Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces
Abstract
:1. Introduction
2. Results
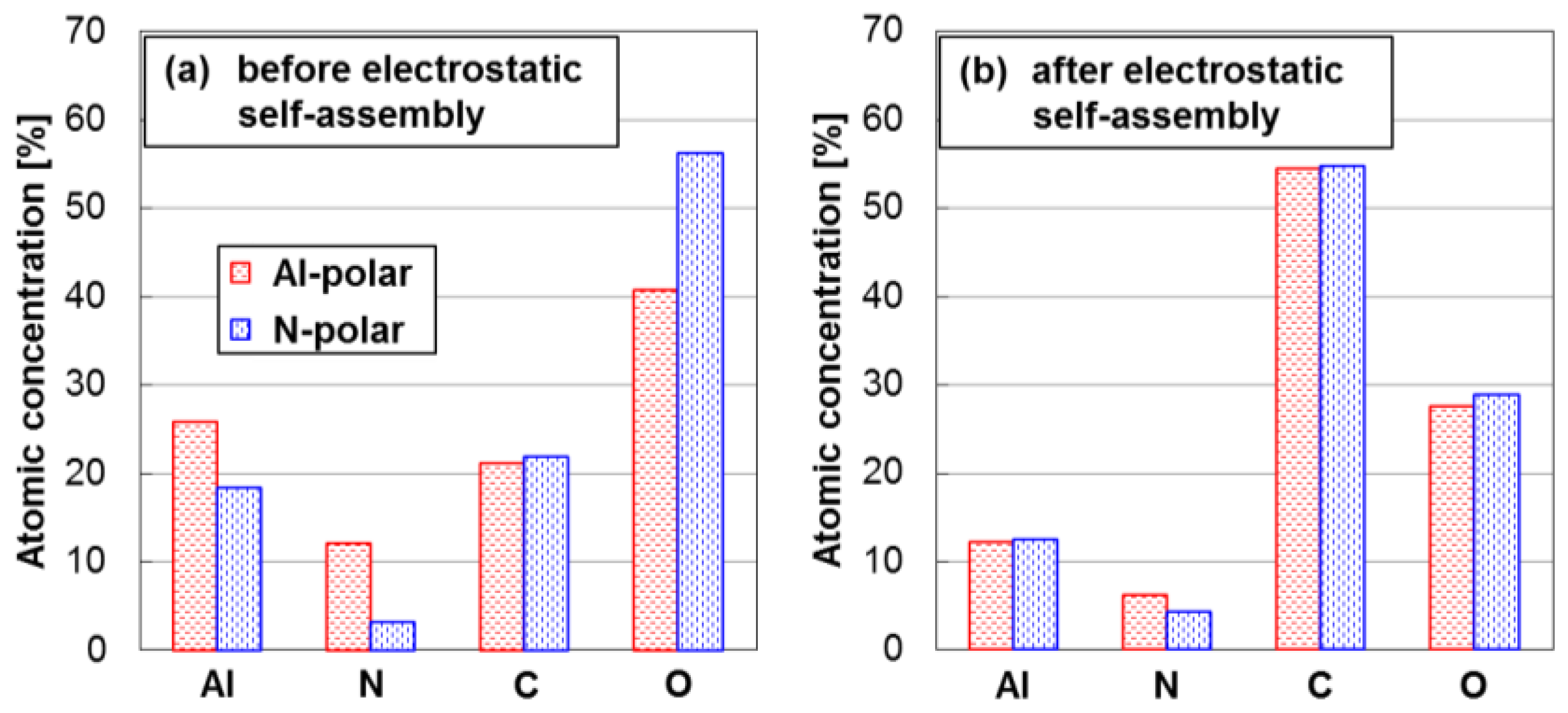
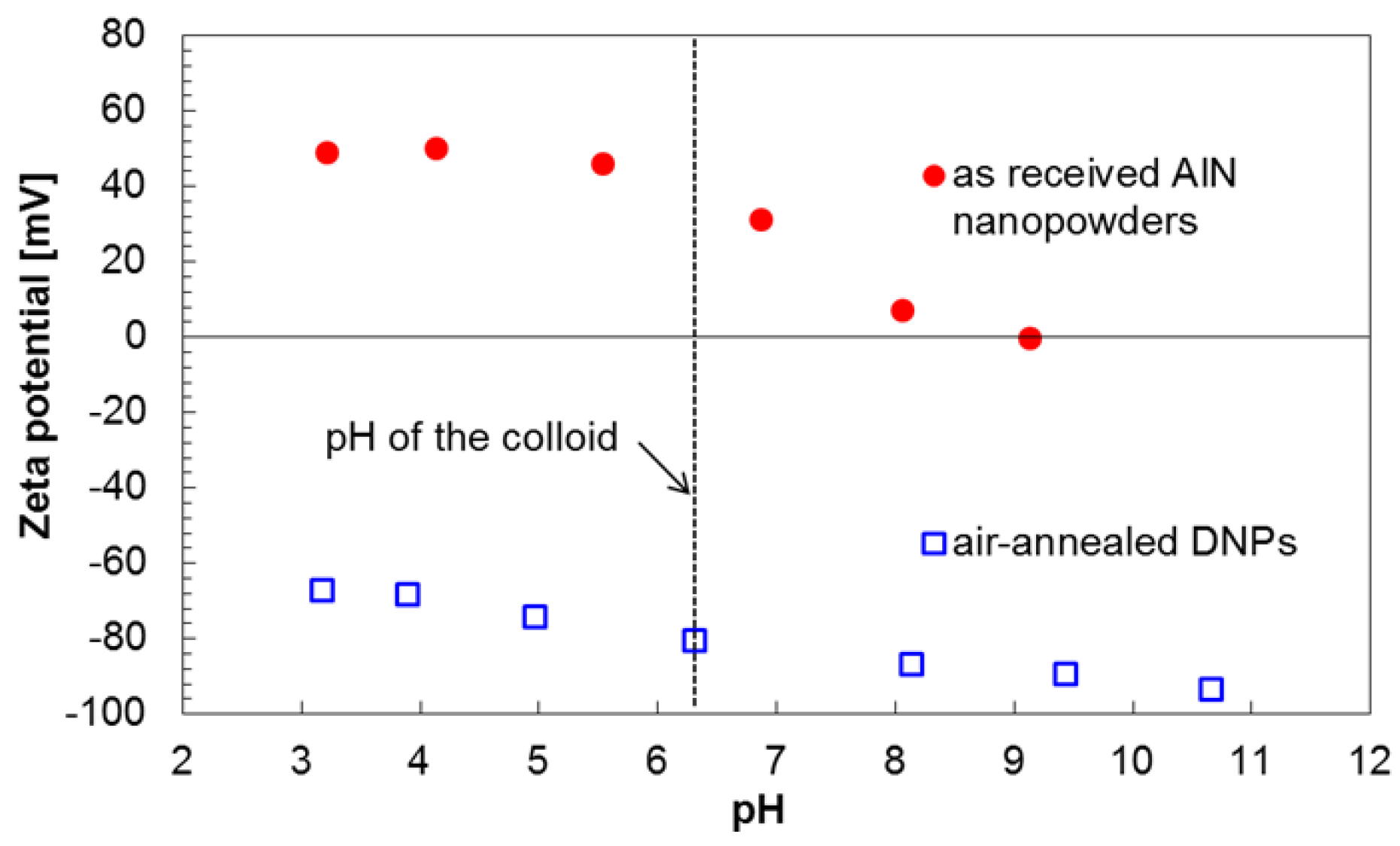
2.1. Properties of an Aqueous Colloid of DNPs
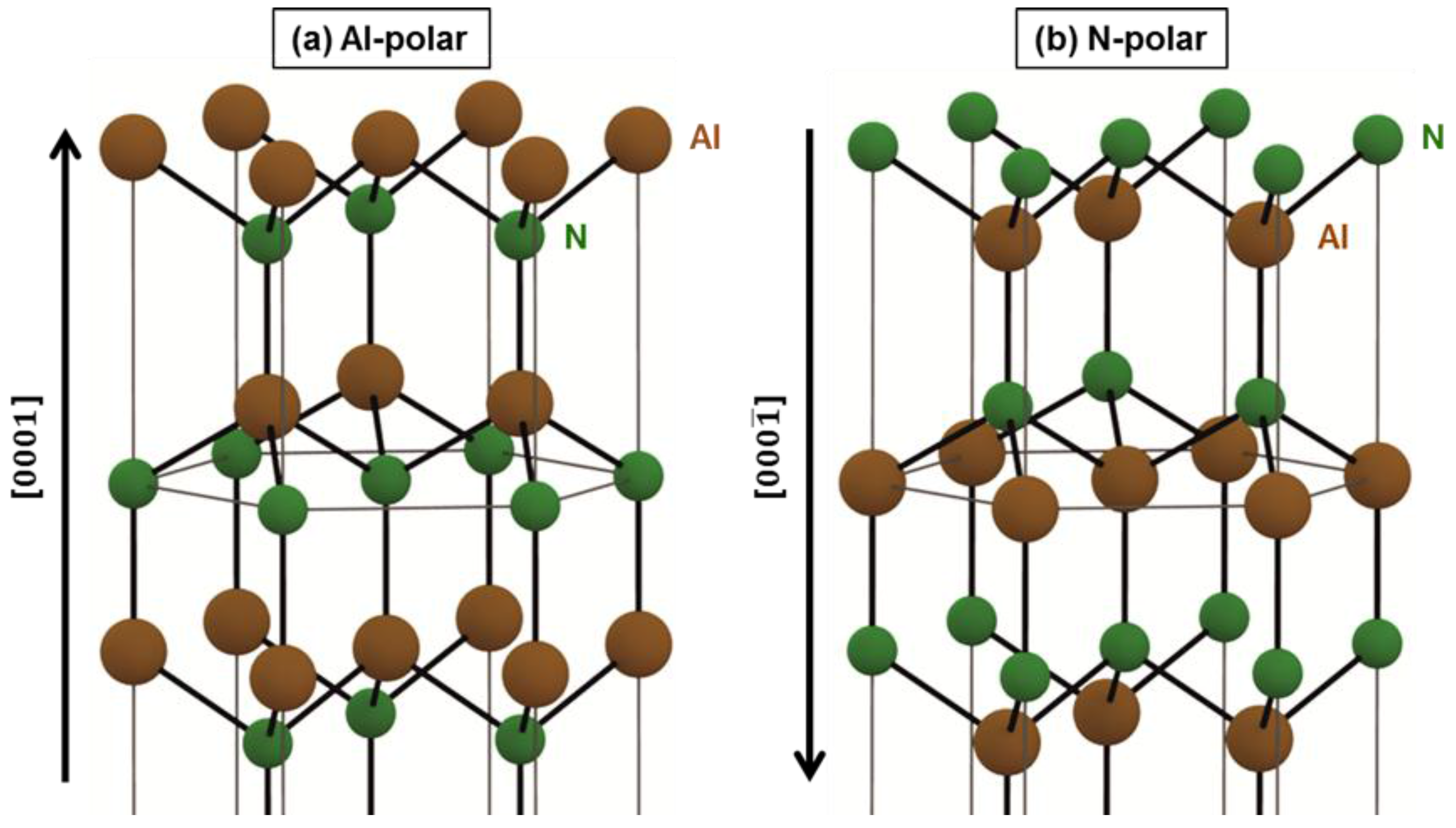
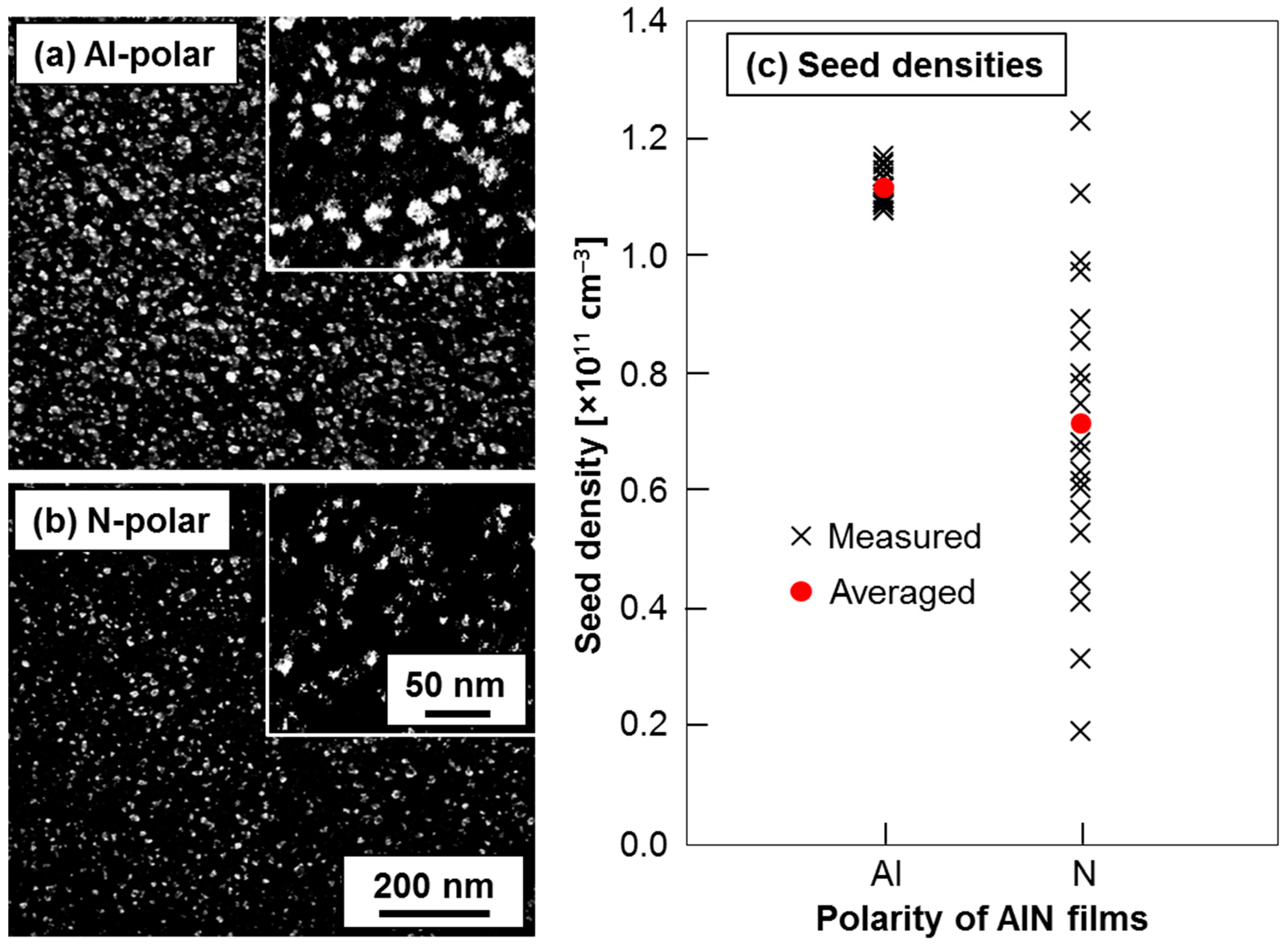
2.2. Densities and Distributions of DNPs Assembled onto Al- and N-Polar Sputtered AlN Film Surfaces
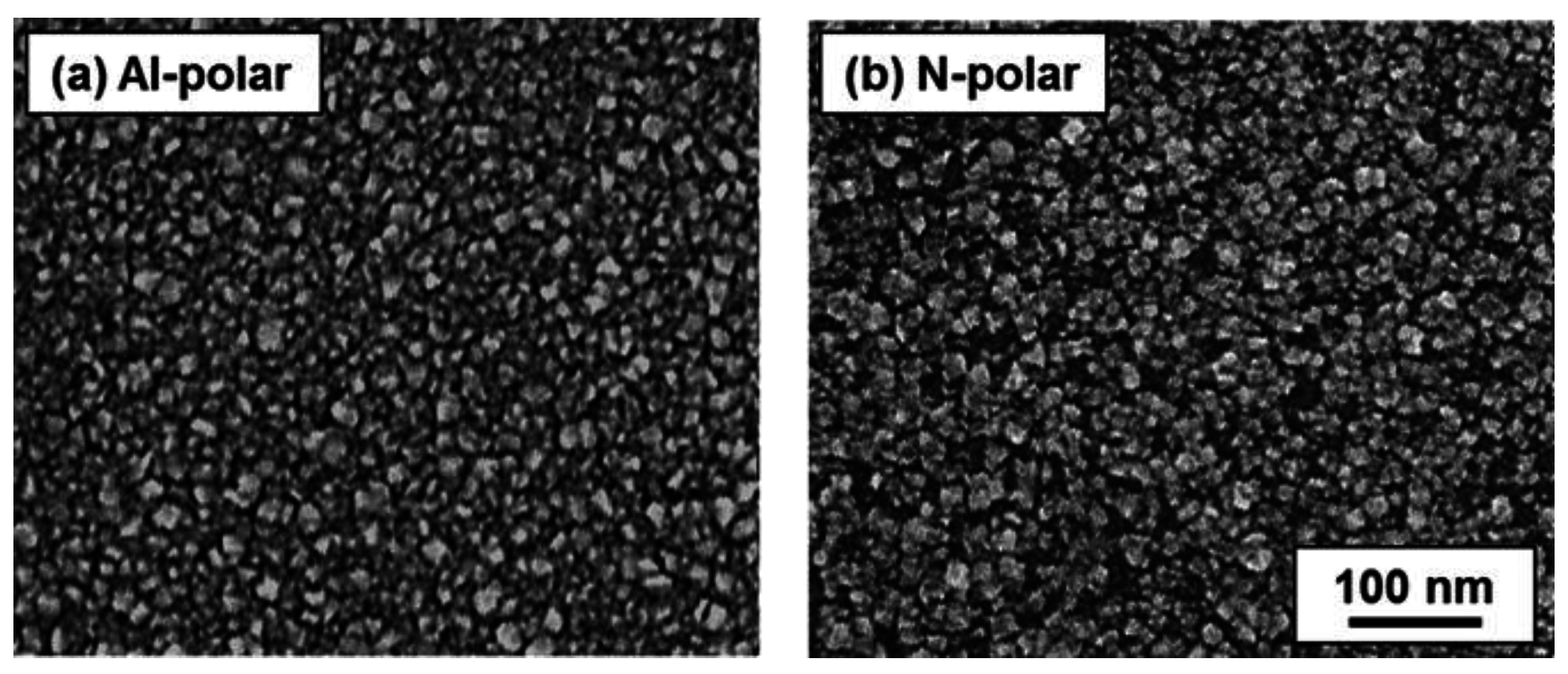
2.3. Ultrathin NCD Film Growth on Al- and N-Polar Sputtered AlN Film Surfaces
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CVD | Chemical vapor deposition |
| NCD | Nanocrystalline diamond |
| AlN | Aluminum nitride |
| DLS | Dynamic light scattering |
| SEM | Scanning electron microscope |
| MOEMS | Micro-opto-electro-mechanical system |
| XPS | X-ray photoelectron spectroscopy |
| DI | Deionized |
References
- Hees, J.; Heidrich, N.; Pletschen, W.; Sah, R.E.; Wolfer, M.; Williams, O.A.; Lebedev, V.; Nebel, C.E.; Ambacher, O. Piezoelectric actuated micro-resonators based on the growth of diamond on aluminum nitride thin films. Nanotechnology 2013, 24, 025601. [Google Scholar] [CrossRef] [PubMed]
- Bénédic, F.; Assouar, M.B.; Mohasseb, F.; Elmazria, O.; Alnot, P.; Gicquel, A. Surface acoustic wave devices based on nanocrystalline diamond and aluminium nitride. Diam. Relat. Mater. 2004, 13, 347–353. [Google Scholar] [CrossRef]
- Heidrich, N.; Iankov, D.; Hees, J.; Pletschen, W.; Sah, R.E.; Kirste, L.; Zuerbig, V.; Nebel, C.; Ambacher, O.; Lebedev, V. Enhanced mechanical performance of AlN/nanodiamond micro-resonators. J. Micromech. Microeng. 2013, 23, 125017–125022. [Google Scholar] [CrossRef]
- Zuerbig, V.; Pletschen, W.; Hees, J.; Sah, R.E.; Kirste, L.; Heidrich, N.; Nebel, C.E.; Ambacher, O.; Lebedev, V. Transparent diamond electrodes for tunable micro-optical devices. Diam. Relat. Mater. 2013, 38, 101–103. [Google Scholar] [CrossRef]
- Godbole, V.P.; Jagannadham, K.; Narayan, J. Nucleation and growth of diamond films on aluminum nitride coated nickel. Appl. Phys. Lett. 1995, 67, 1322–1324. [Google Scholar] [CrossRef]
- Cui, J.B.; Ma, Y.R.; Zhang, J.F.; Chen, H.; Fang, R.C. Growth and characterization of diamond film on aluminum nitride. Mater. Res. Bull. 1996, 31, 781–785. [Google Scholar] [CrossRef]
- Wang, W.L.; Zhang, R.Q.; Liao, K.J.; Sun, Y.W.; Wang, B.B. Nucleation and growth of diamond films on aluminum nitride by hot filament chemical vapor deposition. Diam. Relat. Mater. 2000, 9, 1660–1663. [Google Scholar] [CrossRef]
- Pobedinskas, P.; Degutis, G.; Dexters, W.; Janssen, W.; Janssens, S.D.; Conings, B.; Ruttens, B.; D’Haen, J.; Boyen, H.G.; Hardy, A.; et al. Surface plasma pretreatment for enhanced diamond nucleation on AlN. Appl. Phys. Lett. 2013, 102, 201609–201612. [Google Scholar] [CrossRef]
- Reddy, P.; Bryan, I.; Bryan, Z.; Guo, W.; Hussey, L.; Collazo, R.; Sitar, Z. The effect of polarity and surface states on the Fermi level at III-nitride surfaces. J. Appl. Phys. 2014, 116, 123701–123706. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J.H.; Strojek, B.; Chaudhuri, J.; Rek, Z. Defect-selective etching of bulk AlN single crystals in molten KOH/NaOH eutectic alloy. J. Cryst. Growth 2004, 262, 89–94. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J.H. Wet etching of GaN, AlN, and SiC: A review. Mater. Sci. Eng. R Rep. 2005, 48, 1–46. [Google Scholar] [CrossRef]
- Gu, Z.; Edgar, J.H.; Speakman, S.A.; Blom, D.; Perrin, J.; Chaudhuri, J. Thermal oxidation of polycrystalline and single crystalline aluminum nitride wafers. J. Electron. Mater. 2005, 34, 1271–1279. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Gao, F.; Zuerbig, V.; Giese, C.; Nebel, C.E.; Ambacher, O.; Lebedev, V. Pinhole-free ultra-thin nanocrystalline diamond film growth via electrostatic self-assembly seeding with increased salt concentration of nanodiamond colloids. Diam. Relat. Mater. 2016, 63, 103–107. [Google Scholar] [CrossRef]
- Bowen, P.; Highfield, J.G.; Mocellin, A.; Ring, T.A. Degradation of aluminum nitride powder in an aqueous environment. J. Am. Ceram. Soc. 1990, 73, 724–728. [Google Scholar] [CrossRef]
- Dalmau, R.; Collazo, R.; Mita, S.; Sitar, Z. X-ray photoelectron spectroscopy characterization of aluminum nitride surface oxides: Thermal and hydrothermal evolution. J. Electron. Mater. 2007, 36, 414–419. [Google Scholar] [CrossRef]
- Mobley, W.M. Colloidal Properties, Processing and Characterization of Aluminum Nitride Suspensions. Ph.D. Thesis, Alfred University, Alfred, NY, USA, 1996. [Google Scholar]
- Krnel, K.; Kosmač, T. Reactivity of aluminum nitride powder in dilute inorganic acids. J. Am. Ceram. Soc. 2000, 83, 1375–1378. [Google Scholar] [CrossRef]
- Williams, O.A.; Hees, J.; Dieker, C.; Jäger, W.; Kirste, L.; Nebel, C.E. Size-dependent reactivity of diamond nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.; Kriele, A.; Williams, O.A. Electrostatic self-assembly of diamond nanoparticles. Chem. Phys. Lett. 2011, 509, 12–15. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J.H.; Liu, L.; Liu, B.; Walker, L. Wet chemical etching of AlN single crystals. MRS Int. J. Nitride Semicond. Res. 2002, 7, 4–6. [Google Scholar]
- Reusch, M.; Holc, K.; Pletschen, W.; Kirste, L.; Žukauskaite, A.; Yoshikawa, T.; Iankov, D.; Ambacher, O.; Lebedev, V. Analysis and optimization of sputter deposited AlN-layers for flexural plate wave devices. J. Vac. Sci. Technol. B 2016, 34, 052001. [Google Scholar] [CrossRef]
- Akiyama, M.; Kamohara, T.; Kano, K.; Teshigahara, A.; Kawahara, N. Influence of oxygen concentration in sputtering gas on piezoelectric response of aluminum nitride thin films. Appl. Phys. Lett. 2008, 93, 021903. [Google Scholar] [CrossRef]
- Arnault, J.C.; Saada, S.; Williams, O.A.; Haenen, K.; Bergonzo, P.; Nesladek, M.; Polini, R.; Osawa, E. Diamond nanoseeding on silicon: Stability under H2 MPCVD exposures and early stages of growth. Diam. Relat. Mater. 2008, 17, 1143–1149. [Google Scholar] [CrossRef]
- Zeppilli, S.; Arnault, J.C.; Gesset, C.; Bergonzo, P.; Polini, R. Thermal stability and surface modifications of detonation diamond nanoparticles studied with X-ray photoelectron spectroscopy. Diam. Relat. Mater. 2010, 19, 846–853. [Google Scholar] [CrossRef]
- Petit, T.; Girard, H.A.; Trouvé, A.; Battonneau-Gener, I.; Bergonzo, P.; Arnault, J.C. Surface transfer doping can mediate both colloidal stability and self-assembly of nanodiamonds. Nanoscale 2013, 5, 8958–8962. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, T.; Reusch, M.; Zuerbig, V.; Cimalla, V.; Lee, K.-H.; Kurzyp, M.; Arnault, J.-C.; Nebel, C.E.; Ambacher, O.; Lebedev, V. Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces. Nanomaterials 2016, 6, 217. https://doi.org/10.3390/nano6110217
Yoshikawa T, Reusch M, Zuerbig V, Cimalla V, Lee K-H, Kurzyp M, Arnault J-C, Nebel CE, Ambacher O, Lebedev V. Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces. Nanomaterials. 2016; 6(11):217. https://doi.org/10.3390/nano6110217
Chicago/Turabian StyleYoshikawa, Taro, Markus Reusch, Verena Zuerbig, Volker Cimalla, Kee-Han Lee, Magdalena Kurzyp, Jean-Charles Arnault, Christoph E. Nebel, Oliver Ambacher, and Vadim Lebedev. 2016. "Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces" Nanomaterials 6, no. 11: 217. https://doi.org/10.3390/nano6110217
APA StyleYoshikawa, T., Reusch, M., Zuerbig, V., Cimalla, V., Lee, K.-H., Kurzyp, M., Arnault, J.-C., Nebel, C. E., Ambacher, O., & Lebedev, V. (2016). Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces. Nanomaterials, 6(11), 217. https://doi.org/10.3390/nano6110217






