Progress in UV Photodetectors Based on ZnO Nanomaterials: A Review of the Detection Mechanisms and Their Improvement
Abstract
1. Introduction
2. Basic Detection Mechanisms
2.1. Photoconductivity Effect
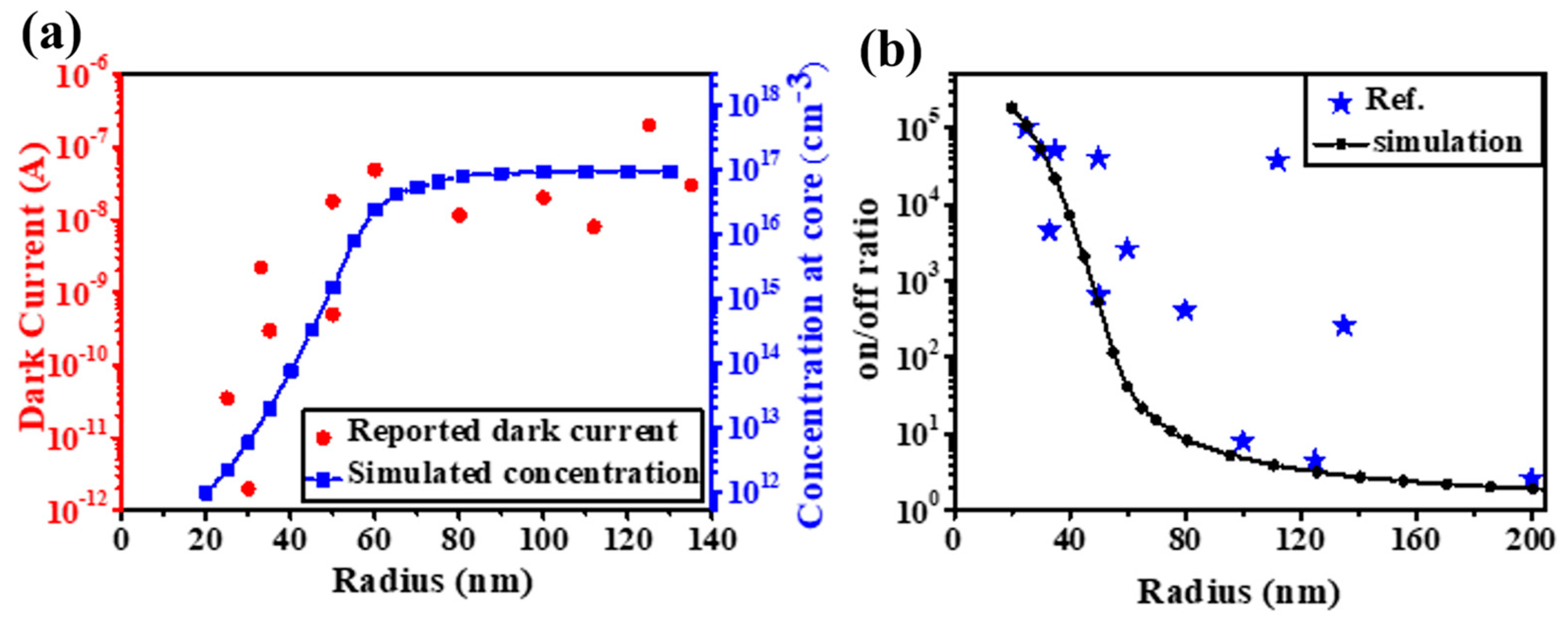
2.2. Threshold Dimension Effect
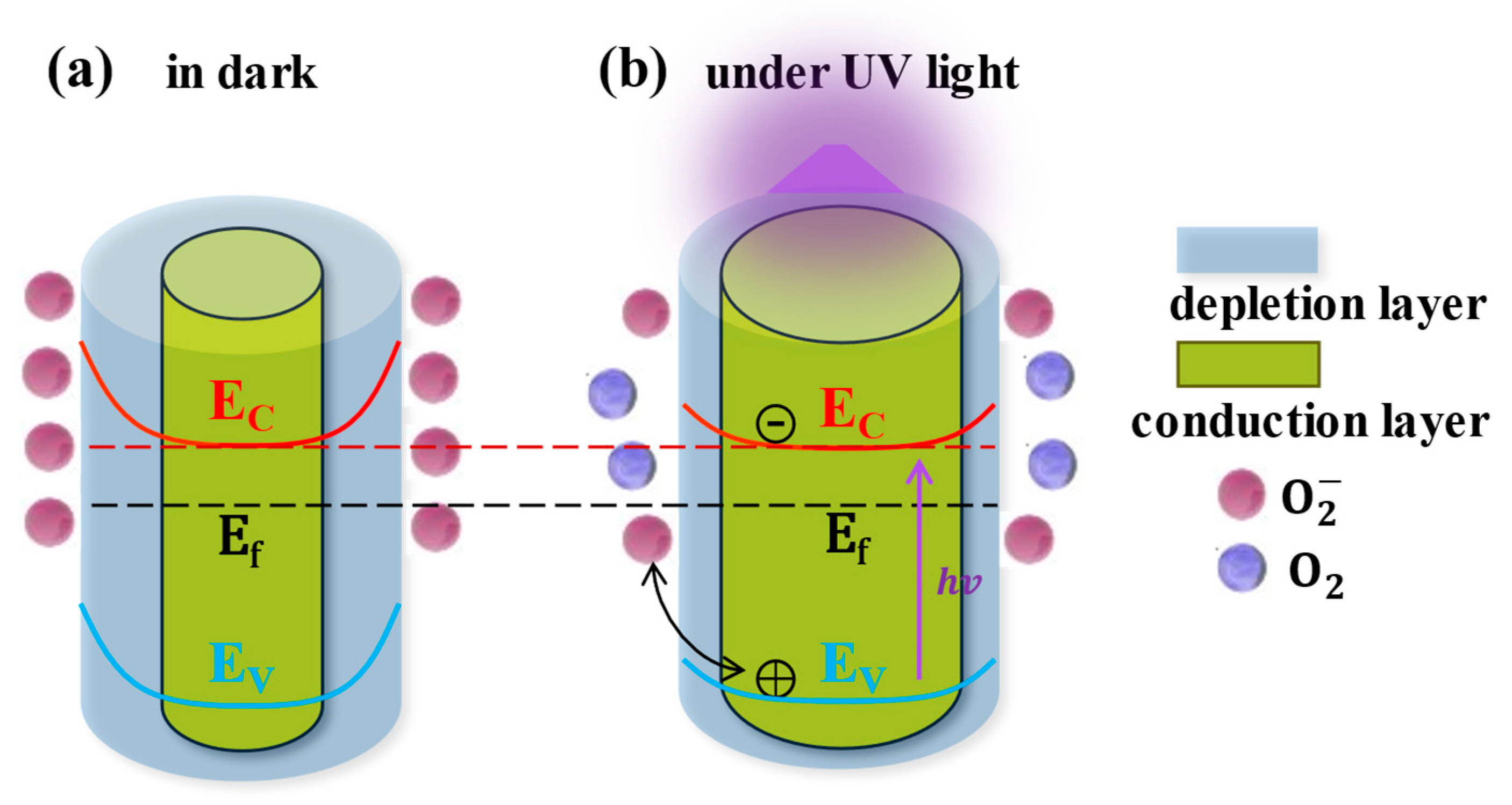
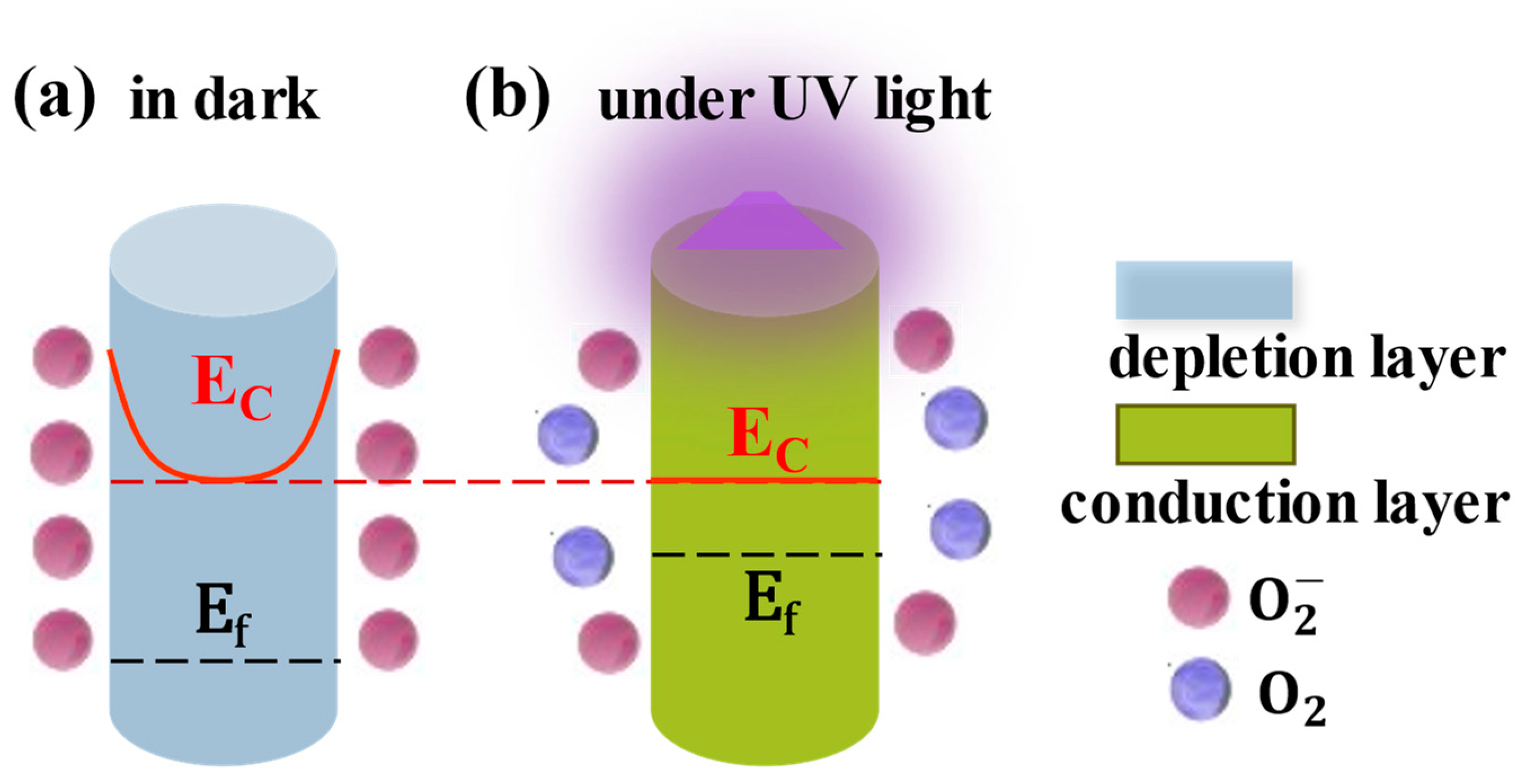
2.3. Light-Regulated Interface Barrier
3. Methods of Improvement
3.1. ZnO–Organic Semiconductor Interfaces
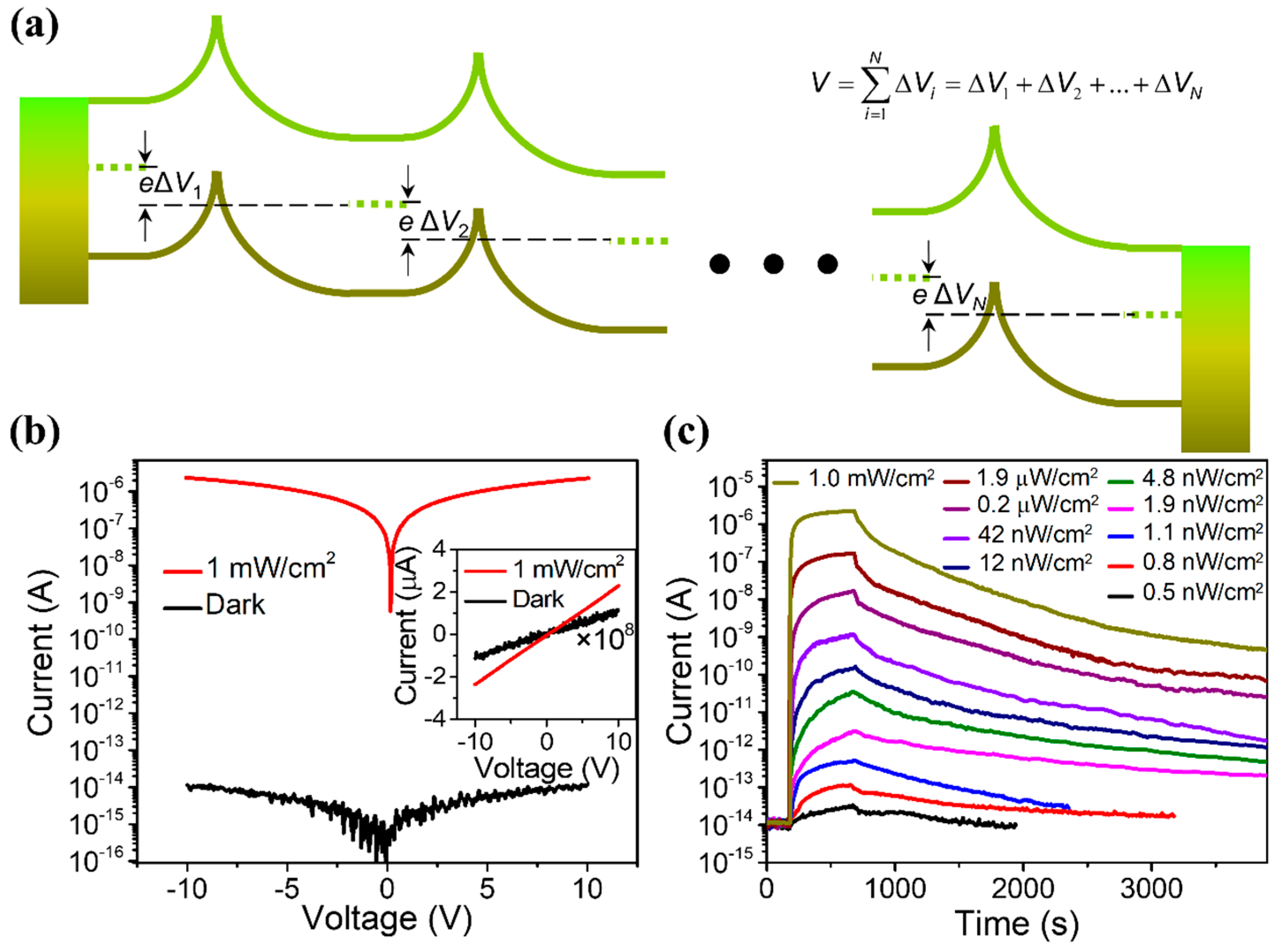
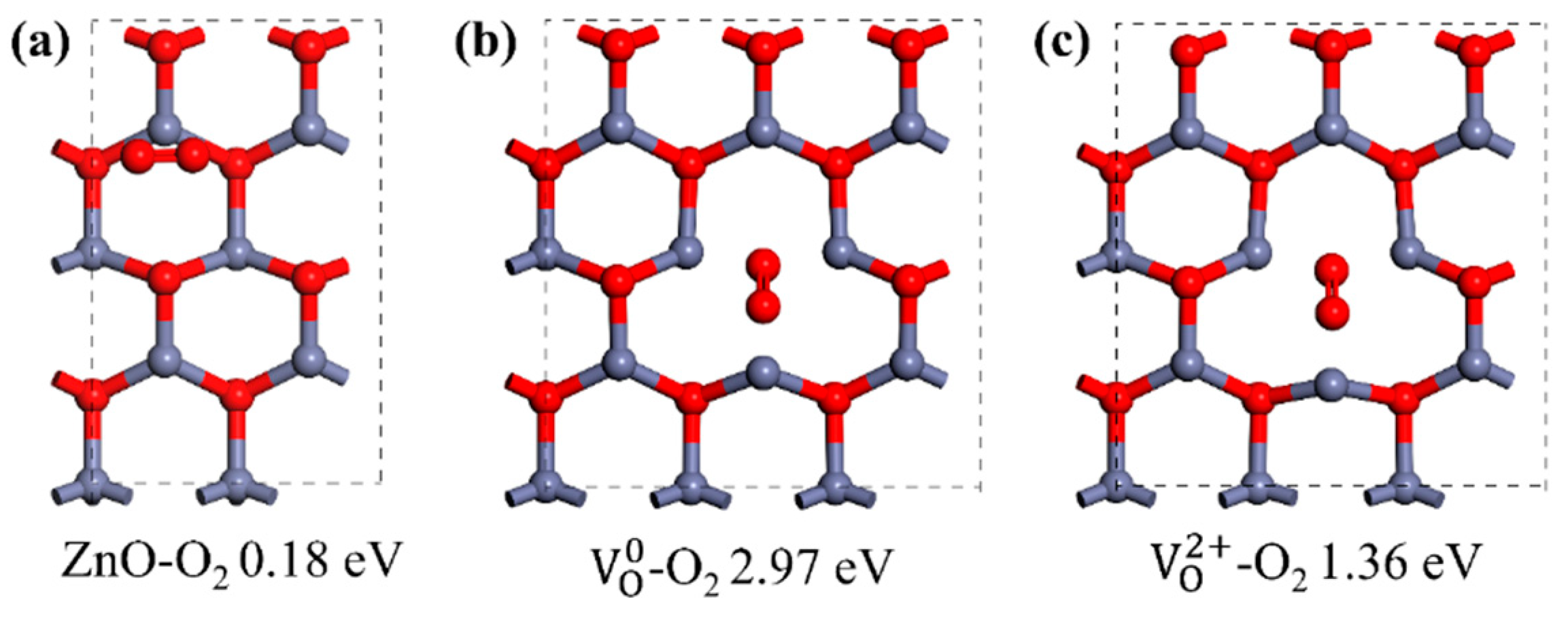
3.2. Defect Engineering and Doping
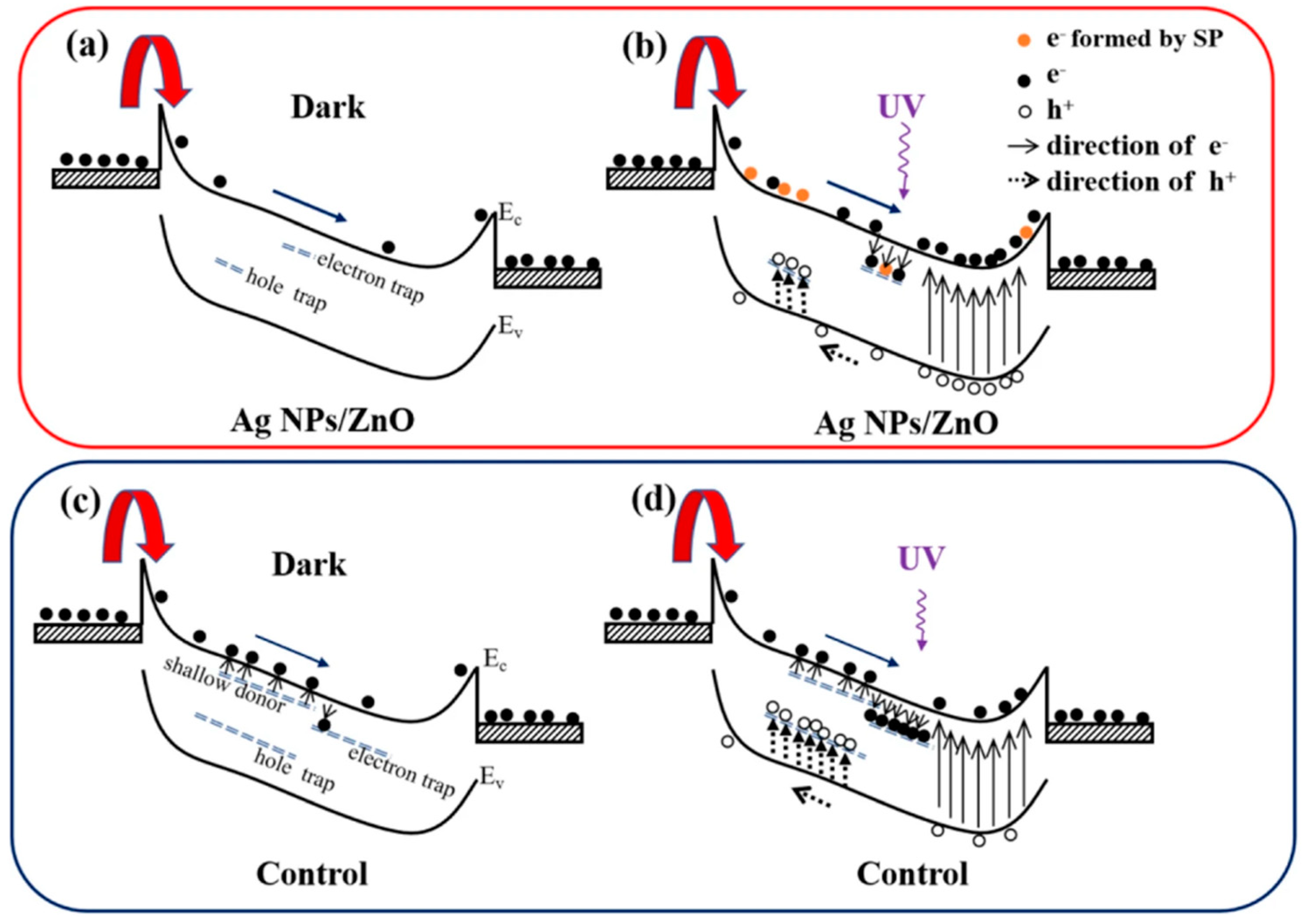
3.3. Surface Modification
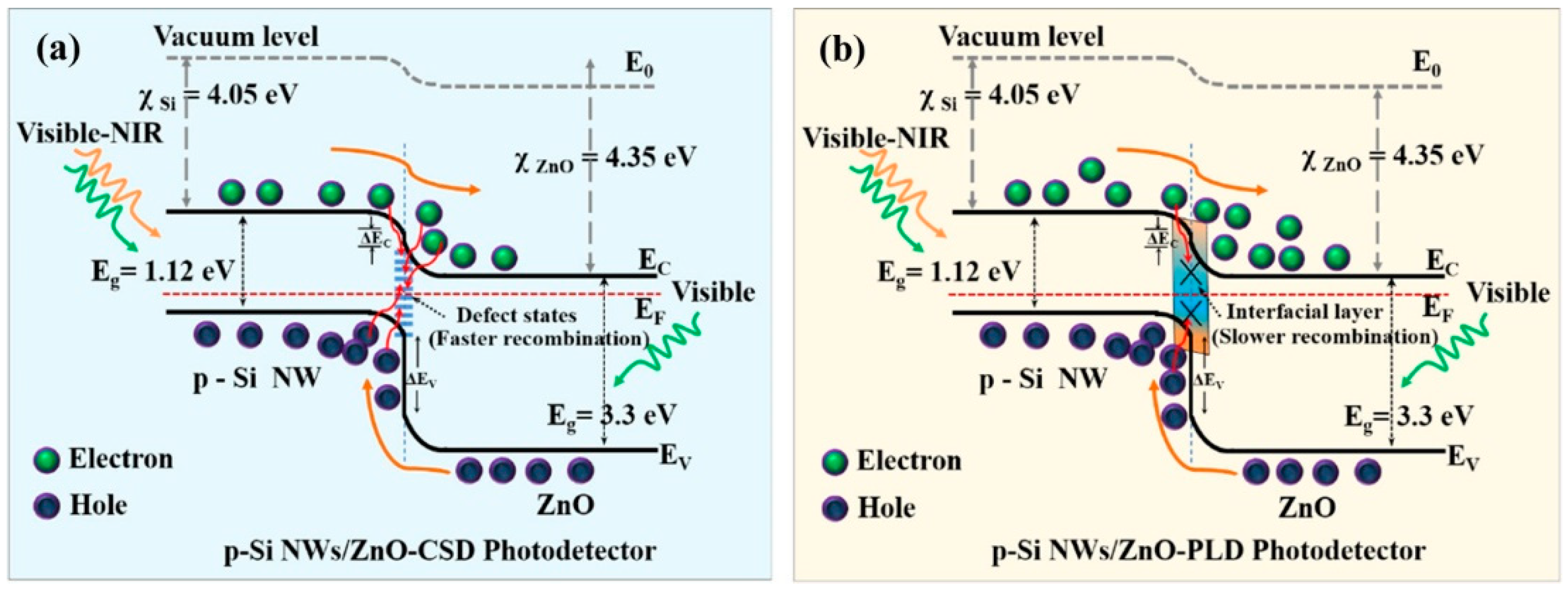
3.4. Heterojunction and the Schottky Barrier
4. Summary and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, F.; Yang, B.; Yuan, Y.; Xiao, Z.; Dong, Q.; Bi, Y.; Huang, J. A nanocomposite ultraviolet photodetector based on interfacial trap-controlled charge injection. Nat. Nanotechnol. 2012, 7, 798–802. [Google Scholar] [CrossRef]
- Lou, Z.; Shen, G. Flexible Photodetectors Based on 1D Inorganic Nanostructures. Adv. Sci. 2016, 3, 1500287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, R.; Wang, X.; Wu, W.; Wang, Z.L. Ultrafast Response p-Si/n-ZnO Heterojunction Ultraviolet Detector Based on Pyro-Phototronic Effect. Adv. Mater. 2016, 28, 6880–6886. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Teng, F.; Fang, X. High Performance BiOCl Nanosheets/TiO2 Nanotube Arrays Heterojunction UV Photodetector: The Influences of Self-Induced Inner Electric Fields in the BiOCl Nanosheets. Adv. Funct. Mater. 2018, 28, 1707178. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Zhu, X.; Chen, L.; Wang, Y.; Teng, F.; Zhang, Z.; Duan, H.; Xie, E. High performance, self-powered UV-photodetector based on ultrathin, transparent, SnO2–TiO2 core–shell electrodes. J. Alloys Compd. 2014, 616, 510–515. [Google Scholar] [CrossRef]
- Chu, J.; Wang, F.; Yin, L.; Lei, L.; Yan, C.; Wang, F.; Wen, Y.; Wang, Z.; Jiang, C.; Feng, L.; et al. High-Performance Ultraviolet Photodetector Based on a Few-Layered 2D NiPS3 Nanosheet. Adv. Funct. Mater. 2017, 27, 1701342. [Google Scholar] [CrossRef]
- Technavio. Available online: https://www.technavio.com/report/ultraviolet-uv-sensor-market-size-industry-analysis (accessed on 21 March 2025).
- Li, X.; Liu, X.; Li, Y.; Gao, D.; Cao, L. Using Novel Semiconductor Features to Construct Advanced ZnO Nanowires-Based Ultraviolet Photodetectors: A Brief Review. IEEE Access 2021, 9, 11954–11973. [Google Scholar] [CrossRef]
- Li, G.; Liu, L.; Wu, G.; Chen, W.; Qin, S.; Wang, Y.; Zhang, T. Self-Powered UV-Near Infrared Photodetector Based on Reduced Graphene Oxide/n-Si Vertical Heterojunction. Small 2016, 12, 5019–5026. [Google Scholar] [CrossRef]
- Wan, X.; Xu, Y.; Guo, H.; Shehzad, K.; Ali, A.; Liu, Y.; Yang, J.; Dai, D.; Lin, C.-T.; Liu, L.; et al. A self-powered high-performance graphene/silicon ultraviolet photodetector with ultra-shallow junction: Breaking the limit of silicon? Npj 2D Mater. Appl. 2017, 1, 4. [Google Scholar] [CrossRef]
- Yang, W.; Hu, K.; Teng, F.; Weng, J.; Zhang, Y.; Fang, X. High-Performance Silicon-Compatible Large-Area UV-to-Visible Broadband Photodetector Based on Integrated Lattice-Matched Type II Se/n-Si Heterojunctions. Nano Lett. 2018, 18, 4697–4703. [Google Scholar] [CrossRef]
- Jehad, A.K.; Fidan, M.; Ünverdi, Ö.; Çelebi, C. CVD graphene/SiC UV photodetector with enhanced spectral responsivity and response speed. Sens. Actuators A Phys. 2023, 355, 114309. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Chen, C.; Li, W.; Wang, L.; Hu, F.; Gao, F.; Yang, W.; Wang, Z.; Chen, S. High-Performance Ultraviolet Photodetector Based on Single-Crystal Integrated Self-Supporting 4H-SiC Nanohole Arrays. ACS Appl. Mater. Interfaces 2023, 15, 23457–23469. [Google Scholar] [CrossRef]
- Aggarwal, N.; Krishna, S.; Sharma, A.; Goswami, L.; Kumar, D.; Husale, S.; Gupta, G. A Highly Responsive Self-Driven UV Photodetector Using GaN Nanoflowers. Adv. Electron. Mater. 2017, 3, 1700036. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liu, B.; Yang, W.; Li, J.; Niu, P.; Jiang, X. Giant UV photoresponse of a GaN nanowire photodetector through effective Pt nanoparticle coupling. J. Mater. Chem. C 2017, 5, 4319–4326. [Google Scholar] [CrossRef]
- Li, G.; Meng, L.; Zhu, X.; Gao, W.; Qin, Y.; Chen, L. Clarifying the high on/off ratio mechanism of nanowire UV photodetector by characterizing surface barrier height. Nanoscale 2018, 10, 2242–2248. [Google Scholar] [CrossRef]
- Meng, L.; Li, G.; Tian, X.; Bai, S.; Xu, Q.; Jia, X.; Cui, X.; Qin, Y.; Wu, W. Ultrasensitive Fiber-Based ZnO Nanowire Network Ultraviolet Photodetector Enabled by the Synergism between Interface and Surface Gating Effects. ACS Appl. Mater. Interfaces 2020, 12, 1054–1060. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Meng, L.; Sun, Z.; Chen, Z.; Huang, X.; Qin, Y. Adjustment of oxygen vacancy states in ZnO and its application in ppb-level NO2 gas sensor. Sci. Bull. 2020, 65, 1650–1658. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.-F.; Zhang, X.; Liu, Z.; Fu, J.; Fan, S.; Bu, R.; Zhang, J.; Wang, W.; Wang, H.-X.; et al. UV-photodetector based on NiO/diamond film. Appl. Phys. Lett. 2018, 112, 032103. [Google Scholar] [CrossRef]
- Patel, M.; Kim, H.S.; Kim, J. All Transparent Metal Oxide Ultraviolet Photodetector. Adv. Electron. Mater. 2015, 1, 1500232. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Shi, S.; Dong, H.; Cheng, Y.; Wei, C.; Zhang, X.; Yin, S.; Li, L. TiO2 Nanorod Arrays Based Self-Powered UV Photodetector: Heterojunction with NiO Nanoflakes and Enhanced UV Photoresponse. ACS Appl. Mater. Interfaces 2018, 10, 11269–11279. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zuo, C.; Fang, X. Application of Nanostructured TiO2 in UV Photodetectors: A Review. Adv. Mater. 2022, 34, e2109083. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Gao, J.; Chow, P.; Sun, H.; Xin, G.; Sharma, P.; Lian, J.; Koratkar, N.A.; Sawyer, S. Organic-Inorganic Heterointerfaces for Ultrasensitive Detection of Ultraviolet Light. Nano Lett. 2015, 15, 3787–3792. [Google Scholar] [CrossRef]
- Yang, L.; Yang, D.; Guo, D.; Zheng, J.; Tao, S.; Li, J.; He, G.; Ma, D. Visible-Blind Deep Ultraviolet Photomultiplication Organic Photodetectors with Ultrahigh Gain for UVB and UVC Light Detection. Adv. Funct. Mater. 2022, 32, 2206993. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Leng, B.; Li, J.; Ma, Z.; Yang, W.; Liu, F.; Liu, B. Enhanced Performances of PVK/ZnO Nanorods/Graphene Heterostructure UV Photodetector via Piezo-Phototronic Interface Engineering. Adv. Mater. Interfaces 2019, 6, 1901365. [Google Scholar] [CrossRef]
- Adinolfi, V.; Ouellette, O.; Saidaminov, M.I.; Walters, G.; Abdelhady, A.L.; Bakr, O.M.; Sargent, E.H. Fast and Sensitive Solution-Processed Visible-Blind Perovskite UV Photodetectors. Adv. Mater. 2016, 28, 7264–7268. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.H.; Lee, S.K.; Kim, S.; Bark, C.W. Practical Demonstration of Deep-Ultraviolet Detection with Wearable and Self-Powered Halide Perovskite-Based Photodetector. ACS Appl. Mater. Interfaces 2021, 13, 57609–57618. [Google Scholar] [CrossRef]
- Zou, T.; Liu, X.; Qiu, R.; Wang, Y.; Huang, S.; Liu, C.; Dai, Q.; Zhou, H. Enhanced UV-C Detection of Perovskite Photodetector Arrays via Inorganic CsPbBr3 Quantum Dot Down-Conversion Layer. Adv. Opt. Mater. 2019, 7, 1801812. [Google Scholar] [CrossRef]
- Bie, Y.Q.; Liao, Z.M.; Zhang, H.Z.; Li, G.R.; Ye, Y.; Zhou, Y.B.; Xu, J.; Qin, Z.X.; Dai, L.; Yu, D.P. Self-powered, ultrafast, visible-blind UV detection and optical logical operation based on ZnO/GaN nanoscale p-n junctions. Adv. Mater. 2011, 23, 649–653. [Google Scholar] [CrossRef]
- Jacopin, G.; De Luna Bugallo, A.; Rigutti, L.; Lavenus, P.; Julien, F.H.; Lin, Y.-T.; Tu, L.-W.; Tchernycheva, M. Interplay of the photovoltaic and photoconductive operation modes in visible-blind photodetectors based on axial p-i-n junction GaN nanowires. Appl. Phys. Lett. 2014, 104, 023116. [Google Scholar] [CrossRef]
- Liu, B.; Chen, D.; Lu, H.; Tao, T.; Zhuang, Z.; Shao, Z.; Xu, W.; Ge, H.; Zhi, T.; Ren, F.; et al. Hybrid Light Emitters and UV Solar-Blind Avalanche Photodiodes based on III-Nitride Semiconductors. Adv. Mater. 2020, 32, e1904354. [Google Scholar] [CrossRef]
- Yuan, J.; Ni, W.J.; Huang, X.; Xu, M.L.; Zhang, J.W.; Niu, X.P.; Li, M.S.; Dou, J.J. A theoretical study of two novel SiC and GaN ultraviolet avalanche drift detectors with front-illumination. In Proceedings of the 2017 14th China International Forum on Solid State Lighting: International Forum on Wide Bandgap Semiconductors China (SSLChina: IFWS), Beijing, China, 1–3 November 2017; pp. 228–231. [Google Scholar] [CrossRef]
- Deka Boruah, B. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Bo, R.; Chen, H.; White, T.P.; Fu, L.; Tricoli, A. Structural Engineering of Nano-Grain Boundaries for Low-Voltage UV-Photodetectors with Gigantic Photo- to Dark-Current Ratios. Adv. Opt. Mater. 2016, 4, 1787–1795. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Meng, J.; Li, Z. Enhanced Performance of a Self-Powered ZnO Photodetector by Coupling LSPR-Inspired Pyro-Phototronic Effect and Piezo-Phototronic Effect. Adv. Opt. Mater. 2022, 10, 2102468. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, M.; Li, Q.; Liu, Y.; Di, X.; Lu, X.; Meng, J.; Li, Z. High-Performance UV Photodetector via Energy Band Engineering and LSPR-Enhanced Pyro-Phototronic Effect in Au Decorated 2D-PbI2/1D-ZnO Heterojunction. Adv. Opt. Mater. 2024, 12, 2303177. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, Y.; Dong, J.; Fang, Y.; Song, X.; Yan, Z. Cs3Cu2I5/ZnO Heterostructure for Flexible Visible-Blind Ultraviolet Photodetection. ACS Appl. Mater. Interfaces 2022, 14, 43490–43497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Jin, M.; Zhu, Y.; Zhang, N. The carbon dots modified ZnO films photodetector with broadband and fast photoresponse. Opt. Mater. 2023, 135, 113341. [Google Scholar] [CrossRef]
- Chu, L.; Xu, C.; Zeng, W.; Nie, C.; Hu, Y. Fabrication and Application of Different Nanostructured ZnO in Ultraviolet Photodetectors: A Review. IEEE Sens. J. 2022, 22, 7451–7462. [Google Scholar] [CrossRef]
- Lin, S.; Mandavkar, R.; Kulkarni, R.; Burse, S.; Habib, M.A.; Kim, S.H.; Li, M.-Y.; Kunwar, S.; Lee, J. MoS2 Nanoflake and ZnO Quantum Dot Blended Active Layers on AuPd Nanoparticles for UV Photodetectors. ACS Appl. Nano Mater. 2022, 5, 3289–3302. [Google Scholar] [CrossRef]
- Garratt, E.; Prete, P.; Lovergine, N.; Nikoobakht, B. Observation and Impact of a Surface Skin Effect on Lateral Growth of Nanocrystals. J. Phys. Chem. C 2017, 121, 14845–14853. [Google Scholar] [CrossRef]
- Prete, P.; Lovergine, N.; Tapfer, L. Nanostructure size evolution during Au-catalysed growth by carbo-thermal evaporation of well-aligned ZnO nanowires on (100)Si. Appl. Phys. A 2007, 88, 21–26. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Maddaka, R.; Viswanath, S.K.; Banerjee, A.N.; Kim, M.-D.; Joo, S.W. High-responsivity self-powered UV photodetector performance of pristine and V-doped ZnO nano-flowers. Opt. Laser Technol. 2023, 157, 108776. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Young, S.-J.; Liu, C.-J.; Arya, S.; Chu, T.-T. Enhanced UV-Sensing Performances of 2-D Pd/ZnO Nanosheet Photodetectors through Inexpensive Photochemical Synthesis at Room Temperature and Their Humidity Applications. ACS Appl. Electron. Mater. 2025, 7, 129–142. [Google Scholar] [CrossRef]
- Yu, H.; Liao, Q.; Kang, Z.; Wang, Z.; Liu, B.; Zhang, X.; Du, J.; Ou, Y.; Hong, M.; Xiao, J.; et al. Atomic-Thin ZnO Sheet for Visible-Blind Ultraviolet Photodetection. Small 2020, 16, e2005520. [Google Scholar] [CrossRef]
- Yang, D.; Ma, D. Development of Organic Semiconductor Photodetectors: From Mechanism to Applications. Adv. Opt. Mater. 2018, 7, 1800522. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J. Ultrahigh Gain, Low Noise, Ultraviolet Photodetectors with Highly Aligned Organic Crystals. Adv. Opt. Mater. 2015, 4, 264–270. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q. Enhancing the performance of photomultiplication-type organic photodetectors using solution-processed ZnO as an interfacial layer. J. Mater. Chem. C 2019, 7, 1544–1550. [Google Scholar] [CrossRef]
- Maity, S.; Thomas, T. Hybrid-Organic-Photodetector Containing Chemically Treated ZnMgO Layer With Promising and Reliable Detectivity, Responsivity and Low Dark Current. IEEE Trans. Device Mater. Reliab. 2019, 19, 193–200. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, F.; Xiao, Z.; Huang, J. Large Gain, Low Noise Nanocomposite Ultraviolet Photodetectors with a Linear Dynamic Range of 120 dB. Adv. Opt. Mater. 2014, 2, 348–353. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Gorai, P.; Seebauer, E.G.; Ertekin, E. Mechanism and energetics of O and O2 adsorption on polar and non-polar ZnO surfaces. J. Chem. Phys. 2016, 144, 184708. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.G.; Lavrov, E.V.; Weber, J. Interplay between interstitial and substitutional hydrogen donors in ZnO. Phys. Rev. B 2014, 89, 235203. [Google Scholar] [CrossRef]
- Xue, X.; Wang, T.; Jiang, X.; Jiang, J.; Pan, C.; Wu, Y. Interaction of hydrogen with defects in ZnO nanoparticles—Studied by positron annihilation, Raman and photoluminescence spectroscopy. CrystEngComm 2014, 16, 1207–1216. [Google Scholar] [CrossRef]
- Gurylev, V.; Su, C.-Y.; Perng, T.-P. Hydrogenated ZnO nanorods with defect-induced visible light-responsive photoelectrochemical performance. Appl. Surf. Sci. 2017, 411, 279–284. [Google Scholar] [CrossRef]
- Look, D.C.; Leedy, K.D.; Vines, L.; Svensson, B.G.; Zubiaga, A.; Tuomisto, F.; Doutt, D.R.; Brillson, L.J. Self-compensation in semiconductors: The Zn vacancy in Ga-doped ZnO. Phys. Rev. B 2011, 84, 115202. [Google Scholar] [CrossRef]
- Doni Pon, V.; Joseph Wilson, K.S.; Hariprasad, K.; Ganesh, V.; Elhosiny Ali, H.; Algarni, H.; Yahia, I.S. Enhancement of optoelectronic properties of ZnO thin films by Al doping for photodetector applications. Superlattices Microstruct. 2021, 151, 106790. [Google Scholar] [CrossRef]
- Shkir, M. Enhancement in optical and electrical properties of ZnO thin films via Co doping for photodetector applications. Mater. Sci. Eng. B 2022, 284, 115861. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Li, H.-H.; Hsueh, T.-J. Water- and Humidity-Enhanced UV Detector by Using p-Type La-Doped ZnO Nanowires on Flexible Polyimide Substrate. ACS Appl. Mater. Interfaces 2013, 5, 11142–11151. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Angappane, S. Synthesis, characterization and photoresponse study of undoped and transition metal (Co, Ni, Mn) doped ZnO thin films. Mater. Sci. Eng. B 2013, 178, 1068–1075. [Google Scholar] [CrossRef]
- Safa, S.; Mokhtari, S.; Khayatian, A.; Azimirad, R. Improving ultraviolet photodetection of ZnO nanorods by Cr doped ZnO encapsulation process. Opt. Commun. 2018, 413, 131–135. [Google Scholar] [CrossRef]
- Shatnawi, M.; Alsmadi, A.M.; Bsoul, I.; Salameh, B.; Mathai, M.; Alnawashi, G.; Alzoubi, G.M.; Al-Dweri, F.; Bawa’aneh, M.S. Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles. Results Phys. 2016, 6, 1064–1071. [Google Scholar] [CrossRef]
- Das, J.; Mishra, D.K.; Srinivasu, V.V.; Sahu, D.R.; Roul, B.K. Photoluminescence and Raman studies for the confirmation of oxygen vacancies to induce ferromagnetism in Fe doped Mn:ZnO compound. J. Magn. Magn. Mater. 2015, 382, 111–116. [Google Scholar] [CrossRef]
- Deka Boruah, B.; Misra, A. Energy-Efficient Hydrogenated Zinc Oxide Nanoflakes for High-Performance Self-Powered Ultraviolet Photodetector. ACS Appl. Mater. Interfaces 2016, 8, 18182–18188. [Google Scholar] [CrossRef]
- Ding, H.; Shao, J.; Ding, Y.; Liu, W.; Tian, H.; Li, X. One-Dimensional Au-ZnO Heteronanostructures for Ultraviolet Light Detectors by a Two-Step Dielectrophoretic Assembly Method. ACS Appl. Mater. Interfaces 2015, 7, 12713–12718. [Google Scholar] [CrossRef]
- Klochko, N.P.; Klepikova, K.S.; Khrypunova, I.V.; Kopach, V.R.; Tyukhov, I.I.; Petrushenko, S.I.; Dukarov, S.V.; Sukhov, V.M.; Kirichenko, M.V.; Khrypunova, A.L. Solution-processed flexible broadband ZnO photodetector modified by Ag nanoparticles. Sol. Energy 2022, 232, 1–11. [Google Scholar] [CrossRef]
- Wang, H.C.; Hong, Y.; Chen, Z.; Lao, C.; Lu, Y.; Yang, Z.; Zhu, Y.; Liu, X. ZnO UV Photodetectors Modified by Ag Nanoparticles Using All-Inkjet-Printing. Nanoscale Res. Lett. 2020, 15, 176. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Liu, Y.-H.; Chu, T.-T.; Young, S.-J. Improved UV-Sensing of Au-Decorated ZnO Nanostructure MSM Photodetectors. IEEE Sens. J. 2022, 22, 5644–5650. [Google Scholar] [CrossRef]
- Bera, A.; Ghosh, T.; Basak, D. Enhanced photoluminescence and photoconductivity of ZnO nanowires with sputtered Zn. ACS Appl. Mater. Interfaces 2010, 2, 2898–2903. [Google Scholar] [CrossRef]
- Bera, A.; Basak, D. Effect of surface capping with poly(vinyl alcohol) on the photocarrier relaxation of ZnO nanowires. ACS Appl. Mater. Interfaces 2009, 1, 2066–2070. [Google Scholar] [CrossRef]
- Srivastava, A.; Jit, S.; Tripathi, S. High-Performance Pentacene/ZnO UV-Visible Photodetector Using Solution Method. IEEE Trans. Electron. Devices 2021, 68, 3439–3445. [Google Scholar] [CrossRef]
- Samanta, C.; Ghatak, A.; Raychaudhuri, A.K.; Ghosh, B. Surface/Interface Defect Engineering on Charge Carrier Transport toward Broadband (UV-NIR) Photoresponse in the Heterostructure Array of p-Si NWs/ZnO Photodetector. ACS Appl. Electron. Mater. 2023, 5, 865–876. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, D.; Zhao, M.; Wang, N.; Duan, Y.; Wang, W.; Li, M.; Li, Q.; Shan, C.; Sun, J. Polarization induced two-dimensional electron gas in ZnO/ZnMgO Heterointerface for High-performance enhanced UV photodetector. J. Alloys Compd. 2020, 820, 153416. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Cheng, B.; Zhang, H.; Zhu, X.; Yang, D. Progress in UV Photodetectors Based on ZnO Nanomaterials: A Review of the Detection Mechanisms and Their Improvement. Nanomaterials 2025, 15, 644. https://doi.org/10.3390/nano15090644
Li G, Cheng B, Zhang H, Zhu X, Yang D. Progress in UV Photodetectors Based on ZnO Nanomaterials: A Review of the Detection Mechanisms and Their Improvement. Nanomaterials. 2025; 15(9):644. https://doi.org/10.3390/nano15090644
Chicago/Turabian StyleLi, Gaoda, Bolang Cheng, Haibo Zhang, Xinghua Zhu, and Dingyu Yang. 2025. "Progress in UV Photodetectors Based on ZnO Nanomaterials: A Review of the Detection Mechanisms and Their Improvement" Nanomaterials 15, no. 9: 644. https://doi.org/10.3390/nano15090644
APA StyleLi, G., Cheng, B., Zhang, H., Zhu, X., & Yang, D. (2025). Progress in UV Photodetectors Based on ZnO Nanomaterials: A Review of the Detection Mechanisms and Their Improvement. Nanomaterials, 15(9), 644. https://doi.org/10.3390/nano15090644






