Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans
Abstract
:1. Introduction
2. Results and Discussion
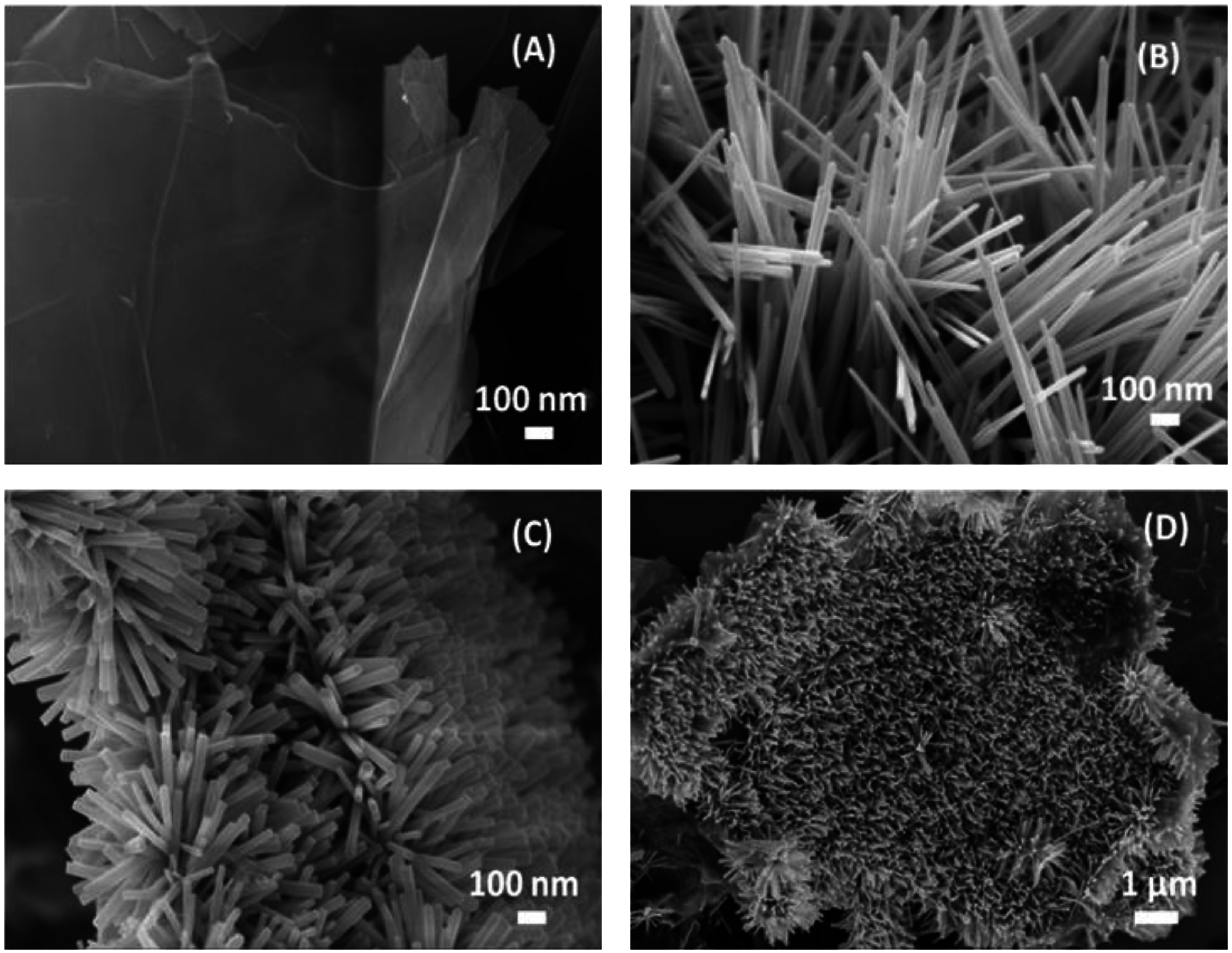
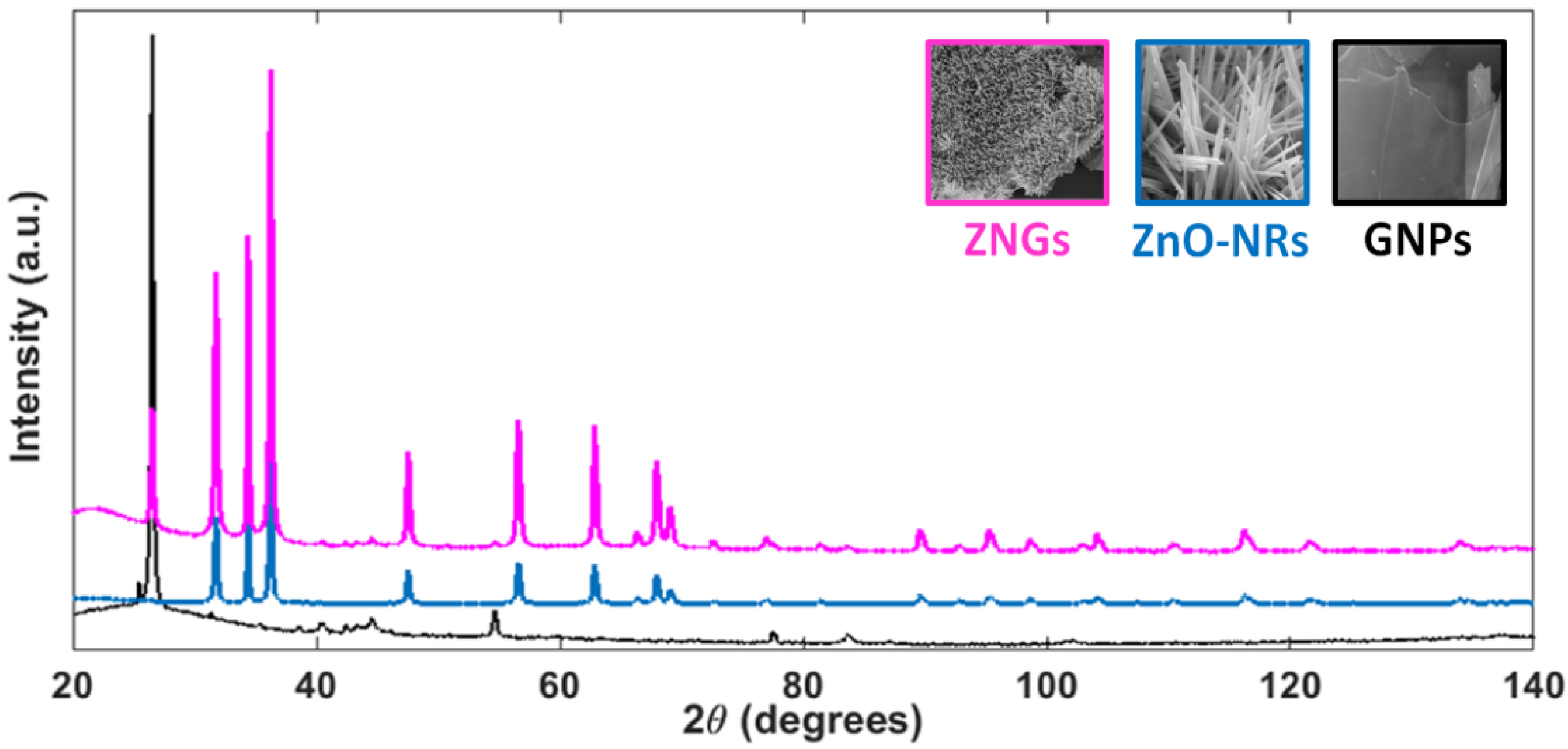
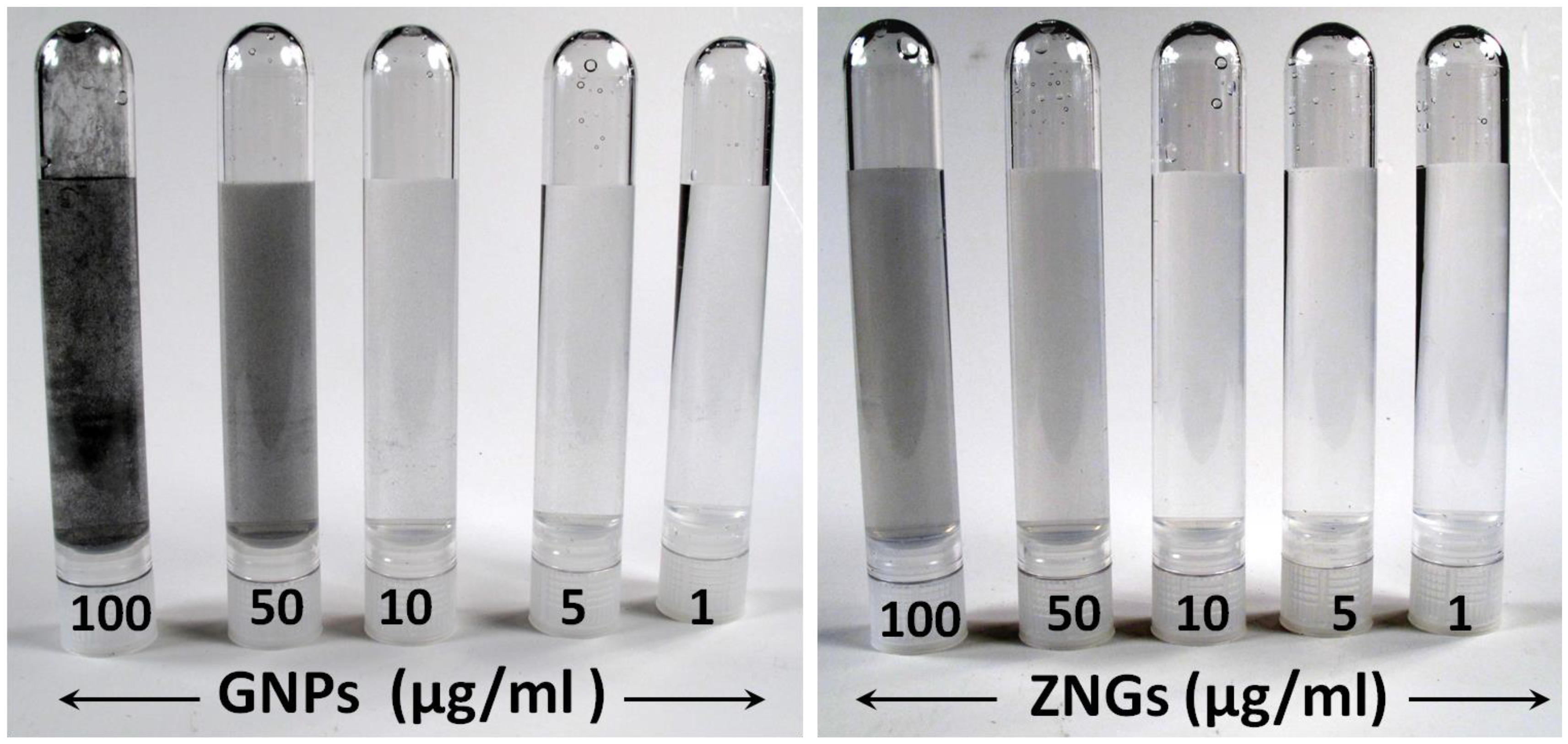
2.1. Morphological and Structural Properties
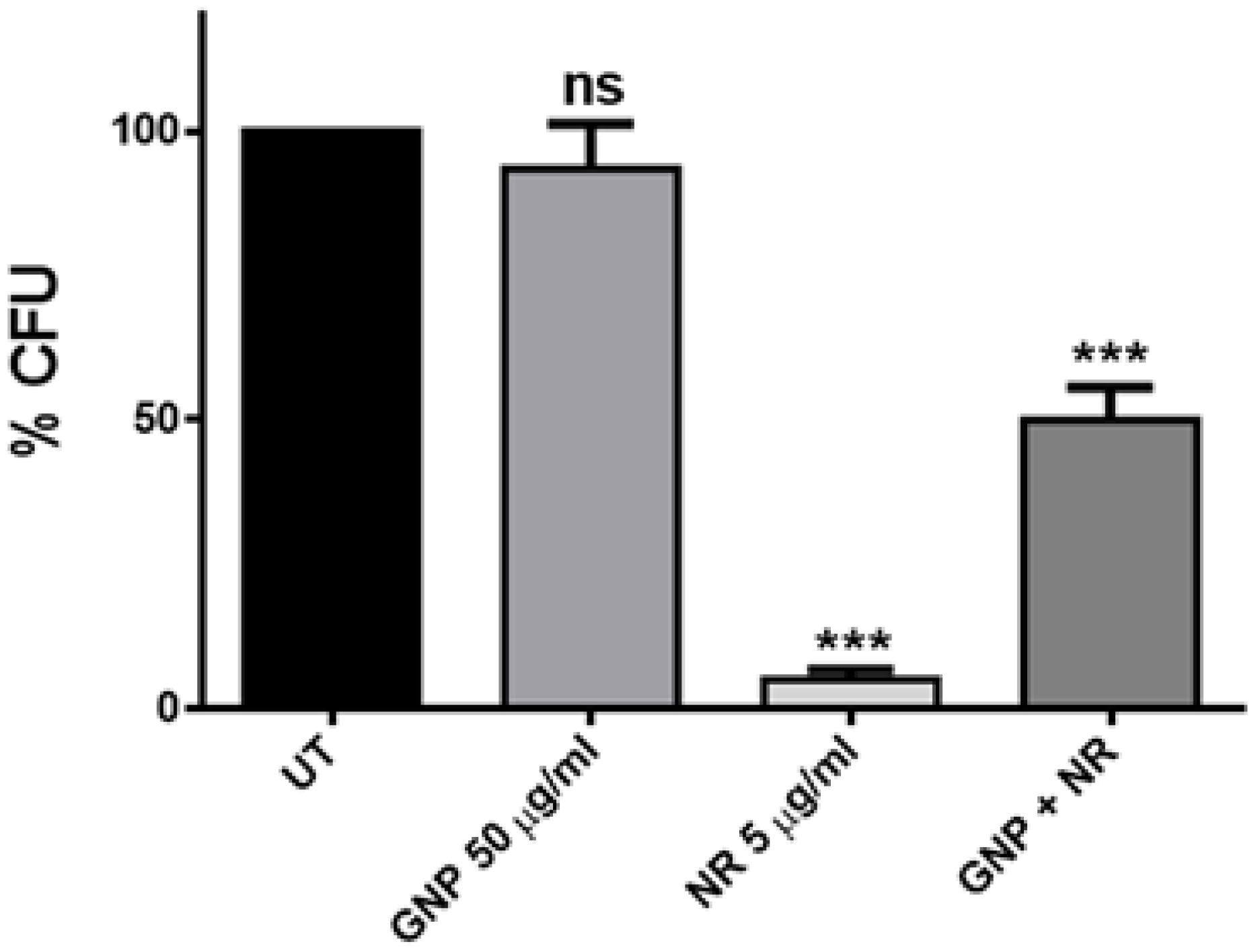
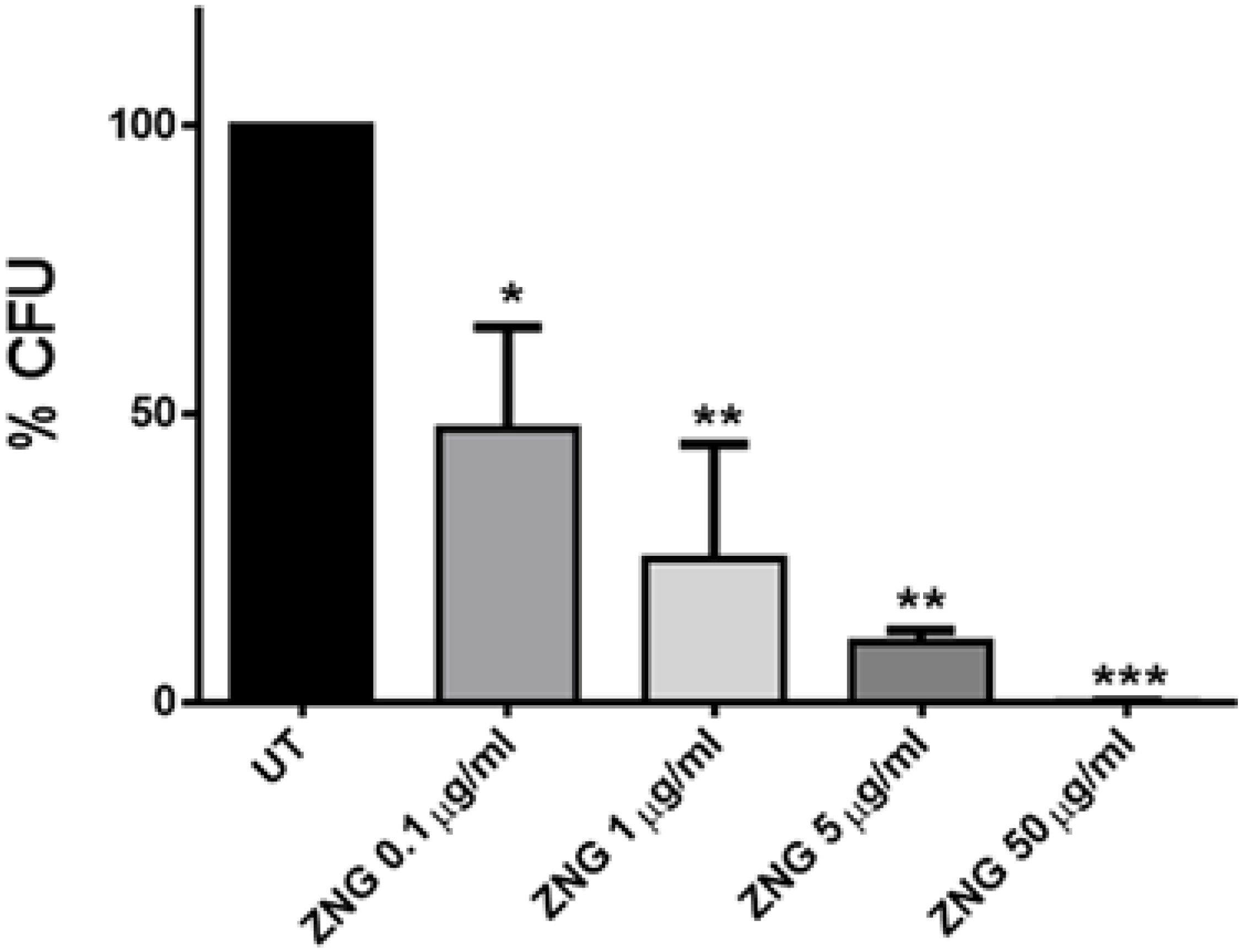
2.2. Antimicrobial Activity
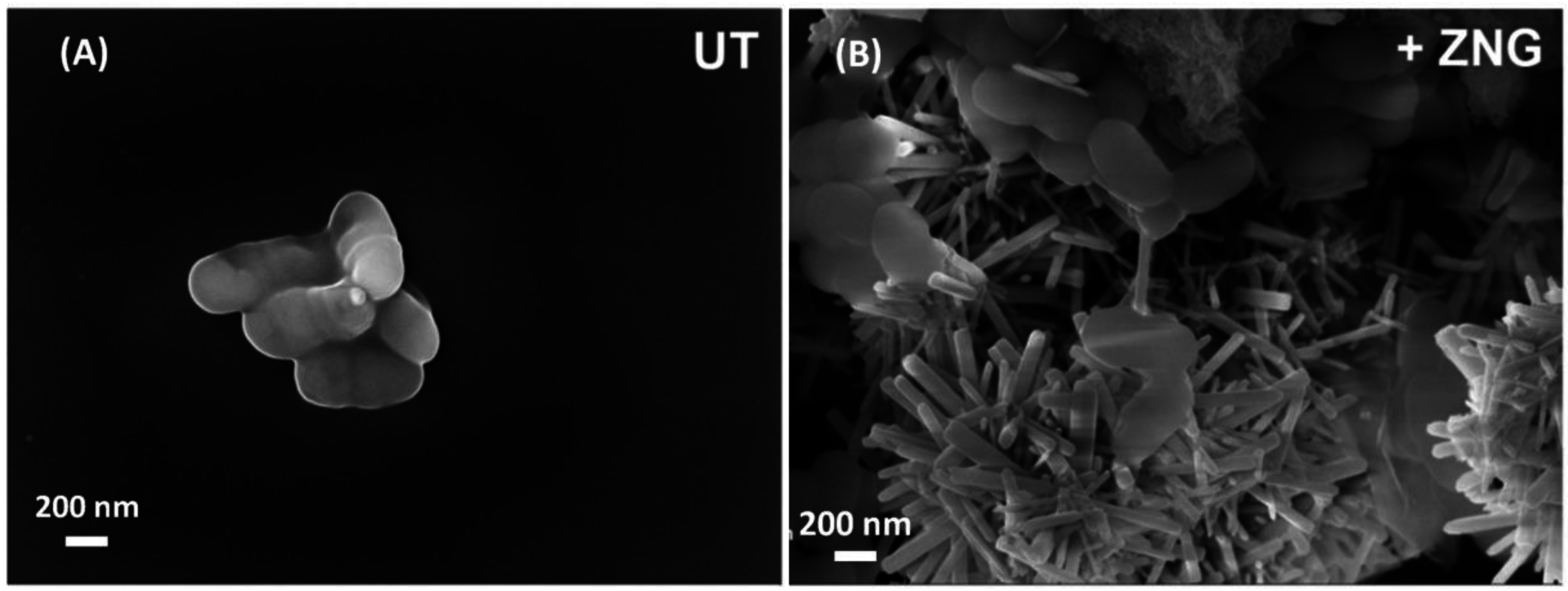
2.3. Field Emission Scanning Electron Microscopy Analysis of Cells Interaction with Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets
2.4. ROS Analysis
2.5. Inductively Coupled Plasma Mass Spectrometry Analysis
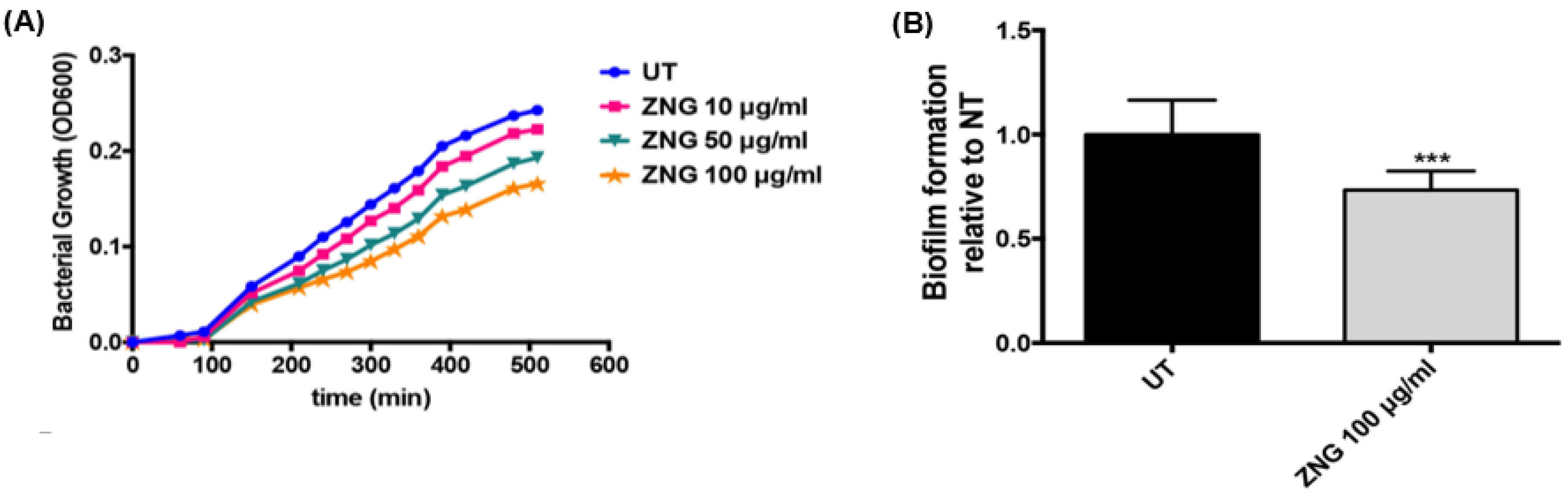
2.6. Bacterial Growth Inhibition by ZnO-NRs-decorated GNPs
3. Materials and Methods
3.1. Materials
3.2. Production of Nanostructures and Suspensions
- suspensions of GNPs;
- suspensions of pristine ZnO-NRs;
- suspensions of mixture of both pristine GNPs and pristine ZnO-NRs;
- suspensions of ZnO-NRs-decorated GNPs (ZNGs).
3.3. Characterization of ZNGs
3.4. Strains and Growth Culture
3.5. Cells Viability Test
3.6. FE-SEM Microscopy Imaging of Bacterial Cells
3.7. ROS Estimation
3.8. Zn2+ Release
3.9. Bacterial Growth Analysis
3.10. Estimation of Biofilm Production
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, H.; Tenovuo, J.; Huovinen, P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob. Agents Chemother. 1993, 37, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Dacarro, C.; Gazzani, G. Antibacterial activity of red and white wine against oral streptococci. J. Agric. Food Chem. 2007, 55, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B 2016, 146, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Soomin, P.; Jinkyu, R.; Sujin, K.; Kyunghee, C.; Jongheop, Y.; Younghun, K.; Jeyong, Y. Biofilm-inactivating activity of silver nanoparticles: A comparison with silver ions. J. Ind. Eng. Chem. 2013, 19, 614–619. [Google Scholar] [CrossRef]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023–9029. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Bai, H.; Wang, Y.; Sun, D.D. Gram-scale production of graphene oxide-TiO2 nanorod composites: Towards high-activity photocatalytic materials. Appl. Catal. B. Environ. 2011, 106, 76–82. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, G.; Tamburrano, A.; Dinescu, A.; Santarelli, M.L.; Sarto, M.S. Electromagnetic properties of composites containing graphite nanoplatelets at radio frequency. Carbon 2011, 49, 4291–4300. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.J. Chemical Preparation of Graphene-Based Nanomaterials and Their Applications in Chemical and Biological Sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Dong, S.J. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite Nanoplatelets and Caenorhabditis elegans: Insights from an in Vivo Model. Nanoletters 2012, 12, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Rago, I.; Bregnocchi, A.; Zanni, E.; D’Aloia, A.G.; de Angelis, F.; Bossu, M.; De Bellis, G.; Polimeni, A.; Uccelletti, D.; Sarto, M.S. Antimicrobial activity of graphene nanoplatelets against Streptococcus mutans. Proc. IEEE NANO 2015. [Google Scholar] [CrossRef]
- Wellings, J.S.; Chaure, N.B.; Heavens, S.N.; Dharmadasa, I.M. Growth and characterisation of electrodeposited ZnO thin films. Thin Solid Films 2008, 516, 3893–3898. [Google Scholar] [CrossRef]
- Ma, M.G.; Zhu, Y.J.; Cheng, G.F.; Huang, Y.H. Microwave synthesis and characterization of ZnO with various morphologies. Mater. Lett. 2008, 62, 507–510. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled Growth of ZnO Nanowires and Their Optical Properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Li, S.Y.; Lee, C.Y.; Tseng, T.Y. Copper-catalyzed ZnO nanowires on silicon (100) grown by vapor-liquid-solid process. J. Cryst. Growth 2003, 247, 357–362. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.W.; Yi, G.C. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Park, W.I.; Yi, G.C.; Kim, M.; Pennycook, S.J. ZnO nanoneedles grown vertically on Si substrates by non-catalytic vapor-phase epitaxy. Adv. Mater. 2002, 14, 1841–1843. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Xie, Y.; Xiong, Y.J.; Zhang, R.; He, W. Reverse micelle-assisted route to control diameters of ZnO nanorods by selecting different precursors. Chem. Lett. 2003, 32, 760–761. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Balijepalli, S.K.; Kaciulis, S.; Caneve, L.; Sarto, F.; Sarto, M.S. Synthesis and characterization of ZnO nanorods with a narrow size distribution. RSC Adv. 2015, 5, 49861–49870. [Google Scholar] [CrossRef]
- Zanni, E.; De Palma, S.; Chandraiahgari, C.R.; De Bellis, G.; Cialfi, S.; Talora, C.; Palleschi, C.; Sarto, M.S.; Uccelletti, D.; Mancini, P. In vitro toxicity studies of zinc oxide nano- and micro rods on mammalian cells: A comparative analysis. Mater. Lett. 2016, 179, 90–94. [Google Scholar] [CrossRef]
- Rago, I.; Chandraiahgari, C.R.; Bracciale, M.P.; De Bellis, G.; Zanni, E.; Guidi, M.C.; Sali, D.; Broggi, A.; Palleschi, C.; Sarto, M.S.; et al. Zinc oxide microrods and nanorods: Different antibacterial activity and their mode of action against Gram-positive bacteria. RSC Adv. 2014, 4, 56031–56040. [Google Scholar] [CrossRef]
- Chandraiahgari, C.R.; De Bellis, G.; Balijepallic, S.K.; Kaciulisc, S.; Ballirano, P.; Migliori, A.; Morandi, V.; Caneve, L.; Sarto, F.; Sarto, M.S. Control of the size and density of ZnO-nanorods grown onto graphene nanoplatelets in aqueous suspensions. RSC Adv. 2016, 6, 83217–83225. [Google Scholar] [CrossRef]
- Shin, D.M.; Tsege, E.L.; Kang, S.H.; Seung, W.; Kim, S.W.; Kim, H.K.; Hong, S.W.; Hwang, Y.H. Freestanding ZnO nanorod/graphene/ZnO nanorod epitaxial double heterostructure for improved piezoelectric nanogenerators. Nano Energy 2015, 12, 268–277. [Google Scholar] [CrossRef]
- Bratthall, D. Serological Studies on Streptococcus mutans. Odontol. Revy. Suppl. 1972, 23, 401–410. [Google Scholar]
- Nyvad, B.; Kilian, M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990, 24, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Simmonds, R.S.; Tagg, J.R. Dental caries is a preventable infectious disease. Aust. Dent. J. 2000, 45, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane, and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristobal, L.F.; Martínez-Castañón, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater. Lett. 2009, 63, 2603–2606. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eshed, M.; Lellouche, J.; Matalon, S.; Gedanken, A.; Banin, E. Sonochemical coatings of ZnO and CuO nanoparticles inhibit Streptococcus mutans biofilm formation on teeth model. Langmuir 2012, 28, 12288–12295. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.; De Leon, A.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir-Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.G.; O’Brien-Simpson, N.M.; Cross, K.J.; Paolini, R.A.; Hoffmann, B.; Catmull, D.V.; Malkoski, M.; Reynolds, E.C. Divalent metal cations increase the activity of the antimicrobial Peptide kappacin. Antimicrob. Agents Chemother. 2005, 49, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Watsson, G.K.; Cummins, D.; van der Ouderaa, F.J. Inhibition of acid production by Streptococcus mutans NCTC 10449 by zinc and the effect of metal speciation. Caries Res. 1991, 25, 431–437. [Google Scholar] [CrossRef]
- Shaw, J.H. Causes and control of dental caries. N. Engl. J. Med. 1987, 317, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Catizone, A.; Ricci, G.; Frioni, A.; Natalizi, T.; Valenti, P.; Polimeni, A. Streptococcus mutans and Streptococcus sobrinus are able to adhere and invade human gingival fibroblast cell line. Int. J. Immunopathol. Pharmacol. 2010, 23, 1253–1260. [Google Scholar] [PubMed]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing Bacterial Interaction with Copper Surfaces through Graphene and Hexagonal-Boron Nitride Coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kang, F.; Gao, Y.; Mao, X.; Hu, X. Sequestration of nanoparticles by an EPS matrix reduces the particle-specific bactericidal activity. Sci. Rep. 2016, 6, 21379. [Google Scholar] [CrossRef] [PubMed]
- Topas, V. 4.2 General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]


| Nanostructure Type | Nanostructure Concentration (µg/mL) | Treated with S. mutans Cells | Zn2+ Concentration (μg/mL) |
|---|---|---|---|
| ZnO-NRs | 5 | no | 2.58 |
| ZNGs | 5 | no | 1.24 |
| ZnO-NRs | 5 | yes | 1.94 |
| ZNGs | 5 | yes | 0.61 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. https://doi.org/10.3390/nano6100179
Zanni E, Chandraiahgari CR, De Bellis G, Montereali MR, Armiento G, Ballirano P, Polimeni A, Sarto MS, Uccelletti D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials. 2016; 6(10):179. https://doi.org/10.3390/nano6100179
Chicago/Turabian StyleZanni, Elena, Chandrakanth Reddy Chandraiahgari, Giovanni De Bellis, Maria Rita Montereali, Giovanna Armiento, Paolo Ballirano, Antonella Polimeni, Maria Sabrina Sarto, and Daniela Uccelletti. 2016. "Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans" Nanomaterials 6, no. 10: 179. https://doi.org/10.3390/nano6100179
APA StyleZanni, E., Chandraiahgari, C. R., De Bellis, G., Montereali, M. R., Armiento, G., Ballirano, P., Polimeni, A., Sarto, M. S., & Uccelletti, D. (2016). Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials, 6(10), 179. https://doi.org/10.3390/nano6100179









