Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge
Abstract
:1. Introduction
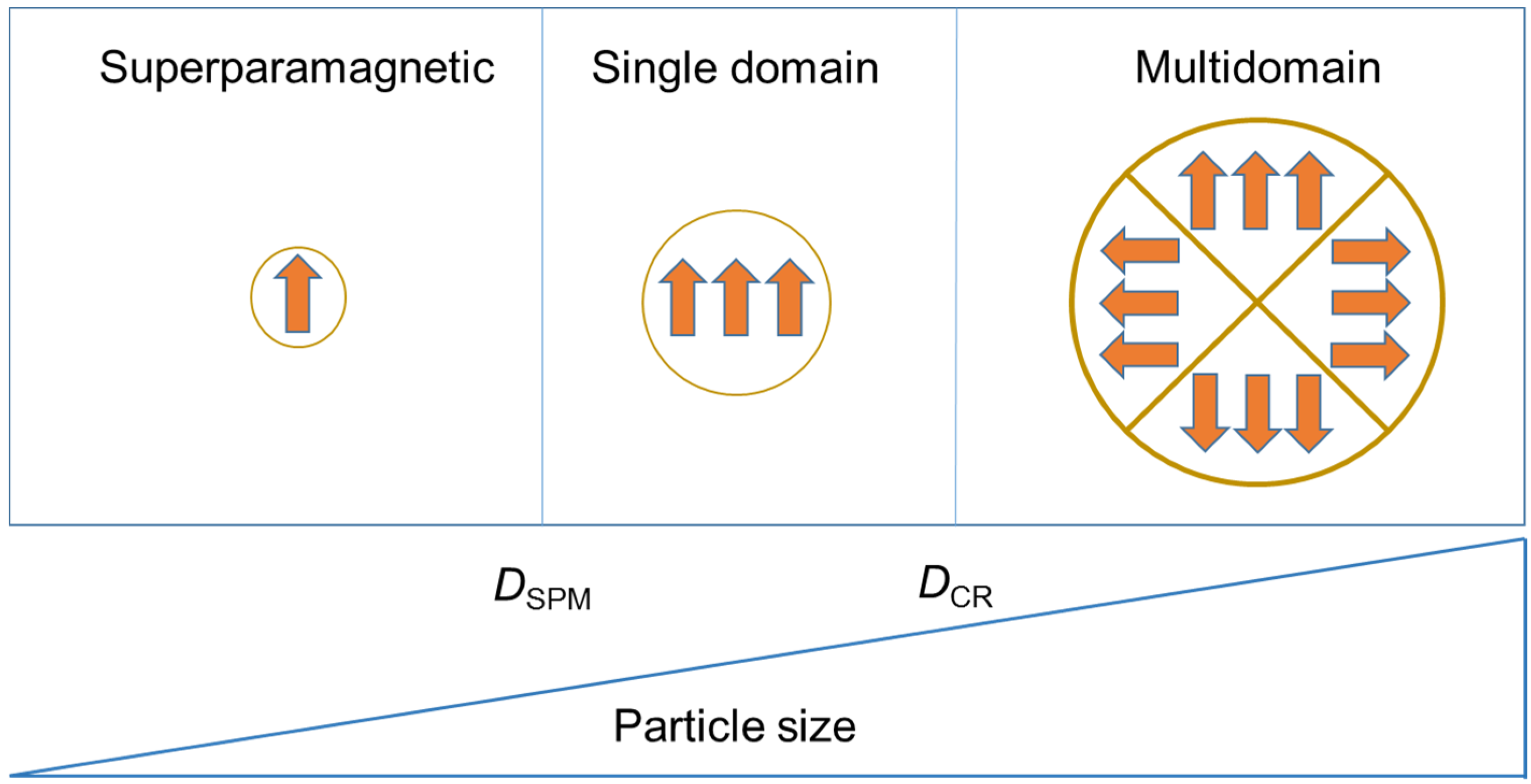
2. Magnetic Nanoparticles
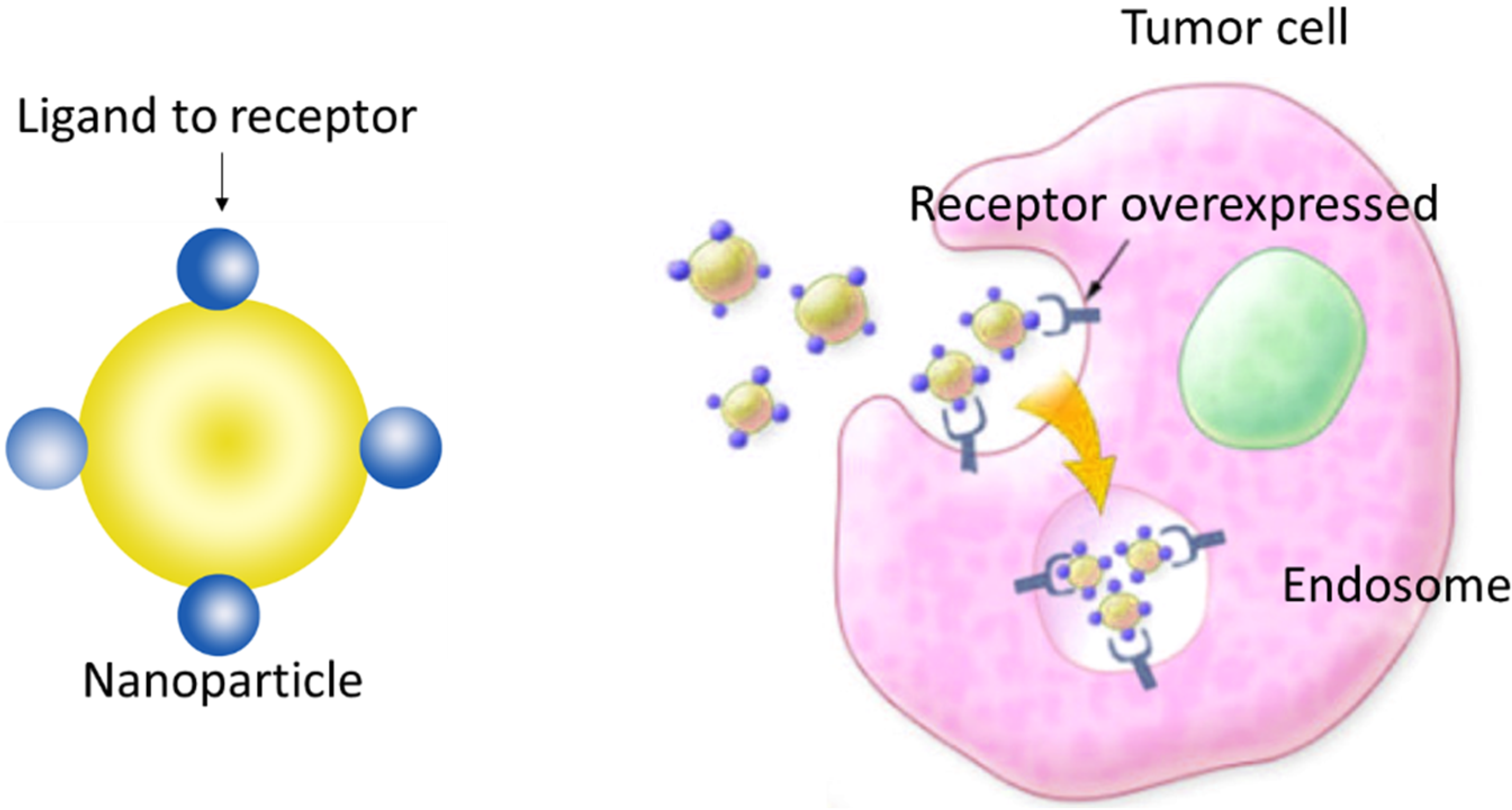
3. SPIONs Modified with Penetrating Peptides that Help to Penetrate Brain Cells
4. Transport of MNPs through Brain Cells by the Effect of an External Magnet
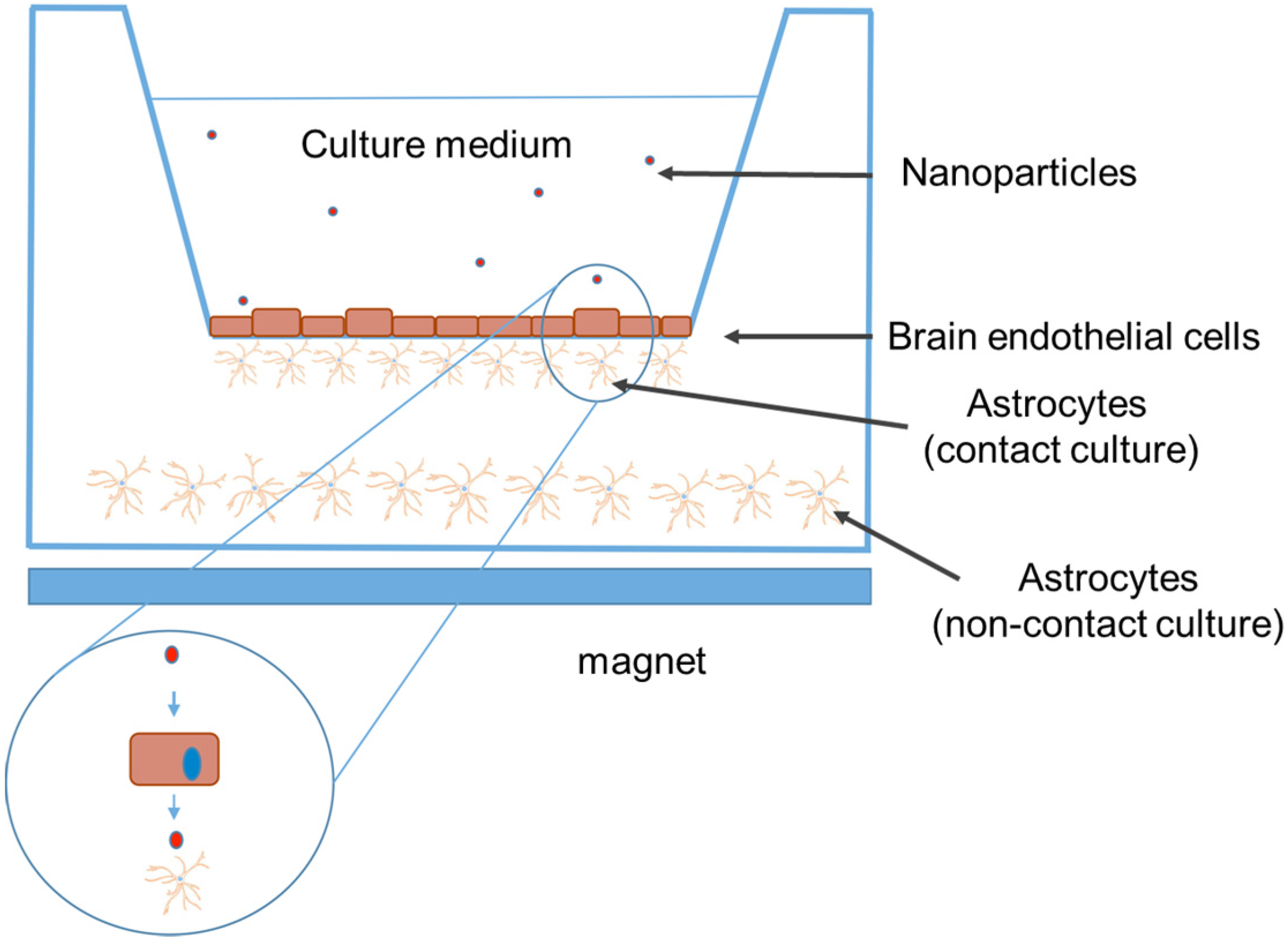
4.1. In Vitro Studies
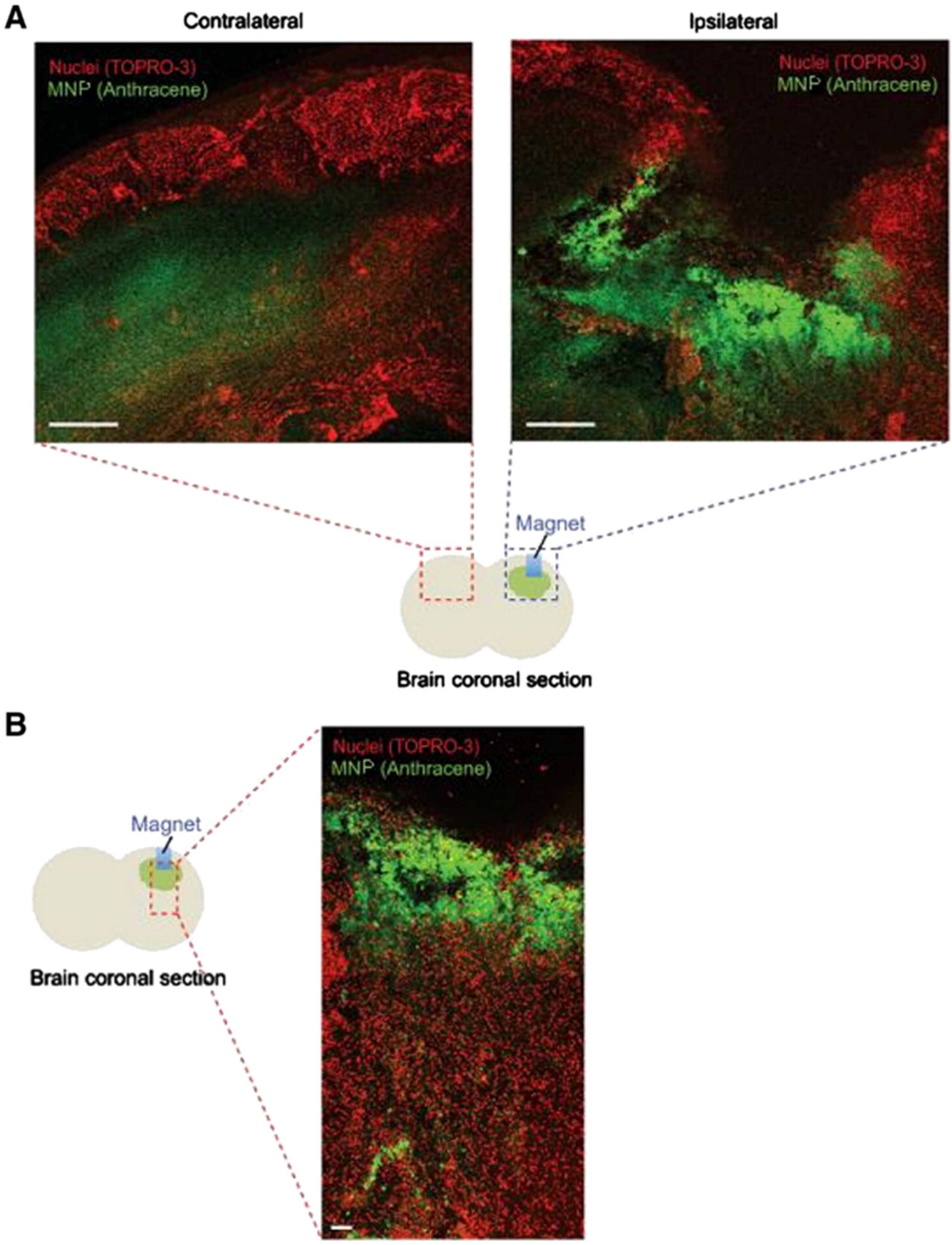
4.2. In Vivo Studies
5. Increase of BBB Permeability by Magnetic Heating of Nanoparticles
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, G.A. Nanotechnology approaches to crossing the blood-brian barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Sahoo, S.K. Therapeutic approaches of magnetic nanoparticles for the central nervous system. Drug Discov. Today 2015, 20, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, W.; Gunasekera, S.M.; Constantine, G.R.; Perera, S.; Perera, B.M.; Kamaladiwela, R. Brain targeted transcranial administration of diazepam and shortening of sleep latency in healthy human volunteers. Indian J. Pharm. Sci. 2011, 73, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Michaud, K.; Fortin, D. A new method of quantitatively assessing the opening of the blood-brain barrier in murine animal models. J. Neurosci. Methods 2012, 207, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lossinsky, A.S.; Vorbrodt, A.W.; Wisniewski, H.M. Scanning and transmission electron microscopic studies of microvascular pathology in the osmotically impaired blood-brain barrier. J. Neurocytol. 1995, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1994, 674, 171–174. [Google Scholar] [CrossRef]
- Alyautdin, R.; Gothier, D.; Petrov, V.; Kharkevich, D.; Kreuter, J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 1995, 41, 44–48. [Google Scholar]
- Wilson, B. Brain targeting PBCA nanoparticles and the blood-brain barrier. Nanomedicine 2009, 4, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A. Transferrin receptor on glioblastoma multiforme. J. Neurosurg. 1991, 74, 313–314. [Google Scholar] [PubMed]
- Eavarone, D.A.; Yu, X.; Bellamkonda, R.V. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J. Biomed. Mater. Res. 2000, 51, 10–14. [Google Scholar] [CrossRef]
- Huang, R.Q.; Ke, W.L.; Qu, Y.H.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, J.; Shen, Y.; Lu, W.; Gao, X.; Zhang, Q.; Jiang, X. Lactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. J. Control. Release 2009, 134, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Hafeli, U.O.; Mahmoudi, M. Crucial ignored parameters on nanotoxicology: The importance of toxicity assay modifications and “cell vision”. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.A.; Hosseinkhani, M. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: Cell “vision” versus physicochemical properties of nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef] [PubMed]
- Kiyatkin, E.A.; Sharma, H.S. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience 2009, 161, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Gonzales-Weimuller, M.; Zeisberger, M.; Krishnan, M. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Gazeau, F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials 2008, 29, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Kruger, A.; Gansbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Dormer, K.; Rutel, I.B. A Two-Magnet System to Push Therapeutic Nanoparticles. AIP Conf. Proc. 2010, 1311, 77–88. [Google Scholar] [PubMed]
- Wui, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimeno, S.; Escribano, E.; Queralt, J.; Estelrich, J. External magnetic field-induced selective biodistribution of magnetoliposomes in mice. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, V.; Denizot, B.; Pouliquen, D.; Jallet, P.; le Jeune, J.J. Investigation of blood-brain barrier permeability to magnetite-dextran nanoparticles (MD3) after osmotic disruption in rats. Magma 1997, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Worden, M.; Wroczynskyj, Y.; Yathindranath, V.; van Lierop, J.; Hegmann, T.; Miller, D.W. Magnetic field enhanced convective diffusion of iron oxide nanoparticles in an osmotically disrupted cell culture model of the blood-brain barrier. Int. J. Nanomed. 2014, 9, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; McMillan, J.M.; Kabanov, A.V.; Sokolsky-Papkov, M.; Gendelman, H.E. Bench-to-bedside translation of magnetic nanoparticles. Nanomedicine 2014, 9, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Maric, G.; Annis, M.G.; Dong, Z.; Rose, A.A.; Ng, S.; Perkins, D.; MacDonald, P.A.; Ouellet, V.; Russo, C.; Siegel, P.M. GPNMB cooperates with neuropilin-1 to promote mammary tumor grwth and engages integrin α5β1 for efficient breast cancer metastasis. Oncogene 2015, 34, 5494–5504. [Google Scholar] [CrossRef] [PubMed]
- Szulzewsky, F.; Pelz, A.; Feng, X.; Synowitz, M.; Markovic, D.; Langmann, T.; Holtman, I.R.; Wang, X.; Eggen, B.J.; Boddeke, H.W.; et al. Glioma associated microglia/macrophages display an expression profile different from M1 and M2 polarisation and highly express Gpnmb and Spp1. PLoS ONE 2015, 10, e0116644. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. Egfrviii antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Drummond, D.C.; Greiser, U.; Hong, K.; Kirpotin, D.B.; Marks, J.D.; Park, J.W. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003, 63, 3154–3161. [Google Scholar] [PubMed]
- Kim, I.Y.; Kang, Y.S.; Lee, D.S.; Park, H.J.; Choi, E.K.; Oh, Y.K.; Son, H.J.; Kim, J.S. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J. Control. Release 2009, 140, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int. J. Nanomed. 2014, 9, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaissa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Jia, Q.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, Y.; He, S.; Ku, S.; Gu, W.; Ye, L. Transferrin-conjugated, fluorescein-loaded magnetic nanoparticles for targeted delivery across the blood-brain barrier. J. Mater. Sci. Mater. Med. 2013, 24, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.B.; Feng, W.; Kang, C.S.; Pu, P.Y.; Betbeder, D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Su, Y.-Z.; Hsu, C.; Lo, Y.-L.; Huang, S.-J.; Ke, J.-H.; Kuo, Y.-C.; Wang, L.-F. Angiopep-pluronic F127-conjugated superparamagnetic iron oxide nanoparticles as nanotheranostic agents for BBB targeting. J. Mater. Chem. B 2014, 2, 5666–5675. [Google Scholar] [CrossRef]
- Gandhi, N.; Saiyed, Z.M.; Napuri, J.; Samikkannu, T.; Reddy, P.V.; Agudelo, M.; Khatavkar, P.; Saxena, S.K.; Nair, M.P. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific TAT protein and cocaine in blood-brain barrier dysfunction: Implications for HIV-1-associated neurocognitive disorder. J. Neurovirol. 2010, 16, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Liao, Z.; Wang, C.; Liu, Y.; Feng, S.; Jiang, X.; Chang, J. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials 2010, 31, 6589–6596. [Google Scholar] [CrossRef] [PubMed]
- Ansciaux, E.; Burtea, C.; Laurent, S.; Crombez, D.; Nonclerc, D.; Vander Elst, L.; Muller, R.N. In vitro and in vivo characterization of several functionalized ultrasmall particles of iron oxide, vectorized against amyloid plaques and potentially able to cross the blood-brain barrier: Toward earlier diagnosis of alzheimer's disease by molecular imaging. Contrast Media Mol. Imaging 2015, 10, 211–224. [Google Scholar] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wang, Y.X.; Chow, A.H.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Koyanagi, Y.; Miles, S.; Wiley, C.; Vinters, H.V.; Chen, I.S. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature 1990, 343, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.C.; Price, R.W. Human immunodeficiency virus and the central nervous system. Annu. Rev. Microbiol. 1992, 46, 655–693. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Destache, C.J.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 2006, 108, 2827–2835. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.J.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroaids. J. Immunol. 2009, 183, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mishra, V.; Singh, P.; Dubey, P.K.; Saraf, D.K.; Vyas, S.P. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 2003, 261, 43–55. [Google Scholar] [CrossRef]
- Saiyed, Z.M.; Gandhi, N.H.; Nair, M.P. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrier. Int. J. Nanomed. 2010, 5, 157–166. [Google Scholar]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Sagar, V.; Agudelo, M.; Pilakka-Kanthikeel, S.; Atluri, V.S.; Raymond, A.; Samikkannu, T.; Nair, M.P. Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 2014, 25, 055101. [Google Scholar] [CrossRef] [PubMed]
- Ranney, D.F.; Huffaker, H.H. Magnetic microspheres for the targeted controlled release of drugs and diagnostic agents. Ann. N. Y. Acad. Sci. 1987, 507, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liang, C.; Li, A.; Chang, J.; Wang, H.; Yan, R.; Zhang, J.; Tai, J. Magnetic paclitaxel nanoparticles inhibit glioma growth and improve the survival of rats bearing glioma xenografts. Anticancer Res. 2010, 30, 2217–2223. [Google Scholar] [PubMed]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for mri monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intracarotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; David, A.E.; Yang, V.C. Brain tumor targeting of magnetic nanoparticles for potential drug delivery: Effect of administration route and magnetic field topography. J. Control. Release 2011, 155, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.F.; Morris, R.M.; Senyei, A.E.; Widder, K.J.; Heller, G.S. Magnetic targeting of microspheres in blood flow. Microvasc. Res. 1984, 27, 353–369. [Google Scholar] [CrossRef]
- Marie, H.; Lemaire, L.; Franconi, F.; Lajnef, S.; Frapart, I.-M.; Nicolas, V.; Frébourg, G.; Trichet, M.; Ménager, C.; Lessieur, S. Superparamagnetic liposomes for MRI monitoring and external magnetic field-induced selective targeting of malignant brain tumors. Adv. Funct. Mater. 2015, 25, 1258–1269. [Google Scholar] [CrossRef]
- Philosof-Mazor, L.; Dakwar, G.R.; Popov, M.; Kolusheva, S.; Shames, A.; Linder, C.; Greenberg, S.; Heldman, E.; Stepensky, D.; Jelinek, R. Bolaamphiphilic vesicles encapsulating iron oxide nanoparticles: New vehicles for magnetically targeted drug delivery. Int. J. Pharm. 2013, 450, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Duchemin, S.; Girouard, H.; Martel, S. Towards MR-navigable nanorobotic carriers for drug delivery into the brain. In Proceedings of the 2012 IEEE International Conference on Robotics and Automation (ICRA), Saint Paul, MN, USA, 14–18 May 2012; pp. 727–732.
- Tabatabaei, S.N.; Girouard, H.; Carret, A.S.; Martel, S. Remote control of the permeability of the blood-brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Bae, Y.; Pittman, T.A.; Yokel, R.A. Alternating magnetic field-induced hyperthermia increases iron oxide nanoparticle cell association/uptake and flux in blood-brain barrier models. Pharm. Res. 2015, 32, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busquets, M.A.; Espargaró, A.; Sabaté, R.; Estelrich, J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials 2015, 5, 2231-2248. https://doi.org/10.3390/nano5042231
Busquets MA, Espargaró A, Sabaté R, Estelrich J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials. 2015; 5(4):2231-2248. https://doi.org/10.3390/nano5042231
Chicago/Turabian StyleBusquets, Maria Antònia, Alba Espargaró, Raimon Sabaté, and Joan Estelrich. 2015. "Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge" Nanomaterials 5, no. 4: 2231-2248. https://doi.org/10.3390/nano5042231
APA StyleBusquets, M. A., Espargaró, A., Sabaté, R., & Estelrich, J. (2015). Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials, 5(4), 2231-2248. https://doi.org/10.3390/nano5042231





