Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens
Abstract
:1. Introduction
1.1. Aim
1.2. Pore Formation Mechanism
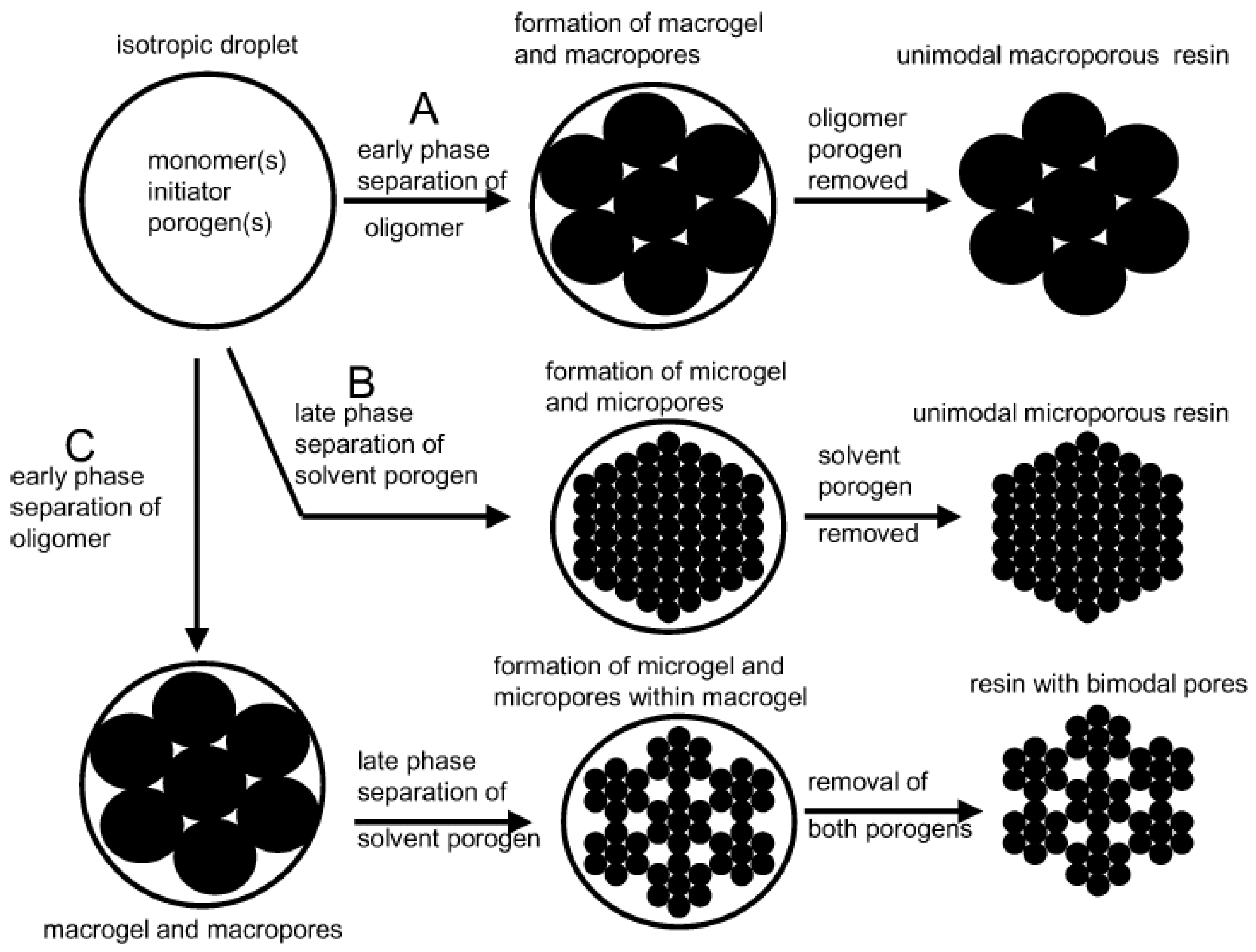
1.3. Review Motivation
1.4. Criteria for Porogen Selection
2. Synthesis and Characterization
2.1. Synthesis
2.2. Characterization
2.2.1. Porosimetry
2.2.2. Solvent Uptake
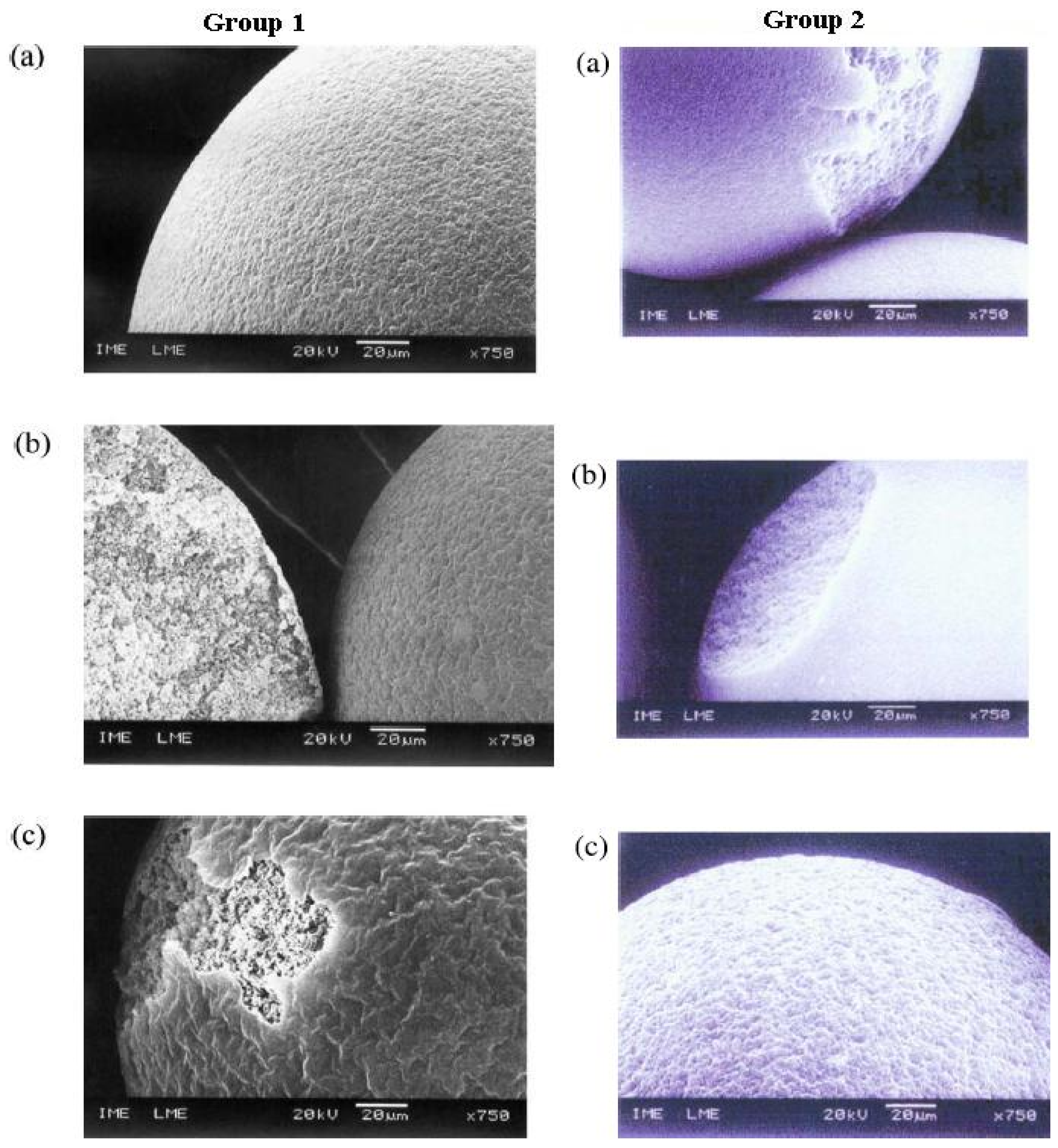
2.2.3. Microscopy
3. Solubility Parameter
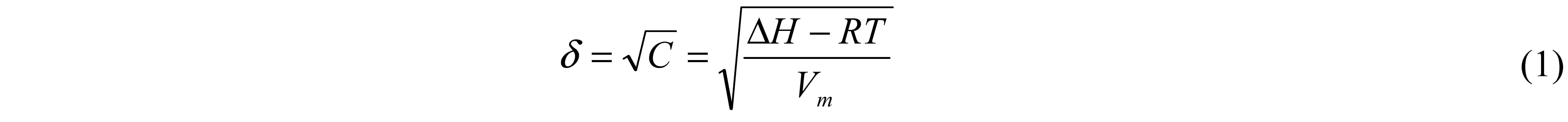
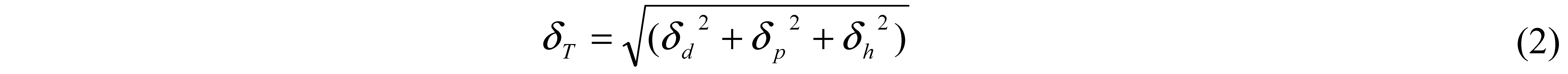


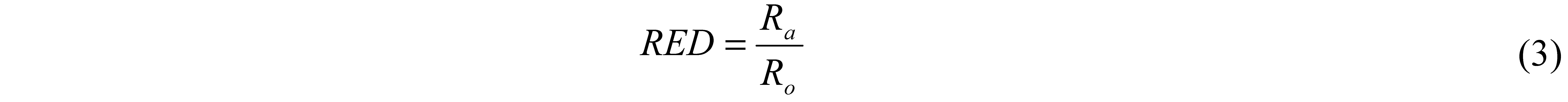

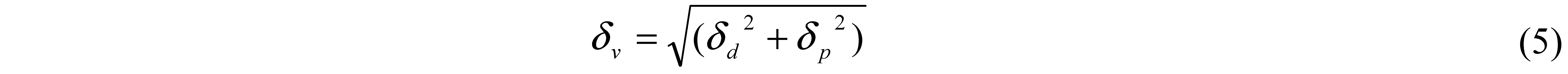
4. Pore Formation Mechanism
5. Influence of the Solvent (Porogen)
5.1. Pure Solvents
| Polymer type | Porogen | Surface area (m2/g) | Pore volume (cm3/g) | Porosity(%) | Average pore diameter (nm) | Reference |
|---|---|---|---|---|---|---|
| Poly(styrene- co-divinylbenzene) – 1:3 | Cyclohexanone | 25 | 0.22 | NM * | ND + | [16] |
| 2-ethylhexanol | 242 | 1.13 | 20–45 | |||
| Toluene | 599 | 0.44 | <4 | |||
| Glycidyl methacrylate- co-trimethylolpropane trimethacrylate – 1:1 | Octan-2-one | 174 | 1.31 | NM * | 10–100 | [15] |
| n-butyl acetate | 170 | 1.27 | ||||
| p-xylene | 139 | 1.50 | ||||
| Toluene | 145 | 1.02 | ||||
| Ethyl acetate | 110 | 0.66 | ||||
| Benzonitrile | <1 | 0.07 | ||||
| Cyclohexanone | 0.2 | 0.16 | ||||
| Dodecan-1-ol | ND + | ND + | ||||
| Poly(hydroxyethyl methacrylate- co-ethylene glycol dimethacrylate) - 1:20 | Cyclohexanol | 250 | 0.396 | NM * | 6.3 | [10] |
| Dodecanol | 28 | 0.06 | 8.6 | |||
| Poly(divinylbenzene); mixtures of isomers | Heptane | 482 | NM * | 45.3 a | 6.9 a | [46] |
| 69.0 b | 86.8 b | |||||
| Cyclohexane | 640 | NM * | 37.9 a | 3.8 a | ||
| 47.7 b | 72.4 b | |||||
| 1-chlorodecane | 720 | NM * | 56.3 a | 7.2 a | ||
| 66.7 b | 53.4 b | |||||
| Toluene | 701 | NM * | 35.9 a | 3.2 a | ||
| 50.5 b | 68.1 b | |||||
| Dibutyl phthalate | 661 | NM * | 37.1 a | 3.6 a | ||
| 61.8 b | 60.2 b | |||||
| Cyclohexanol | 493 | NM * | 29.1 a | 3.3 a | ||
| 47.6 b | 77.8 b |
5.2. Solvent Mixtures

| Polymer type | Porogen A | Surface area (m2/g) | Pore volume (cm3/g) | Average pore diameter (nm) | Reference |
|---|---|---|---|---|---|
| Poly(styrene- co-divinylbenzene) – 1:3 | 2-ethylhexanol: DMF (70%:30%) | 375 | NM * | 20.0 | [16] |
| Toluene: DMF (70%:30%) | 555 | NM * | |||
| Glycidyl methacrylate- co-trimethylolpropane trimethacrylate – 1:1 | Cyclohexanone : Dodecan-1-ol (9:1) | 140 | 1.13 | 10–100 | [15] |
| 2-vinylpyridine- co-styrene-co-divinylbenzene – 1:20 | Heptane : Toluene (100%:0%) | ND + | ND + | ND + | [10] |
| Heptane : Toluene (70%:30%) | 131 | 0.5845 | 21.9 |
5.3. Solvent Volume
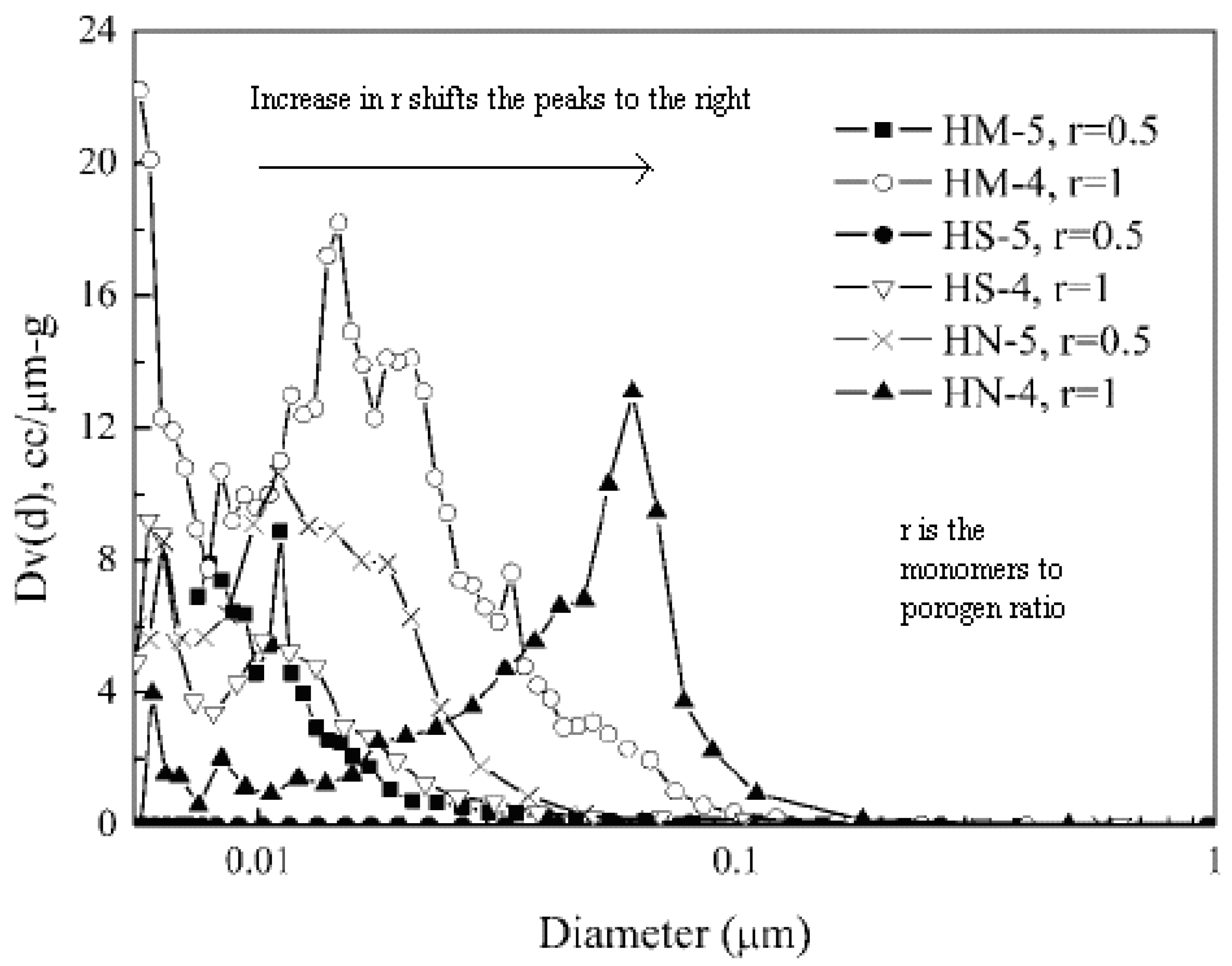
5.4. Solvent Type
5.4.1. Organics
5.4.1.1. Low Molecular Weight
5.4.1.2. High Molecular Weight
5.4.2. Inorganics
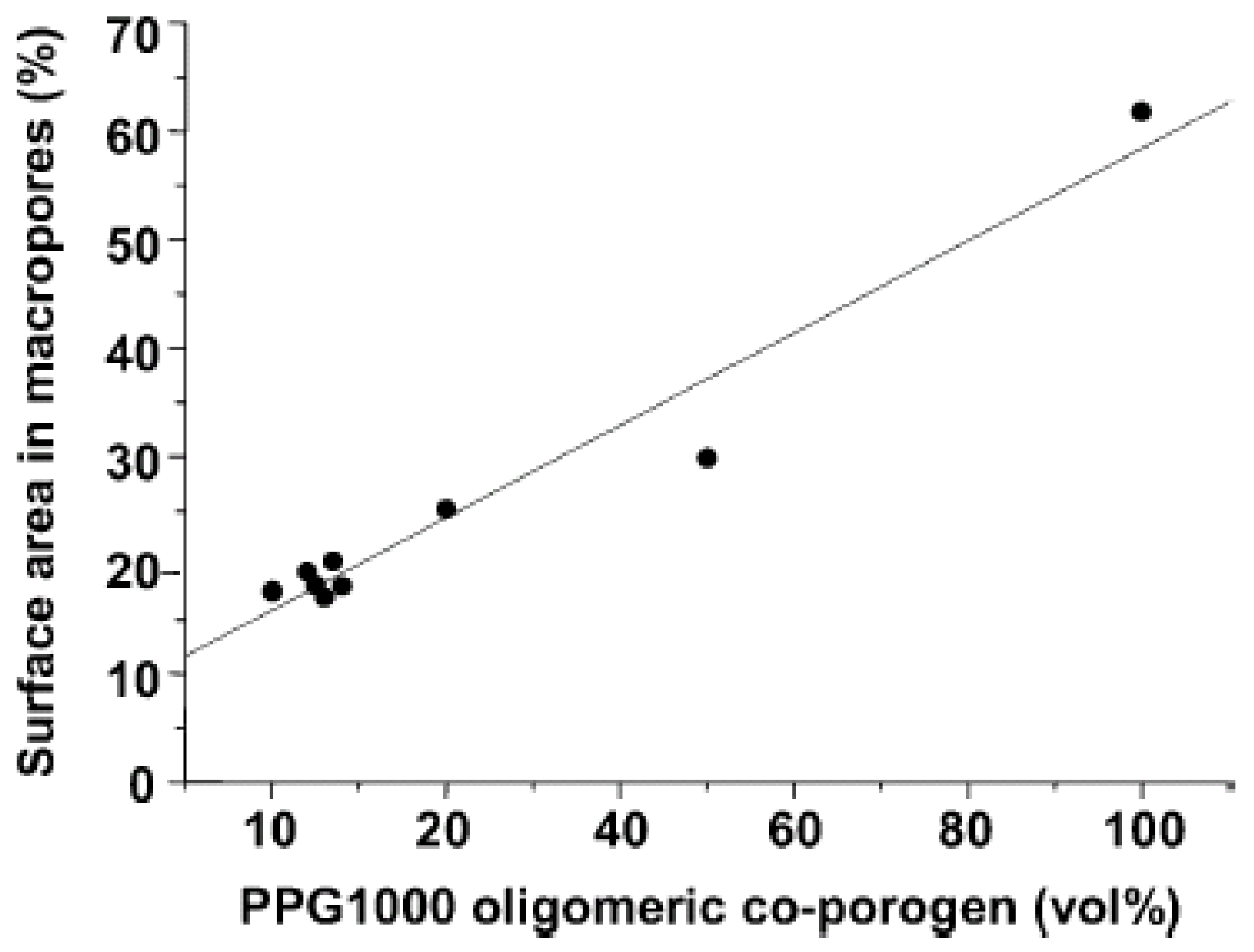
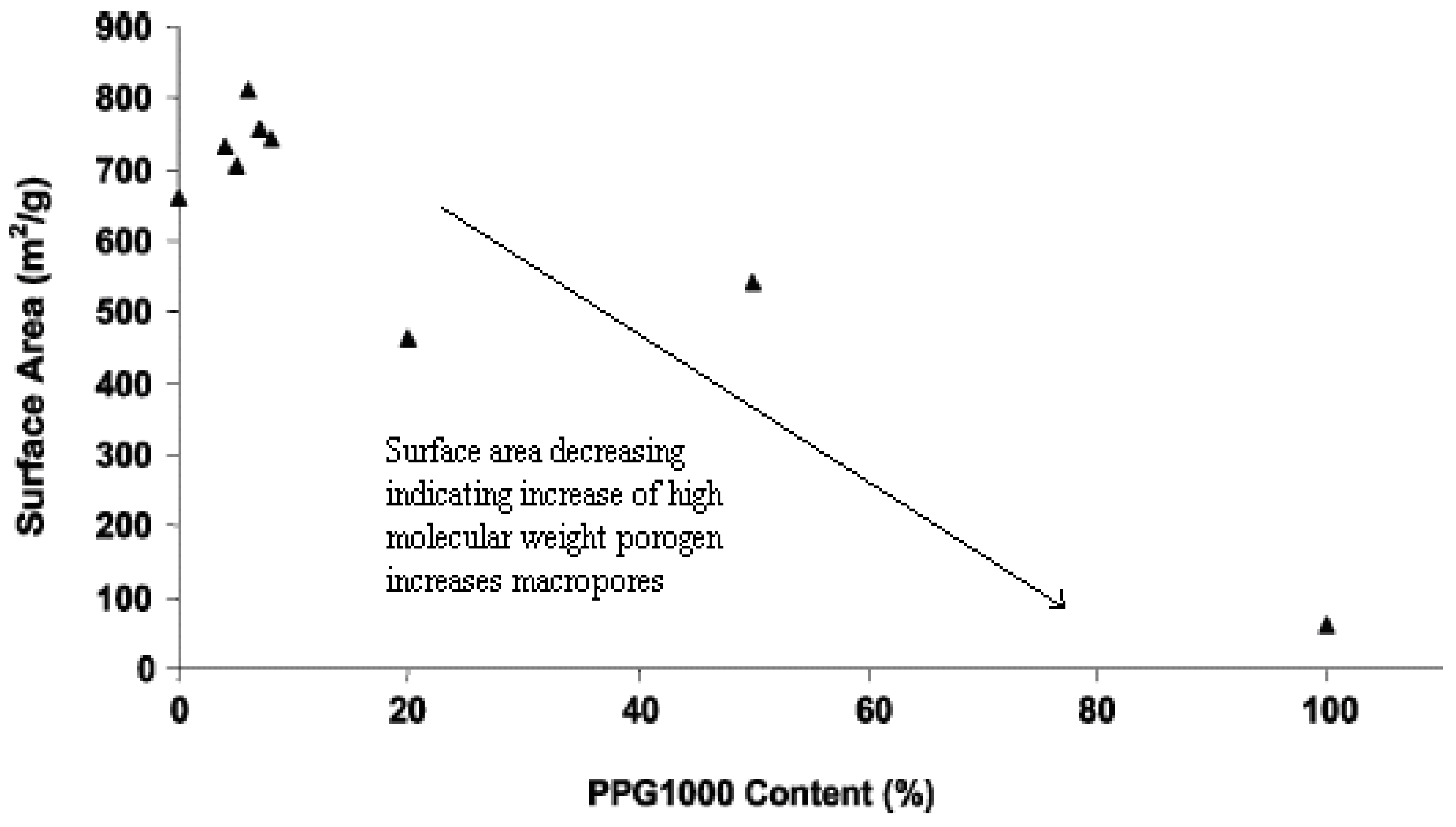
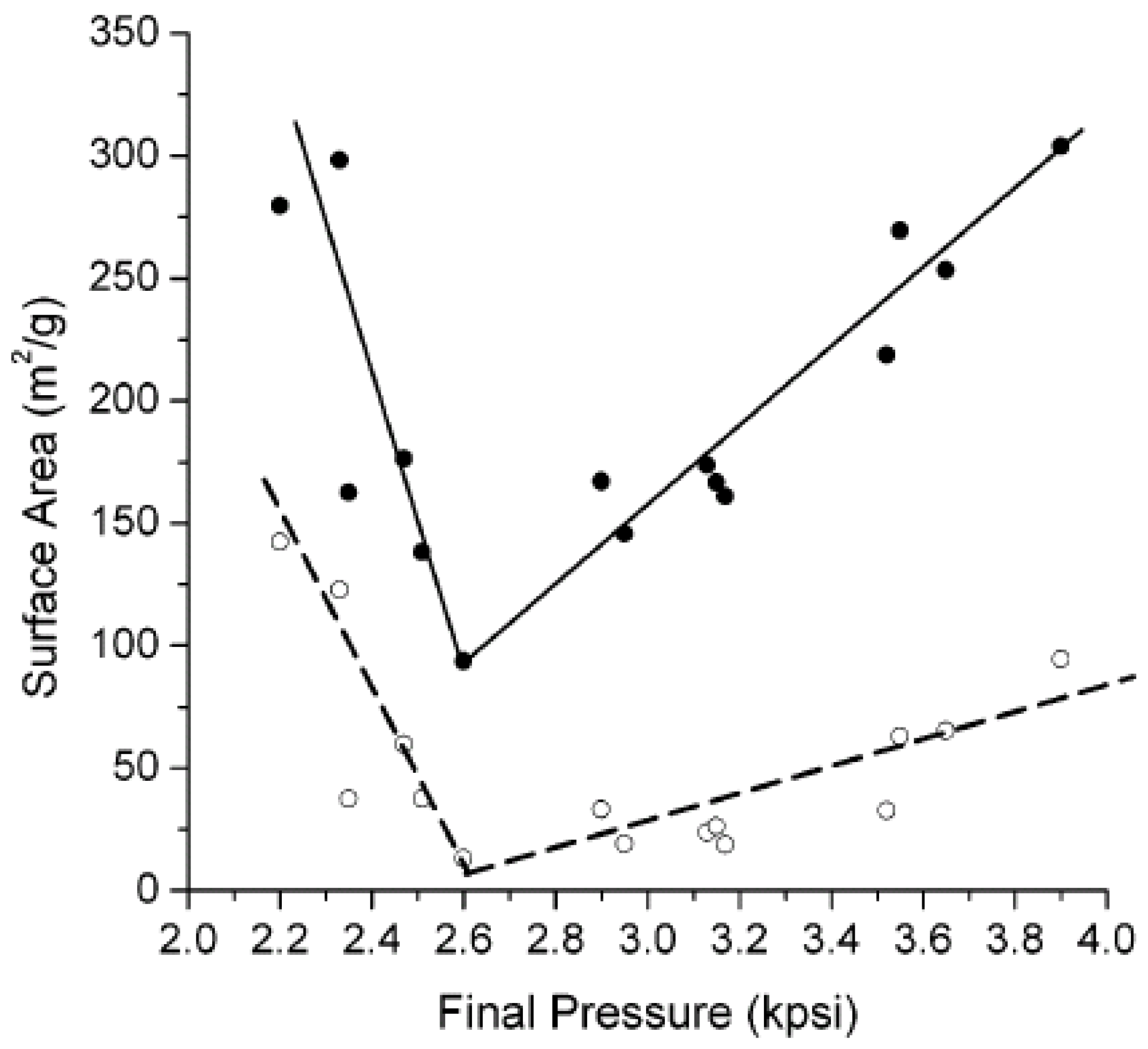
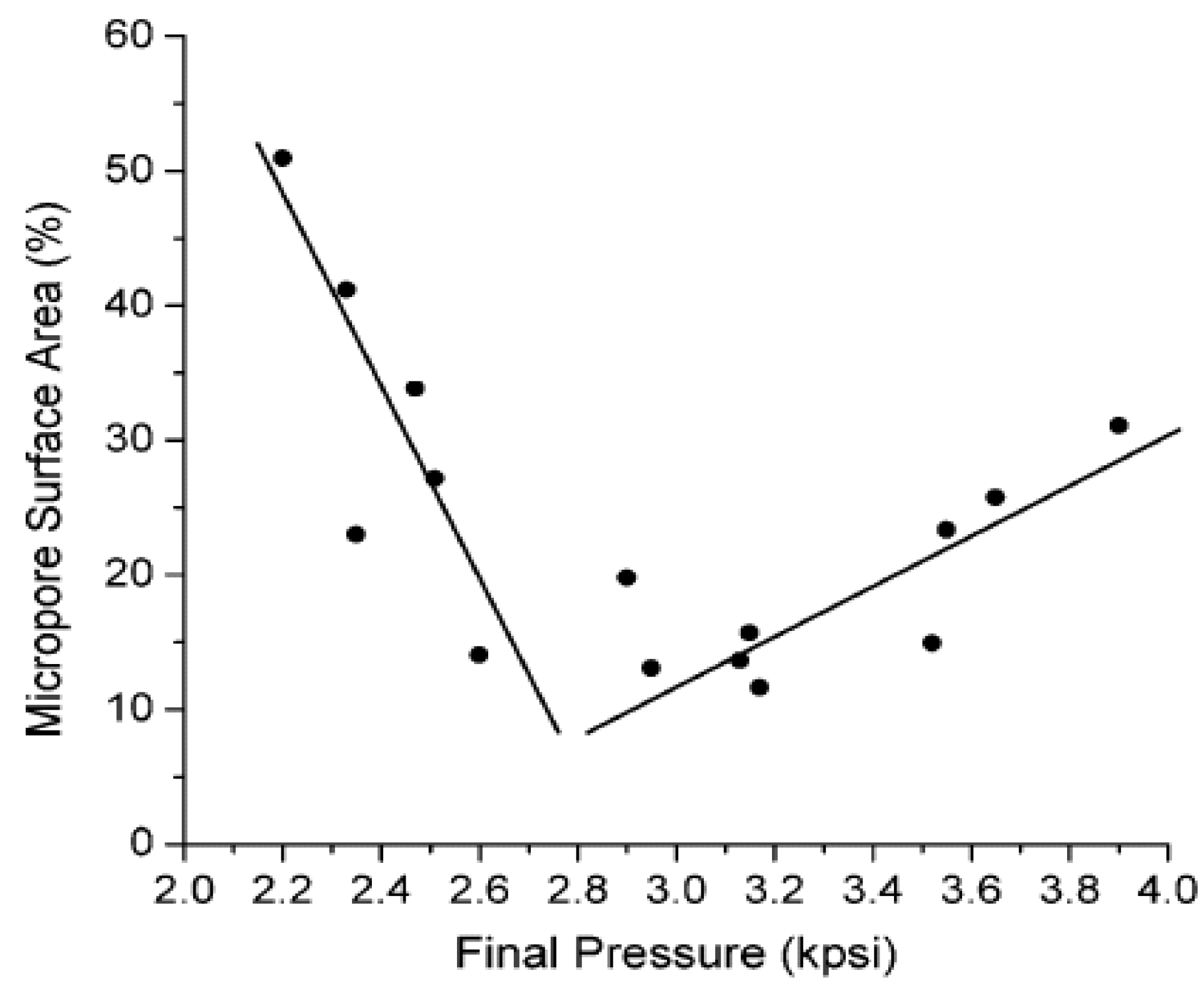
6. Property Changes
6.1. Porosity
6.2. Morphology
7. Hansen Solubility Parameter
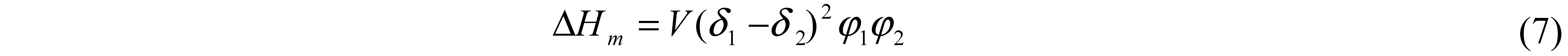
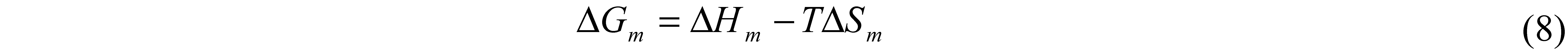
| One-component | Three-component | ||||
|---|---|---|---|---|---|
| Solvent | |Δδ| MPa0.5 | Prediction | Solvent | RED | Prediction |
| Ethyl acetate | 0.2 | “Good” solvents i.e., |Δδ| < 1.0 | Acetophenone | 0.28 | “Good” solvents i.e., |Δδ| < 1.0 |
| Toluene | 0.4 | Diisobutyl phthalate | 0.59 | ||
| Diisobutyl phthalate | 0.4 | Toluene | 0.65 | ||
| Decaline | 0.6 | Diethyl phthalate | 0.65 | ||
| Butyl acetate | 1.2 | Intermediary solvents i.e., 1.0 |Δδ| < 3.0 | Decaline | 0.73 | |
| Methyl-isobutyl ketone | 1.4 | Dioctyl phthalate | 0.75 | ||
| Diethyl phthalate | 1.8 | Benzyl alcohol | 0.87 | Intermediary solvents, i.e., 1.0 |Δδ| < 3.0 | |
| Isoamyl alcohol | 1.9 | Ethyl acetate | 0.90 | ||
| Dioctyl phthalate | 2.4 | Butyl acetate | 0.90 | ||
| Isoamyl acetate | 2.6 | Methyl-isobutyl ketone | 0.94 | ||
| Acetophenone | 3.1 | Isoamyl alcohol | 0.98 | ||
| Heptane | 3.5 | “Bad” solvents i.e., |Δδ| > 3.0 | Heptane | 1.10 | “Bad” solvents i.e., |Δδ| > 3.0 |
| Benzyl alcohol | 6.1 | Isoamyl alcohol | 1.13 | ||
| Solvent | Fixed pore volume (cm3/g) | Surface area (m2/g) | Average pore diameter (nm) |
|---|---|---|---|
| Diethyl phthalate | 0.47 | 149 | 12.6 |
| Benzyl alcohol | 0.65 | 110 | 23.6 |
| Diisobutyl phthalate | 0.57 | 83 | 27.5 |
| Dioctyl phthalate | 0.88 | 75 | 46.9 |
| Heptane | 0.99 | 68 | 58.2 |
| Isoamyl acetate | 0.34 | 30 | 45.3 |
| Isoamyl alcohol | 1.00 | 7 | 143 |
| Toluene | 0.01 | 0 | – |
| Acetophenone | 0.06 | 0 | – |
| Methyl-isobutyl ketone | 0.06 | 0 | – |
| Decaline | 0.12 | 0 | – |
| Ethyl acetate | 0.13 | – | – |
| Butyl acetate | 0.41 | – | – |
8. Knowledge Gaps
9. Conclusions
Acknowledgments
References
- Rajiv Gandhi, M.; Viswanathan, N.; Meenakshi, S. Synthesis and characterization of a few amino-functionalized copolymeric resins and their environmental applications. Ind. Eng. Chem. Res. 2012, 51, 5677–5684. [Google Scholar] [CrossRef]
- Ortiz-Palacios, J.; Cardoso, J.; Manero, O. Production of macroporous resins for heavy-metal removal. I. Nonfunctionalized polymers. J. Appl. Polym. Sci. 2008, 107, 2203–2210. [Google Scholar] [CrossRef]
- Charef, N.; Benmaamar, Z.; Arrar, L.; Baghiani, A.; Zalloum, R.M.; Mubarak, M.S. Preparation of a new polystyrene supported-ethylenediaminediacetic acid resin and its sorption behavior toward divalent metal ions. Solvent Extr. Ion Exch. 2012, 30, 101–112. [Google Scholar] [CrossRef]
- Drake, R.; Dunn, R.; Sherrington, D.C.; Thomson, S.J. Optimisation of polystyrene resin-supported pt catalysts in room temperature, solvent-less, oct-l-ene hydrosilylation using methyldichlorosilane. Comb. Chem. High Throughput Scr. 2002, 5, 201–209. [Google Scholar]
- Siyad, M.A.; Nair, A.S.V.; Kumar, G.S.V. Solid-phase peptide synthesis of endothelin receptor antagonists on novel flexible, styrene-acryloyloxyhydroxypropyl methacrylate-tripropyleneglycol diacrylate [SAT] resin. J. Comb. Chem. 2010, 12, 298–305. [Google Scholar] [CrossRef]
- Park, J.S.; Han, C.; Lee, J.Y.; Kim, S.D.; Kim, J.S.; Wee, J.H. Synthesis of extraction resin containing 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester and its performance for separation of rare earths (gd, tb). Separ. Sci. Technol. 2005, 43, 111–116. [Google Scholar]
- Lin, Z.H.; Guan, C.J.; Feng, X.L.; Zhao, C.X. Synthesis of macroreticular p-(omega-sulfonic-perfluoroalkylated)polystyrene ion-exchange resin and its application as solid acid catalyst. J. Mol. Catal. A Chem. 2006, 247, 19–26. [Google Scholar] [CrossRef]
- Biswas, M.; Packirisamy, S. Synthetic ion-exchange resins. In Key Polymers Properties and Performance; Benoit, H., Cantow, H.-J., Dall’Asta, G., Dusek, K., Ferry, J.D., Fujita, H., Fujita, H., Gordon, M., Henrici-Olive, G., Heublein, H.G., et al., Eds.; Springer-Verlag: New York, NY, USA, 1985; Volume 70, pp. 71–118. [Google Scholar]
- Hentze, H.P.; Antonietti, M. Porous polymers and resins for biotechnological and biomedical applications. Rev. Mol. Biotechnol. 2002, 90, 27–53. [Google Scholar] [CrossRef]
- Blondeau, D.; Bigan, M.; Ferreira, A. Experimental-design study of the influence of the polymerization conditions on the characteristics of copolymers used for liquid chromatography. Chromatographia 2000, 51, 713–721. [Google Scholar] [CrossRef]
- Millar, J.R.; Smith, D.G.; Marr, W.E.; Kressman, T.R.E. 33. Solvent-modified polymer networks. Part I. The preparation and characterisation of expanded-network and macroporous styrene-divinylbenzene copolymers and their sulphonates. J. Chem. Soc. 1963, 218–225. [Google Scholar]
- Kun, K.A.; Kunin, R. Macroreticular resins. III. Formation of macroreticular styrene-divinylbenzene copolymers. J. Polym. Sci. Part A-1: Polym. Chem. 1968, 6, 2689–2701. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Vivaldo-Lima, E.; Wood, P.E.; Hamielec, A.E.; Penlidis, A. An updated review on suspension polymerization. Ind. Eng. Chem. Res. 1997, 36, 939–965. [Google Scholar] [CrossRef]
- Verweij, P.D.; Sherrington, D.C. High-surface-area resins derived from 2,3-epoxypropyl methacrylate cross-linked with trimethylolpropane trimethacrylate. J. Mater. Chem. 1991, 1, 371–374. [Google Scholar] [CrossRef]
- Deleuzel, H.; Schultze, X.; Sherrington, D.C. Porosity analysis of some poly(styrene/divinylbenzene) beads by nitrogen sorption and mercury intrusion porosimetry. Polym. Bull. 2000, 44, 179–189. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, X.; Fan, L. Hyper-cross-linked resins with controllable pore structure. Mater. Lett. 2008, 62, 2392–2395. [Google Scholar] [CrossRef]
- Macintyre, F.S.; Sherrington, D.C. Control of porous morphology in suspension polymerized poly(divinylbenzene) resins using oligomeric porogens. Macromolecules 2004, 37, 7628–7636. [Google Scholar] [CrossRef]
- Wang, Q.C.; Hosoya, K.; Svec, F.; Frechet, J.M.J. Polymeric porogens used in the preparation of novel monodispersed macroporous polymeric separation media for high-performance liquid chromatography. Anal. Chem. 1992, 64, 1232–1238. [Google Scholar] [CrossRef]
- Horák, D.; Labský, J.; Pilař, J.; Bleha, M.; Pelzbauer, Z.; Švec, F. The effect of polymeric porogen on the properties of macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate). Polymer 1993, 34, 3481–3489. [Google Scholar]
- Schmidt, R.H.; Haupt, K. Molecularly imprinted polymer films with binding properties enhanced by the reaction-induced phase separation of a sacrificial polymeric porogen. Chem. Mater. 2005, 17, 1007–1016. [Google Scholar] [CrossRef]
- Negre, M.; Bartholin, M.; Guyot, A. Functionalized resins, 1. Gel and macroporous chloromethylated styrenic resins prepared in the presence of toluene as a pore forming agent. Die Angew. Makromol. Chem. 1982, 106, 67–77. [Google Scholar] [CrossRef]
- Guettaf, H.; Iayadene, F.; Bencheikh, Z.; Rabia, I. Structure and properties of styerene-divinylbenzene-methylmethacrylateterpolymers-ii- effect of methylacrylate at different n-heptane 2-ethyl-1-hexanol diluent composition. Eur. Polym. J. 1998, 34, 241–246. [Google Scholar] [CrossRef]
- Hamid, M.A.; Naheed, R.; Fuzail, M.; Rehman, E. The effect of different diluents on the formation of n-vinylcarbazole-divinylbenzene copolymer beads. Eur. Polym. J. 1999, 35, 1799–1811. [Google Scholar] [CrossRef]
- Chao, A.-C.; Yu, S.-H.; Chuang, G.-S. Using nacl particles as porogen to prepare a highly adsorbent chitosan membranes. J. Membr. Sci. 2006, 280, 163–174. [Google Scholar] [CrossRef]
- Courtois, J.; Szumski, M.; Georgsson, F.; Irgum, K. Assessing the macroporous structure of monolithic columns by transmission electron microscopy. Anal. Chem. 2006, 79, 335–344. [Google Scholar]
- Zhou, Z.; Liu, X.; Liu, Q. A comparative study of preparation of porous poly-l-lactide scaffolds using NaHCO3 and NaCl as porogen materials. J. Macromol. Sci. Part B 2008, 47, 667–674. [Google Scholar] [CrossRef]
- Cooper, A.I.; Holmes, A.B. Synthesis of molded monolithic porous polymers using supercritical carbon dioxide as the porogenic solvent. Adv. Mater. 1999, 11, 1270–1274. [Google Scholar] [CrossRef]
- Wood, C.D.; Cooper, A.I. Synthesis of macroporous polymer beads by suspension polymerization using supercritical carbon dioxide as a pressure-adjustable porogen. Macromolecules 2000, 34, 5–8. [Google Scholar]
- Hebb, A.K.; Senoo, K.; Bhat, R.; Cooper, A.I. Structural control in porous cross-linked poly(methacrylate) monoliths using supercritical carbon dioxide as a “pressure-adjustable” porogenic solvent. Chem. Mater. 2003, 15, 2061–2069. [Google Scholar] [CrossRef]
- Xie, S.; Svec, F.; Fréchet, J.M.J. Preparation of porous hydrophilic monoliths: Effect of the polymerization conditions on the porous properties of poly(acrylamide-co-n,n′-methylenebisacrylamide) monolithic rods. J. Polym. Sci. Part A 1997, 35, 1013–1021. [Google Scholar]
- Viklund, C.; Svec, F.; Fréchet, J.M.J.; Irgum, K. Monolithic, “molded”, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: Control of porous properties during polymerization. Chem. Mater. 1996, 8, 744–750. [Google Scholar] [CrossRef]
- Wernick, D.L. Stereographic display of three-dimensional solubility parameter correlations. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 240–245. [Google Scholar] [CrossRef]
- Rabelo, D.; Coutinho, F.M.B. Structure and properties of styrene-divinylbenzene copolymers. Polym. Bull. 1994, 33, 479–486. [Google Scholar] [CrossRef]
- Krauskopf, L.G. Prediction of plasticizer solvency using hansen solubility parameters. J. Vinyl Addit. Technol. 1999, 5, 101–106. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- zdemir, C.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Svec, F.; Frechet, J.M.J. Kinetic control of pore formation in macroporous polymers. Formation of “molded” porous materials with high flow characteristics for separations or catalysis. Chem. Mater. 1995, 7, 707–715. [Google Scholar] [CrossRef]
- Badyal, J.P.; Cameron, A.M.; Cameron, N.R.; Oates, L.J.; Øye, G.; Steel, P.G.; Davis, B.G.; Coe, D.M.; Cox, R.A. Comparison of the effect of pore architecture and bead size on the extent of plasmachemical amine functionalisation of poly(styrene-co-divinylbenzene) permanently porous resins. Polymer 2004, 45, 2185–2192. [Google Scholar]
- Sherrington, C.D. Preparation, structure and morphology of polymer supports. Chem. Commun. 1998, 2275–2286. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Yan, J. Water-swellable hydrophobic porous copolymer resins based on divinylbenzene and acrylonitrile. II. Pore structure and adsorption behavior. J. Appl. Polym. Sci. 2004, 94, 2050–2056. [Google Scholar] [CrossRef]
- Hao, D.-X.; Gong, F.-L.; Wei, W.; Hu, G.-H.; Ma, G.-H.; Su, Z.-G. Porogen effects in synthesis of uniform micrometer-sized poly(divinylbenzene) microspheres with high surface areas. J. Colloid Interface Sci. 2008, 323, 52–59. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Shen, S.; Xiao, Q.; Chen, L.; Liao, B.; Ou, B.; Ding, Y. Preparation and characterization of crosslinked polymer beads with tunable pore morphology. J. Appl. Polym. Sci. 2011, 121, 654–659. [Google Scholar] [CrossRef]
- Nyhus, A.K.; Hagen, S.; Berge, A. Formation of the porous structure during the polymerization of meta-divinylbenzene and para-divinylbenzene with toluene and 2-ethylhexanoic acid (2-eha) as porogens. J. Polym. Sci. Part A 1999, 37, 3973–3990. [Google Scholar]
- Terada, M.; Marchessault, R.H. Determination of solubility parameters for poly(3-hydroxyalkanoates). Int. J. Biol. Macromol. 1999, 25, 207–215. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Xiao, A.; Yu, H.; Tan, Q.; Ding, J.; Ren, G. Unexpected behavior of 1-chlorodecane as a novel porogen in the preparation of high-porosity poly(divinylbenzene) microspheres. J. Phys. Chem. C 2008, 112, 13171–13174. [Google Scholar]
- Hamedi, M.; Danner, R.P. Prediction of solubility parameters using the group-contribution lattice-fluid theory. J. Appl. Polym. Sci. 2001, 80, 197–206. [Google Scholar] [CrossRef]
- De Santa Maria, L.C.; de Aguiar, A.P.; Aguiar, M.R.M.P.; Jandrey, A.C.; Guimarães, P.I.C.; Nascimento, L.G. Microscopic analysis of porosity of 2-vinylpyridine copolymer networks: 1. Influence of diluent. Mater. Lett. 2004, 58, 563–568. [Google Scholar]
- Lin, Z.H.; Guan, C.J.; Feng, X.L.; Zhao, C.X. Synthesis of macroreticular p-(ω-sulfonic-perfluoroalkylated)polystyrene ion-exchange resin and its application as solid acid catalyst. J. Mol. Catal. A 2006, 247, 19–26. [Google Scholar] [CrossRef]
- Scheler, S. A novel approach to the interpretation and prediction of solvent effects in the synthesis of macroporous polymers. J. Appl. Polym. Sci. 2007, 105, 3121–3131. [Google Scholar] [CrossRef]
- Chee, K.K. Temperature dependence of solubility parameters of polymers. Malays. J. Chem. 2005, 7, 057–061. [Google Scholar]
- Suh, K.W.; Clarke, D.H. Cohesive energy densities of polymers from turbidimetric titrations. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 1671–1681. [Google Scholar] [CrossRef]
- Suh, K.W.; Corbett, J.M. Solubility parameters of polymers from turbidimetric titrations. J. Appl. Polym. Sci. 1968, 12, 2359–2370. [Google Scholar] [CrossRef]
- Tillaev, R.S.; Khasankhanova, M.; Tashmukhamedov, S.A.; Usmanov, K.U. Determination of solubility parameters of chlorinated poly(vinyl chloride) by turbidimetry and viscosimetry. J. Polym. Sci. Part C Polym. Symp. 1972, 39, 107–111. [Google Scholar]
- Ng, S.C.; Chee, K.K. Solubility parameters of copolymers as determined by turbidimetry. Eur. Polym. J. 1997, 33, 749–752. [Google Scholar] [CrossRef]
- Bozdogan, A.E. A method for determination of thermodynamic and solubility parameters of polymers from temperature and molecular weight dependence of intrinsic viscosity. Polymer 2004, 45, 6415–6424. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Wang, H.; Li, X.; Gao, J. Prediction of solubility parameters for polymers by a qspr model. QSAR Comb. Sci. 2006, 25, 156–161. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mohamed, M.H.; Wilson, L.D. Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens. Nanomaterials 2012, 2, 163-186. https://doi.org/10.3390/nano2020163
Mohamed MH, Wilson LD. Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens. Nanomaterials. 2012; 2(2):163-186. https://doi.org/10.3390/nano2020163
Chicago/Turabian StyleMohamed, Mohamed H., and Lee D. Wilson. 2012. "Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens" Nanomaterials 2, no. 2: 163-186. https://doi.org/10.3390/nano2020163
APA StyleMohamed, M. H., & Wilson, L. D. (2012). Porous Copolymer Resins: Tuning Pore Structure and Surface Area with Non Reactive Porogens. Nanomaterials, 2(2), 163-186. https://doi.org/10.3390/nano2020163





