Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization
Abstract
:1. Introduction
| Chemicals | Sequence | Structure |
|---|---|---|
| L-Tryptophan; (Trp) | Trp |  |
| L-Phenylanaline; (Phe) | Phe |  |
| Endomorphin-1; (EMP) | Tyr-Pro-Trp-Phe-NH2 |  |
| W-Nle-R-F-NH2; (WRF) | Trp-Nle-Arg-Phe-NH2 |  |
2. Results and Discussion
2.1. Absorption Spectra

| ONC-Trp | ONC-Phe | |
|---|---|---|
| DO1 | 0.17 | 0.19 |
| CY (%) | 23 | 14 |
2.2. TEM Images
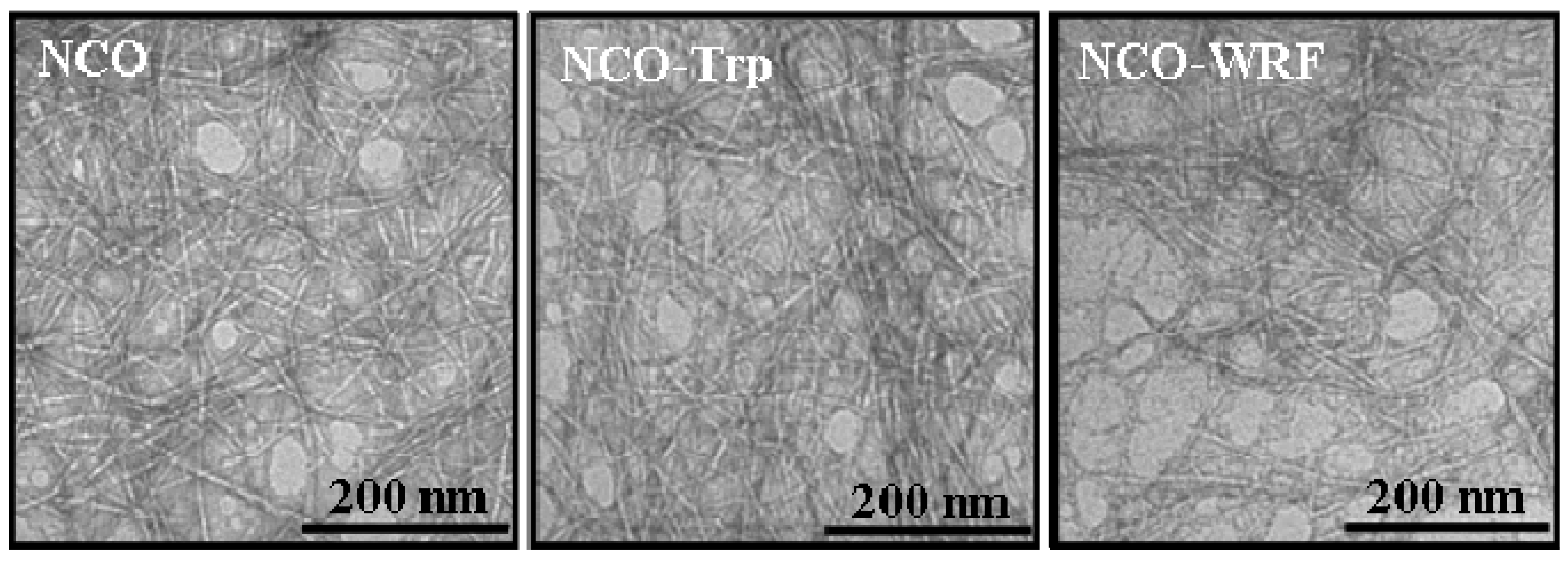
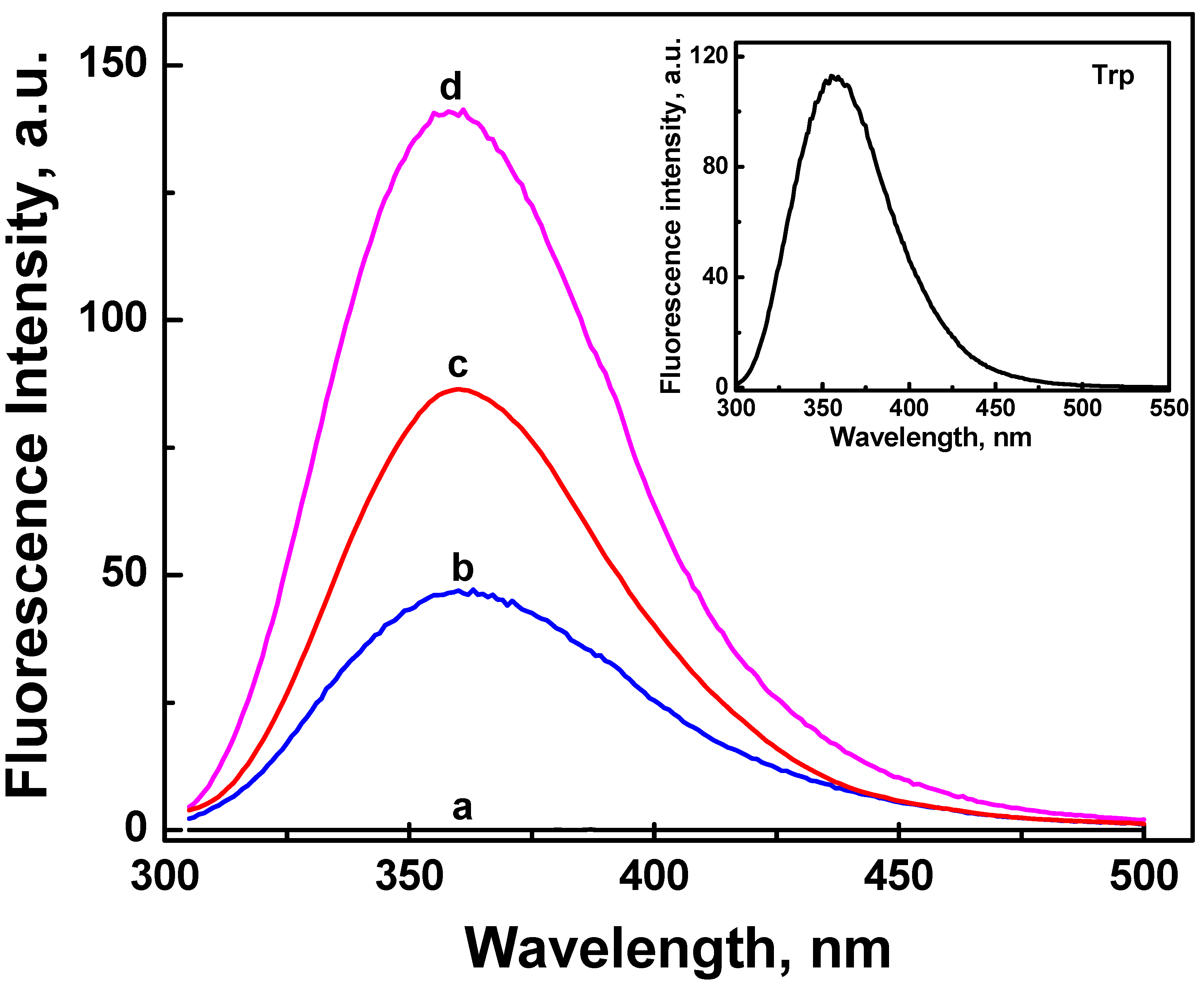
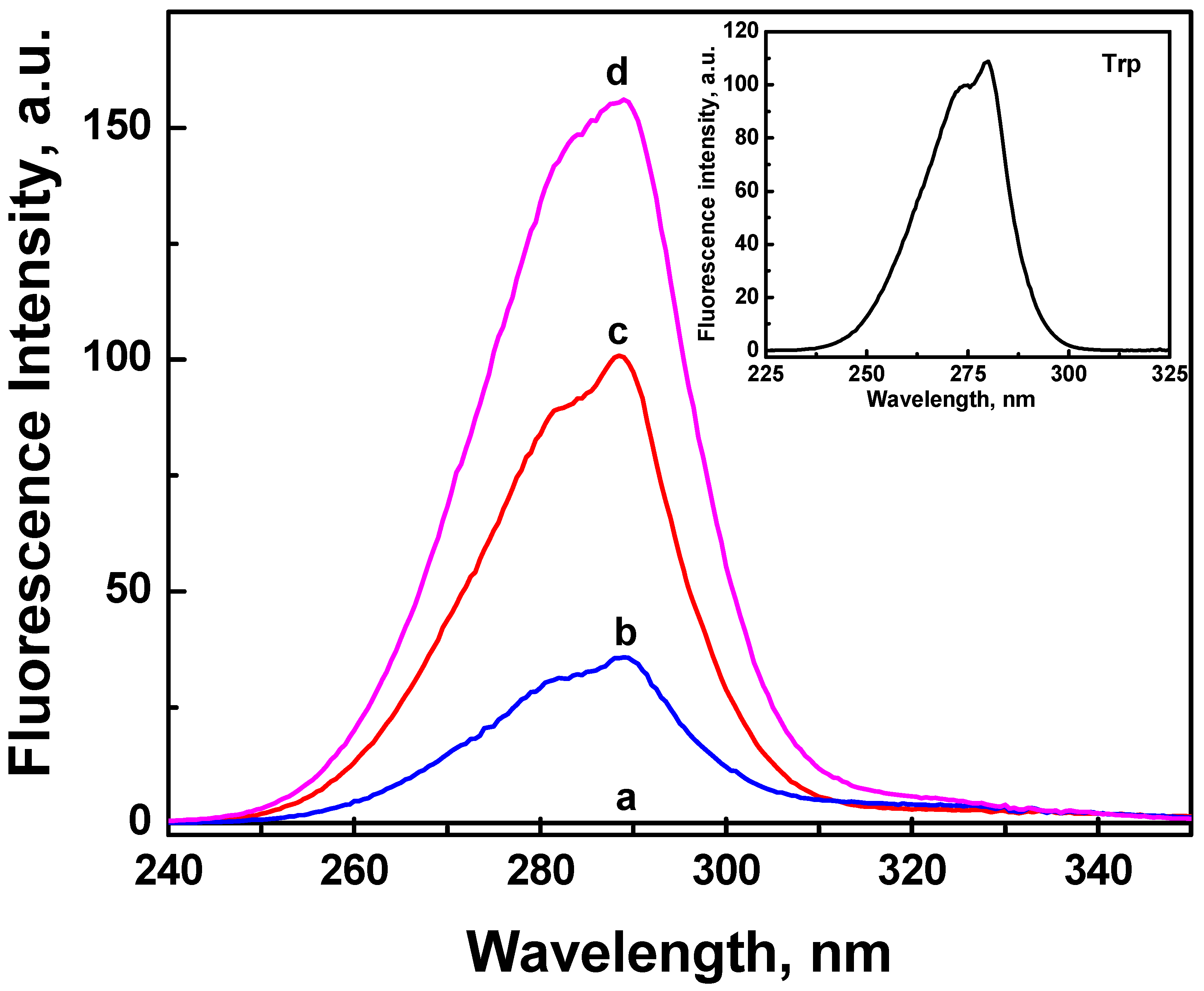
2.3. Fluorescence Properties of ONC and Grafted ONC
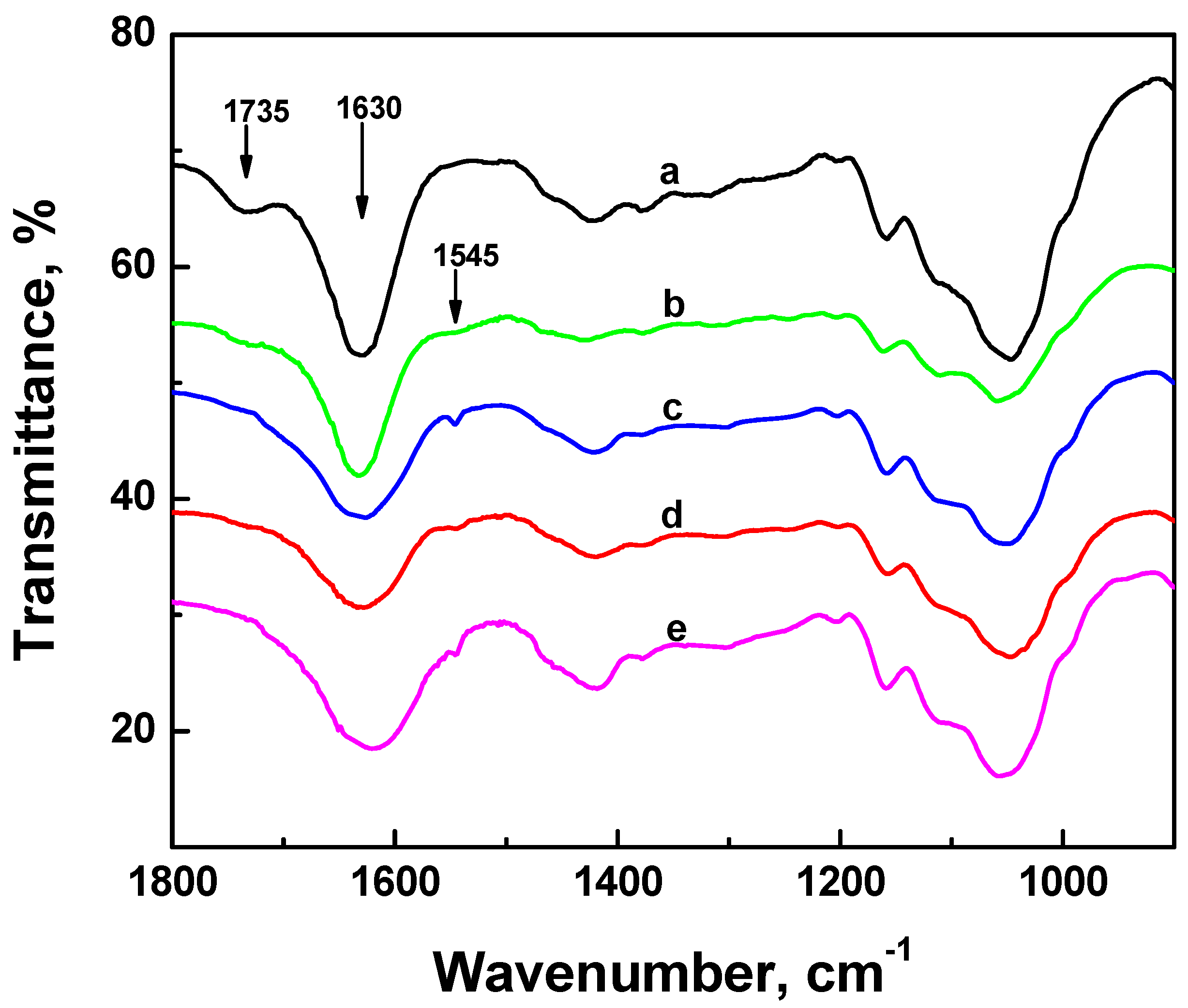
2.4. FTIR Experiments
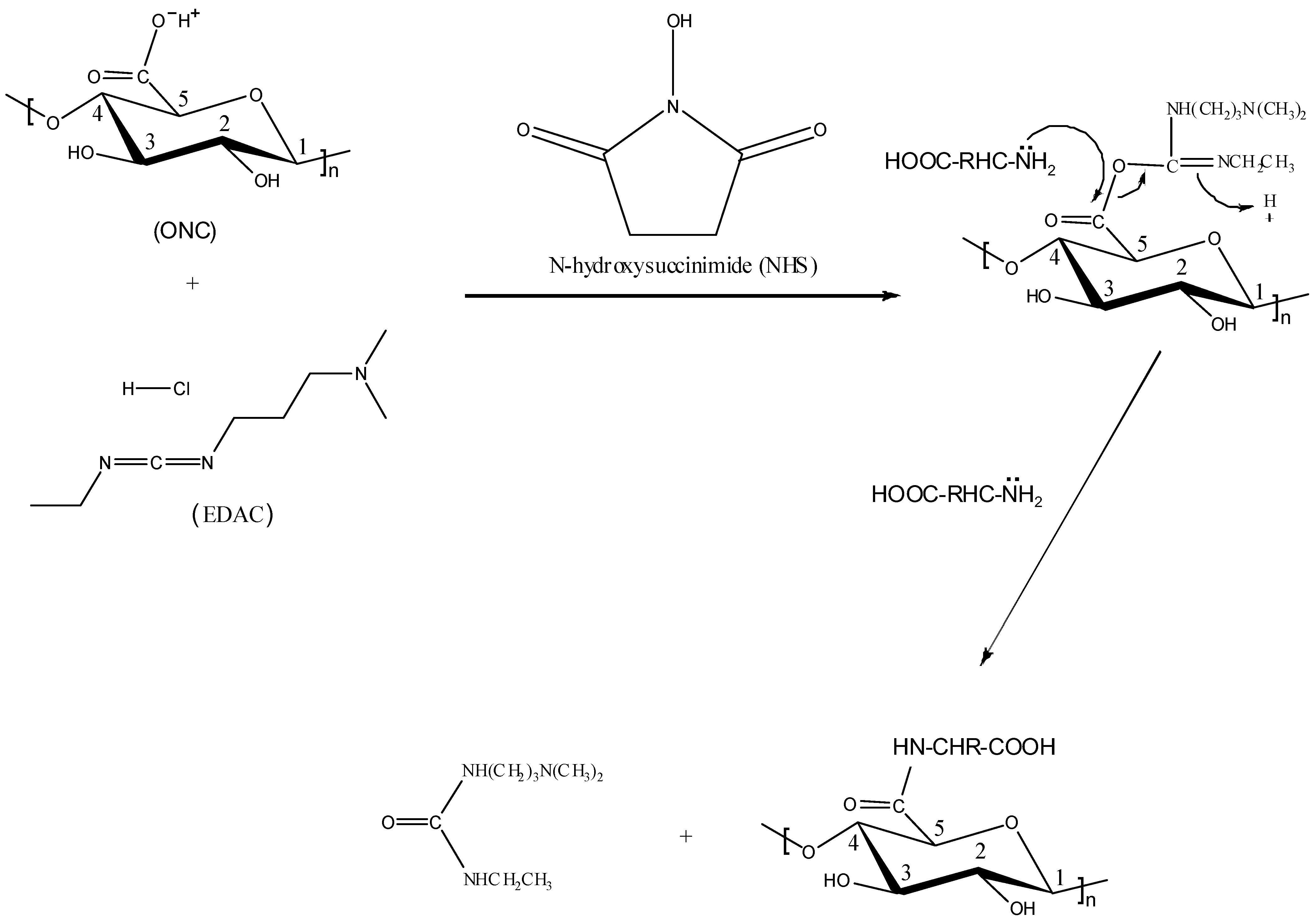
2.5. XPS Results
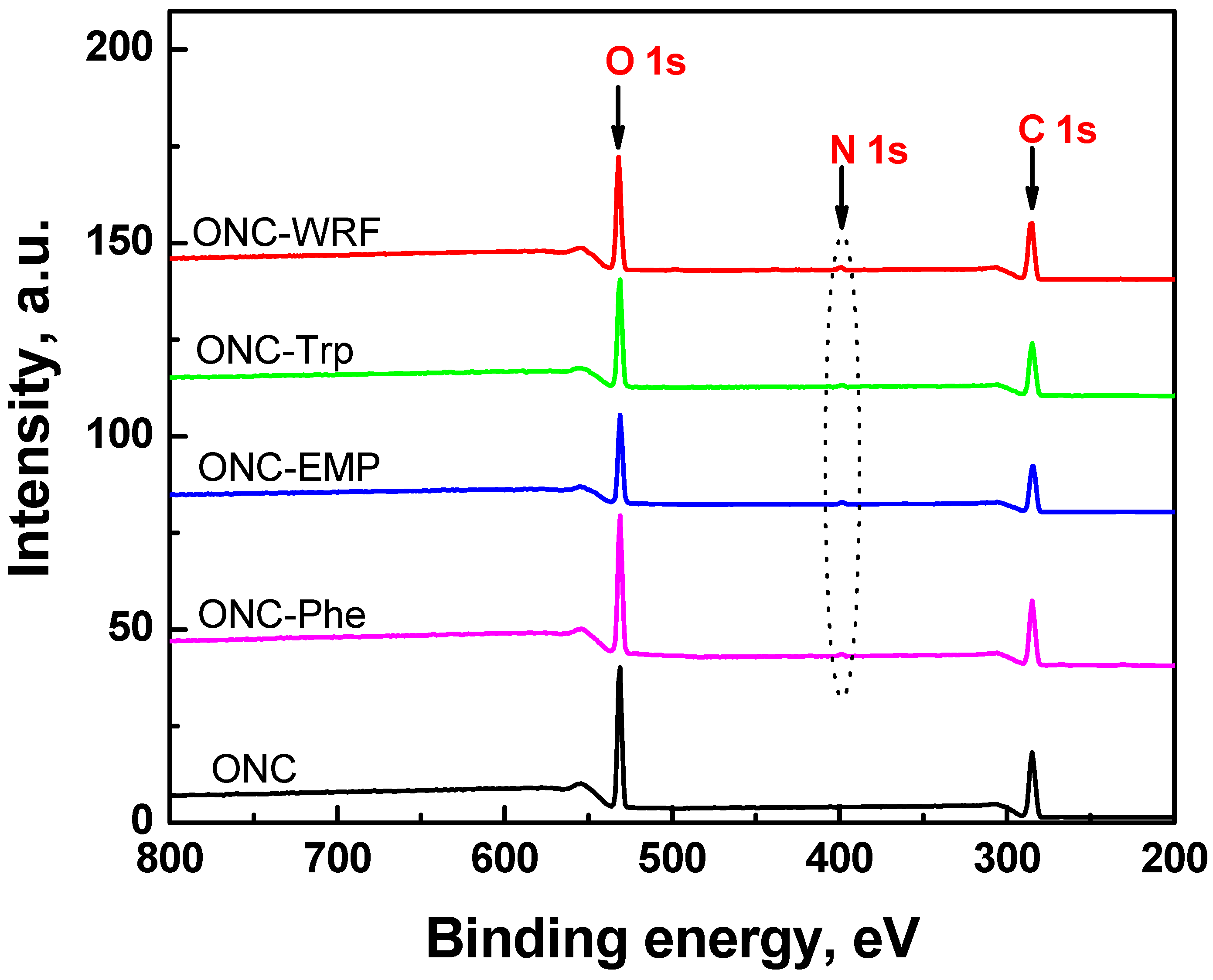
| Sample | Atomic content (%) | O/C | ||
|---|---|---|---|---|
| C | O | N | ||
| ONC | 63.8 | 36.2 | 0 | 0.57 |
| ONC-Trp | 64.4 | 34.2 | 1.4 | 0.53 |
| ONC-Phe | 64.0 | 33.9 | 1.1 | 0.53 |
| ONC-EMP | 67.2 | 31.3 | 1.5 | 0.47 |
| ONC-WRF | 67.1 | 31.2 | 1.7 | 0.46 |

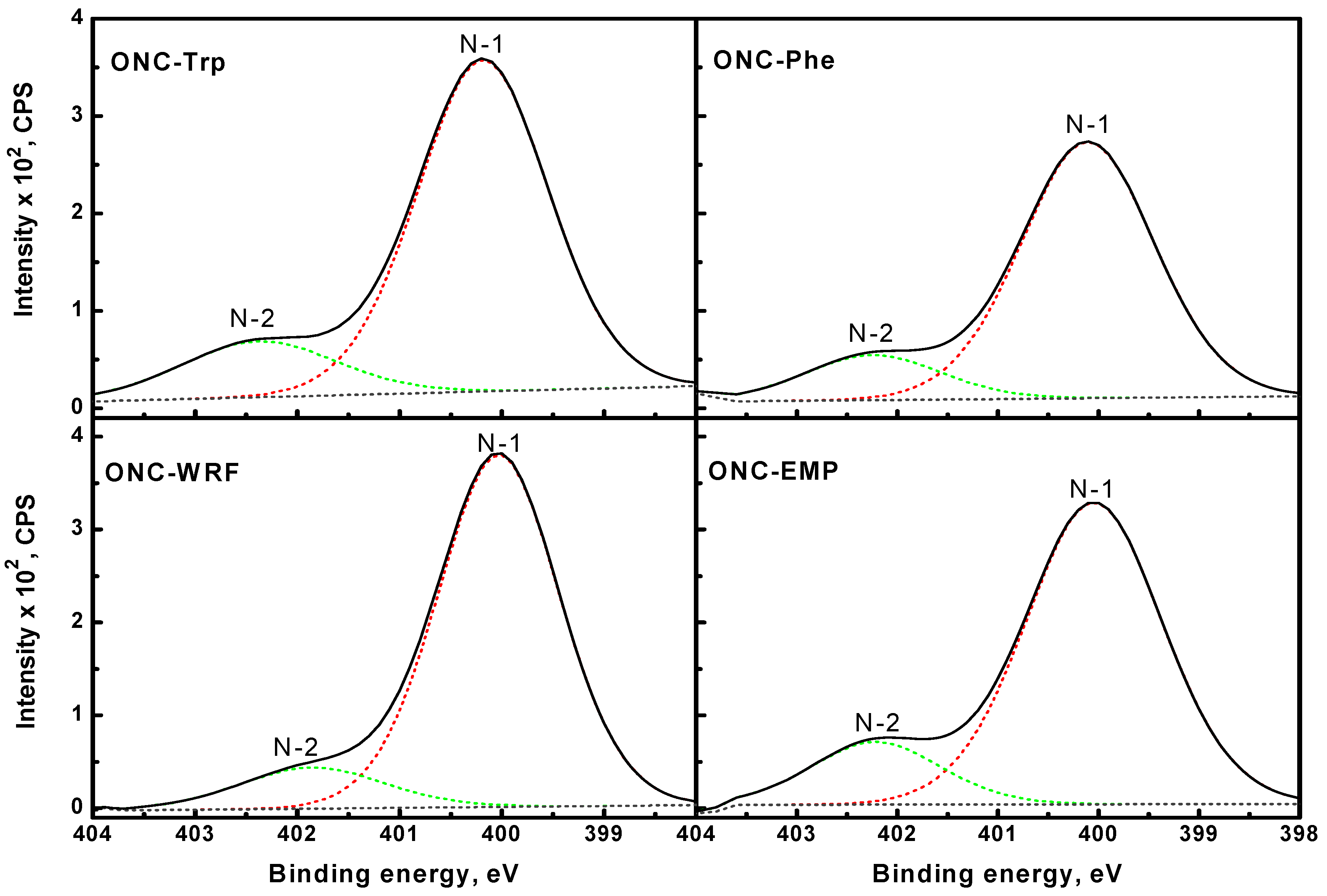
 ) [49]. It should be mentioned that XPS spectra of ONC alone, ONC-amino acids and ONC-Trp-Ps in the absence of coupling agents EDAC-NHS did not show any N 1s signal.
) [49]. It should be mentioned that XPS spectra of ONC alone, ONC-amino acids and ONC-Trp-Ps in the absence of coupling agents EDAC-NHS did not show any N 1s signal. 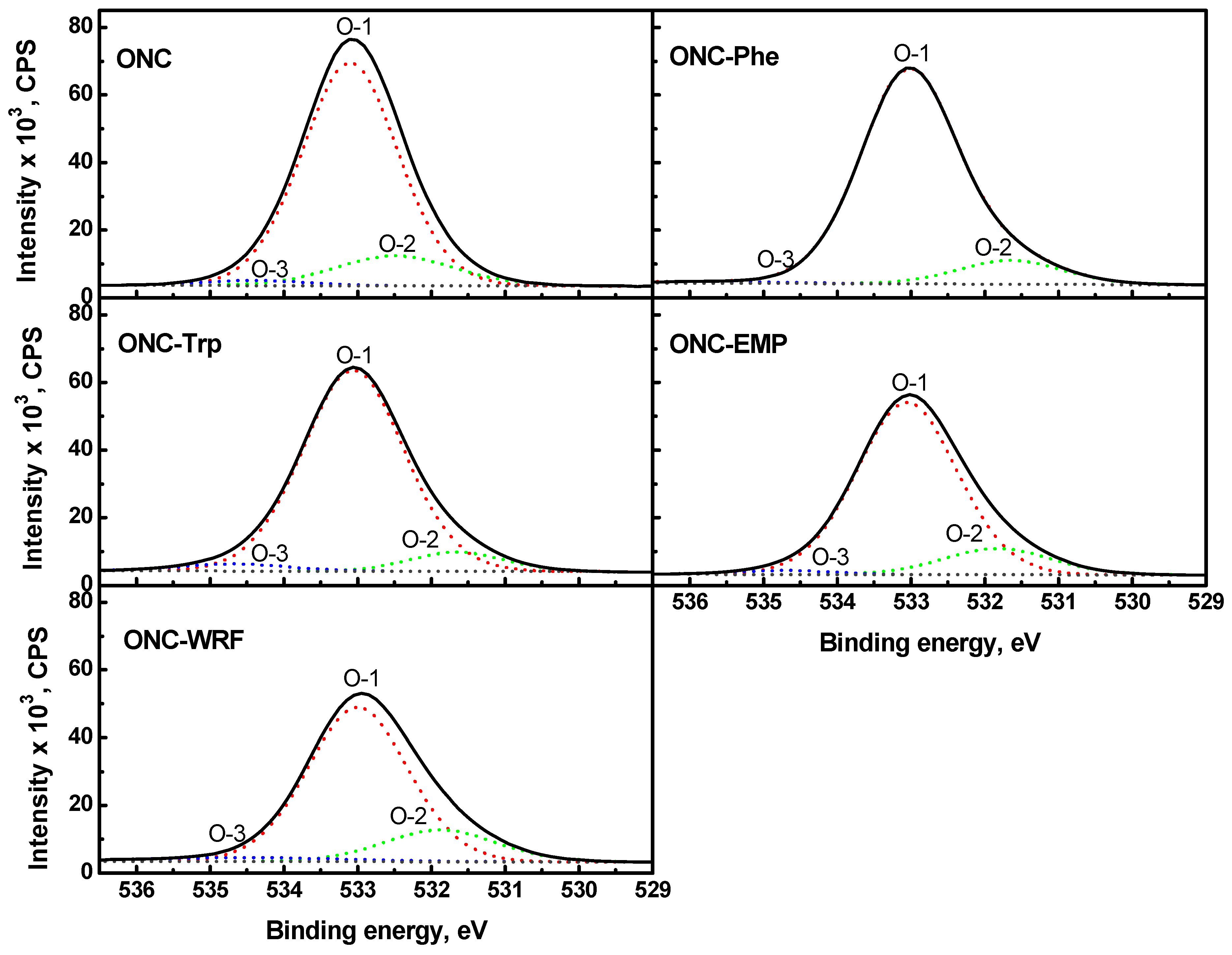
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L. Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J. Appl. Polym. Sci. 2007, 106, 2817–2824. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Chanzy, H. Aspects of cellulose structure. In Cellulose Sources and Exploitation; Kennedy, J.F., Philips, G.O., William, P.A., Eds.; Ellis Horwood Ltd.: New York, NY, USA, 1990; pp. 3–12. [Google Scholar]
- Klemm, D.; Schumann, D.; Kramer, F.; Hessler, N.; Koth, D.; Sultanova, B. Nanocellulose materials: Different cellulose, different functionality. Macromol. Symp. 2009, 280, 60–71. [Google Scholar] [CrossRef]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–805. [Google Scholar]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- de Nooy, A.E.; Besemer, A.C.; van Bekkum, H. Highly selective nitrosyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr. Res. 1995, 69, 89–98. [Google Scholar]
- Chang, P.S.; Robyt, J.F. Oxidation of primary alcohol groups of naturally occurring polysacccharides with 2,2,6,6-tetramethyl-1-piperidine oxoammonium ion. J. Carbohydr. Chem. 1996, 15, 819–830. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Sugiyama, J.; Chanzy, H.; Revol, J.F. On the polarity of cellulose in the cell wall of Valonia. Planta 1994, 193, 260–265. [Google Scholar]
- Isogai, A.; Kato, Y. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Tahiri, C.; Vignon, M.R. TEMPO-oxidation of cellulose: Synthesis and characterization of polyglucuronans. Cellulose 2000, 7, 177–188. [Google Scholar] [CrossRef]
- Saito, T.; Shibata, I.; Isogai, A.; Suguri, N.; Sumikwa, N. Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohydr. Polym. 2005, 61, 414–419. [Google Scholar] [CrossRef]
- Hoare, D.G.; Koshland, D.E. A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J. Biol. Chem. 1967, 242, 2447–2453. [Google Scholar]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Saito, T.; Togawa, E.; Isogai, A. Formation of N-acylureas on the surface of TEMPO oxidizedcellulose nanofibril with carbodiimide in DMF. Cellulose 2011, 18, 1191–1199. [Google Scholar] [CrossRef]
- Azzam, F.; Heux, L.; Putaux, J.L.; Jean, B. Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystal. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef]
- Follain, N.; Montanari, S.; Jeacomine, I.; Gambarelli, S.; Vignon, M.R. Coupling of amines with polyglucuronic acid: Evidence for amide bond formation. Carbohydr. Polym. 2008, 74, 333–343. [Google Scholar] [CrossRef]
- Jiang, K.; Schadler, L.S.; Siegel, R.W.; Zhang, X.; Zhang, H.; Terrones, M. Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J. Mater. Chem. 2004, 14, 37–39. [Google Scholar] [CrossRef]
- Lasseuguette, E. Grafting onto microfibrils of native cellulose. Cellulose 2008, 15, 571–580. [Google Scholar] [CrossRef]
- Carr, M.E.; Hermans, J. Size and density of fibrin fibers from turbidity. Macromolecules 1978, 11, 46–50. [Google Scholar] [CrossRef]
- Danishefsky, I.; Siskovic, E. Conversion of carboxyl groups of mucopolysaccharides into amides of amino acid esters. Carbohydr. Res. 1971, 16, 199–205. [Google Scholar] [CrossRef]
- Rzayev, J.; Hillmyer, M.A. Nanochannel array plastics with tailored surface chemistry. J. Am. Chem. Soc. 2005, 127, 13373–13379. [Google Scholar]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Olmstead, J.A.; Gray, D.G. Fluorescence spectroscopy of cellulose, lignin and mechanical pulps: A review. J. Pulp. Pap. Sci. 1997, 23, J571–J581. [Google Scholar]
- Toner, S.D.; Plitt, K.F. Spectrofluorometric studies of degraded cotton cellulose. Tappi. J. 1962, 45, 681–688. [Google Scholar]
- Albani, J.R. Fluorescence spectroscopy principles. In Principles and Applications of Fluorescence Spectroscopy; Blackwell Science Ltd.: Oxford, UK, 2007; pp. 88–113. [Google Scholar]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Deg. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, L.; Wu, Q.; Yang, S. Preliminary research on structure and properties of nano-cellulose. J. Wuhan. Univ. Tech. Mater. Sci. Ed. 2007, 22, 677–680. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Fardim, P.; Holmbom, B. Surface characterization by XPS, contact angle measurements and ToF-SIMS of cellulose fibers partially esterified with fatty acids. J. Col. Int. Sci. 2006, 301, 205–209. [Google Scholar] [CrossRef]
- Matuana, L.M.; Balatinecz, J.J.; Sodhi, R.N.S.; Park, C.B. Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. [Google Scholar] [CrossRef]
- Hua, X.; Kaliaguine, S.; Kokta, B.V.; Adnot, A. Surface analysis of explosion pulps by ESCA Part 1. Carbon (1s) spectra and oxygen-to-carbon ratios. Wood Sci. Technol. 1993, 27, 449–459. [Google Scholar]
- Dorris, G.M.; Gray, D.G. The surface analysis of paper and wood fibers by ESCA. Cellulose Chem. Technol. 1978, 12, 9–23. [Google Scholar]
- Ahmed, A.; Adnot, A.; Granmaison, J.L.; Kaliaguine, S.; Doucet, J. ESCA analysis of cellulosic materials. Cellulose Chem. Technol. 1987, 21, 483–492. [Google Scholar]
- Barry, A.O.; Zoran, Z. Surface analysis by ESCA of sulfite post-treated CTMP. J. Appl. Polym. Sci. 1990, 39, 31–42. [Google Scholar] [CrossRef]
- Liu, F.P.P.; Rials, T.G.; Simonsen, J. Relationship of wood surface energy to surface composition. Langmuir 1998, 14, 536–541. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Zhang, J.; Adnot, A. Identification of cupric and cuprous copper in copper naphthenate-treated wood by X-ray photoelectron spectroscopy. Holzforschung 2001, 55, 16–20. [Google Scholar]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on biopolymers: Cellulose studies. Surf. Interf. Anal. 2004, 36, 1018–1022. [Google Scholar] [CrossRef]
- Nzokou, P.; Kamdem, D.P. X-ray photoelectron spectroscopy study of red oak-(Quercus rubra), black cherry-(Prunus serotina) and red pine-(Pinus resinosa) extracted wood surfaces. Surf. Interf. Anal. 2005, 37, 689–694. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Riedl, B.; Adnot, A.; Kaliaguine, S. ESCA spectroscopy of poly(methylmethacrylate) grafted onto wood fibers. J. Appl. Polym. Sci. 1991, 43, 1901–1912. [Google Scholar] [CrossRef]
- Jansen, R.J.J.; Van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Zubavichus, Y.; Zharnikov, M.; Shaporenko, A.; Fuchs, O.; Weinhardt, L.; Heske, C.; Umbach, E.; Denlinger, J.D.; Grunze, M. Soft X-ray induced decomposition of phenylalanine and tyrosine: A comparative study. J. Phys. Chem. A 2004, 108, 4557–4565. [Google Scholar]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Barazzouk, S.; Daneault, C. Tryptophan-based peptides grafted onto oxidized nanocellulose. Cellulose 2012, 19, 481–493. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barazzouk, S.; Daneault, C. Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization. Nanomaterials 2012, 2, 187-205. https://doi.org/10.3390/nano2020187
Barazzouk S, Daneault C. Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization. Nanomaterials. 2012; 2(2):187-205. https://doi.org/10.3390/nano2020187
Chicago/Turabian StyleBarazzouk, Saïd, and Claude Daneault. 2012. "Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization" Nanomaterials 2, no. 2: 187-205. https://doi.org/10.3390/nano2020187
APA StyleBarazzouk, S., & Daneault, C. (2012). Amino Acid and Peptide Immobilization on Oxidized Nanocellulose: Spectroscopic Characterization. Nanomaterials, 2(2), 187-205. https://doi.org/10.3390/nano2020187




