monconduit, L. P-Redox Mechanism at the Origin of the High Lithium Storage in NiP2-Based Batteries. Chem. Mater. 2009, 21, 298–308. [Google Scholar] [CrossRef]
- Fan, Q.; Chupas, P.J.; Whittingham, M.S. Characterization of Amorphous and Crystalline Tin–Cobalt Anodes. Electrochem. Solid-State Lett. 2007, 10, A274–A278. [Google Scholar] [CrossRef]
- Tsygankov, V.N.; Safonov, V.V.; Kozlov, A.I.; Gavrilov, V.P. Electrical Properties of GeO2–NiO Materials Containing Ni2GeO4. Inorg. Mater. 2003, 39, 1076–1078. [Google Scholar] [CrossRef]
- Tarte, P. Infra-Red Spectrum of the Spinels Ni2SiO4, Ni2GeO4 and their Solid Solutions. Nature 1962, 193, 971–972. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.F.; Deng, J.W.; Xin, S.; Ji, H.X.; Schmidt, O.G.; Wan, L.J.; Guo, Y.G. Cu-Si Nanocable Arrays as High-Rate Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2011, 23, 4415–4420. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, Y.; Song, H.K.; Lee, K.T.; Cho, J. Carbon-coated single-crystal LiMn2O4 nanoparticle clusters as cathode material for high-energy and high-power lithium-ion batteries. Angew. Chem. Int. Ed. 2012, 51, 8748–8752. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-Structured Phosphorus Composite as High-Capacity Anode Materials for Lithium Batteries. Angew. Chem. Int. Ed. 2012, 51, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Mansour, A.N.; Melendres, C.A. Characterization of α-Ni(OH)2 by XPS. Surf. Sci. Spectra. 1994, 3, 255–260. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Ogino, T. Oxidation of Ge(100) and Ge(111) surfaces: An UPS and XPS study. Surf. Sci. 1995, 325, 263–271. [Google Scholar] [CrossRef]
- Li, C.; Gu, L.; Tong, J.; Tsukimoto, S.; Maier, J. A mesoporous iron based fluoride cathode of tunnel structure for rechargeable lithium batteries. Adv. Funct. Mater. 2011, 21, 1391. [Google Scholar] [CrossRef]
- Ma, D.L.; Cao, Z.Y.; Wang, H.G.; Huang, X.L.; Wang, L.M.; Zhang, X.B. Three-dimensionally ordered macroporous FeF3 and its in situ homogenous polymerization coating for high energy and power density lithium ion batteries. Energy Environ. Sci. 2012, 5, 8538–8542. [Google Scholar] [CrossRef]
- Courtel, F.M.; Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A = Co, Ni, and Zn). J. Mater. Chem. 2011, 21, 10206–10218. [Google Scholar] [CrossRef]
- Ma, D.L.; Yuan, S.; Huang, X.L.; Cao, Z.Y. Synthesis of Ultrathin GeO2–Reduced Graphene Oxide (RGO) Sheets for a High-Capacity Lithium-Ion Battery Anode. Energy Technology 2014, 2, 342–347. [Google Scholar] [CrossRef]
- Seng, K.H.; Park, M.H.; Guo, Z.P.; Liu, H.K.; Cho, J. Catalytic Role of Ge in Highly Reversible GeO2/Ge/C Nanocomposite Anode Material for Lithium Batteries. Nano lett. 2013, 13, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, Y.; Wang, X.; Chen, Q.; Wang, X.; Yang, S.; Yang, Z. Structure and electrochemical performance of FeF3/V2O5 composite cathode material for lithium-ion battery. J. Alloy. Compound. 2009, 486, 93–96. [Google Scholar] [CrossRef]
- Li, C.; Gu, L.; Tsukimoto, S.; van Aken, P.A.; Maier, J. Low-Temperature Ionic-Liquid-Based Synthesis of Nanostructured Iron-Based Fluoride Cathodes for Lithium Batteries. Adv. Mater. 2010, 22, 3650–3654. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Wessells, C.D.; Huggins, R.A.; Cui, Y. Highly Reversible Open Framework Nanoscale Electrodes for Divalent Ion Batteries. Nano lett. 2013, 13, 5748–5752. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Wang, R.Z.; Xu, D.; Wang, Z.L.; Wang, H.G.; Xu, J.J.; Zhang, X.B. Homogeneous CoO on Graphene for Binder-Free and Ultralong-Life Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 4345–4353. [Google Scholar] [CrossRef]
- Liu, H.; Su, D.; Zhou, R.; Sun, B.; Wang, G.; Qiao, S.Z. Highly Ordered Mesoporous MoS2 with Expanded Spacing of the (002) Crystal Plane for Ultrafast Lithium Ion Storage. Adv. Energy Mater. 2012, 2, 970–975. [Google Scholar] [CrossRef]
- Sun, X.L.; Chen, Y.; Chen, Y.; Si, W.P.; Deng, J.W.; Oswald, S.; Liu, L.F.; Schmidt, O.G. Three-Dimensionally “Curved” NiO Nanomembranes as Ultrahigh Rate Capability Anodes for Li-Ion Batteries with Long Cycle Lifetimes. Adv. Energy Mater. 2014, 4, 1300912. [Google Scholar] [CrossRef]
- Sun, X.L.; Si, W.P.; Liu, X.H.; Deng, J.W.; Xi, L.X.; Liu, L.F.; Yan, C.L.; Schmidt, O.G. Multifunctional Ni/NiO hybrid nanomembranes as anode materials for high-rate Li-ion batteries. Nano Energy 2014, 9, 168–175. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Shi, X.; Hu, A. A Facile Method to In-Situ Synthesize Porous Ni2GeO4 Nano-Sheets on Nickel Foam as Advanced Anode Electrodes for Li-Ion Batteries. Nanomaterials 2016, 6, 218. https://doi.org/10.3390/nano6110218
Ma D, Shi X, Hu A. A Facile Method to In-Situ Synthesize Porous Ni2GeO4 Nano-Sheets on Nickel Foam as Advanced Anode Electrodes for Li-Ion Batteries. Nanomaterials. 2016; 6(11):218. https://doi.org/10.3390/nano6110218
Chicago/Turabian StyleMa, Delong, Xiaomin Shi, and Anming Hu. 2016. "A Facile Method to In-Situ Synthesize Porous Ni2GeO4 Nano-Sheets on Nickel Foam as Advanced Anode Electrodes for Li-Ion Batteries" Nanomaterials 6, no. 11: 218. https://doi.org/10.3390/nano6110218
APA StyleMa, D., Shi, X., & Hu, A. (2016). A Facile Method to In-Situ Synthesize Porous Ni2GeO4 Nano-Sheets on Nickel Foam as Advanced Anode Electrodes for Li-Ion Batteries. Nanomaterials, 6(11), 218. https://doi.org/10.3390/nano6110218






