Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior
Abstract
1. Introduction
2. Experimental Procedure and Characterization Techniques
3. Characterization Techniques
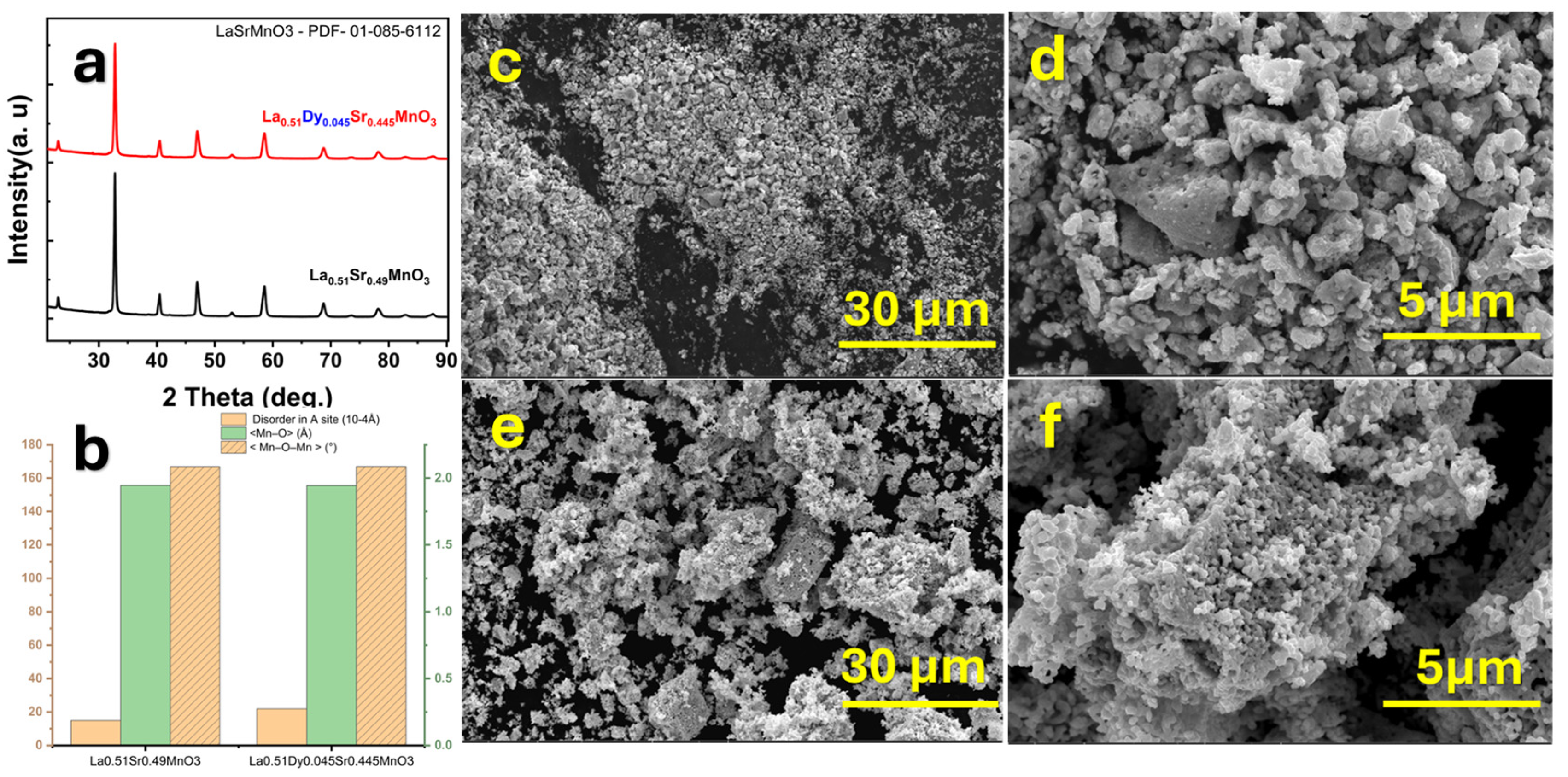
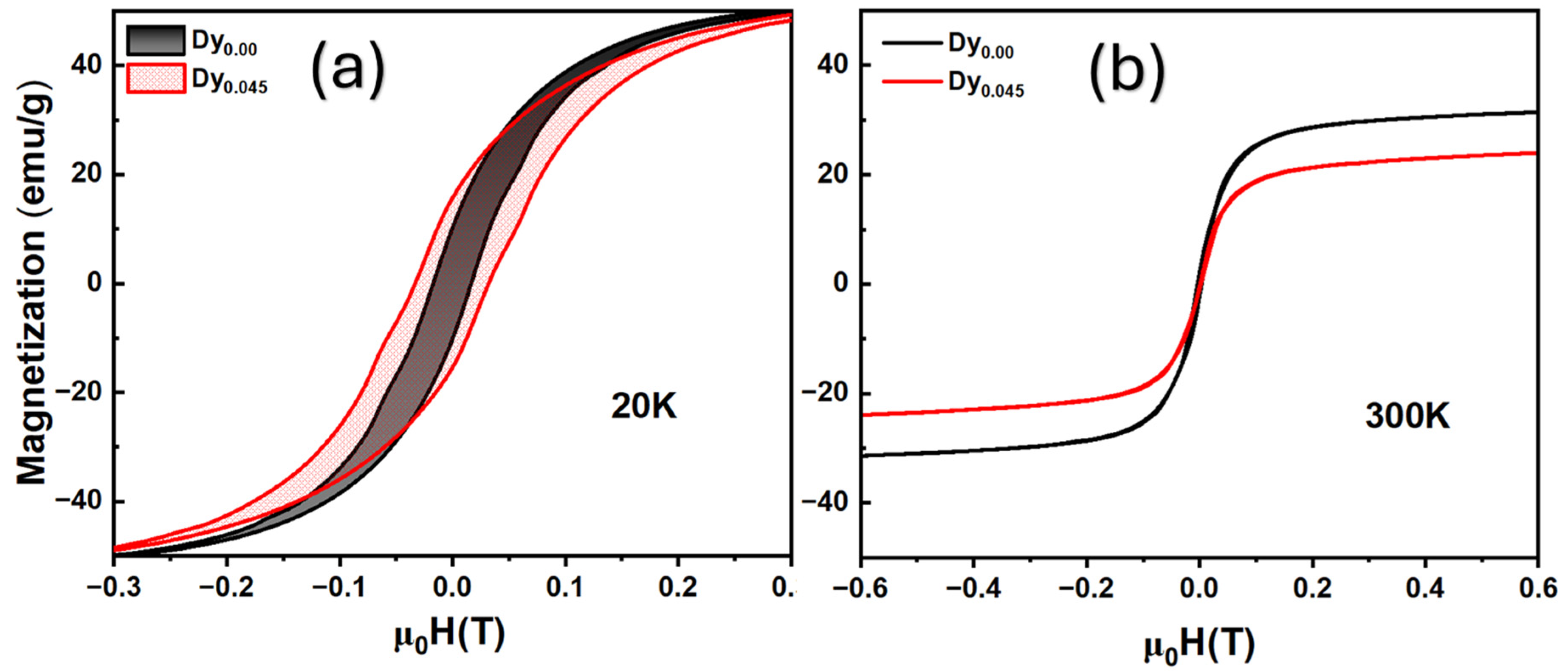
4. Structural and Morphological Analysis
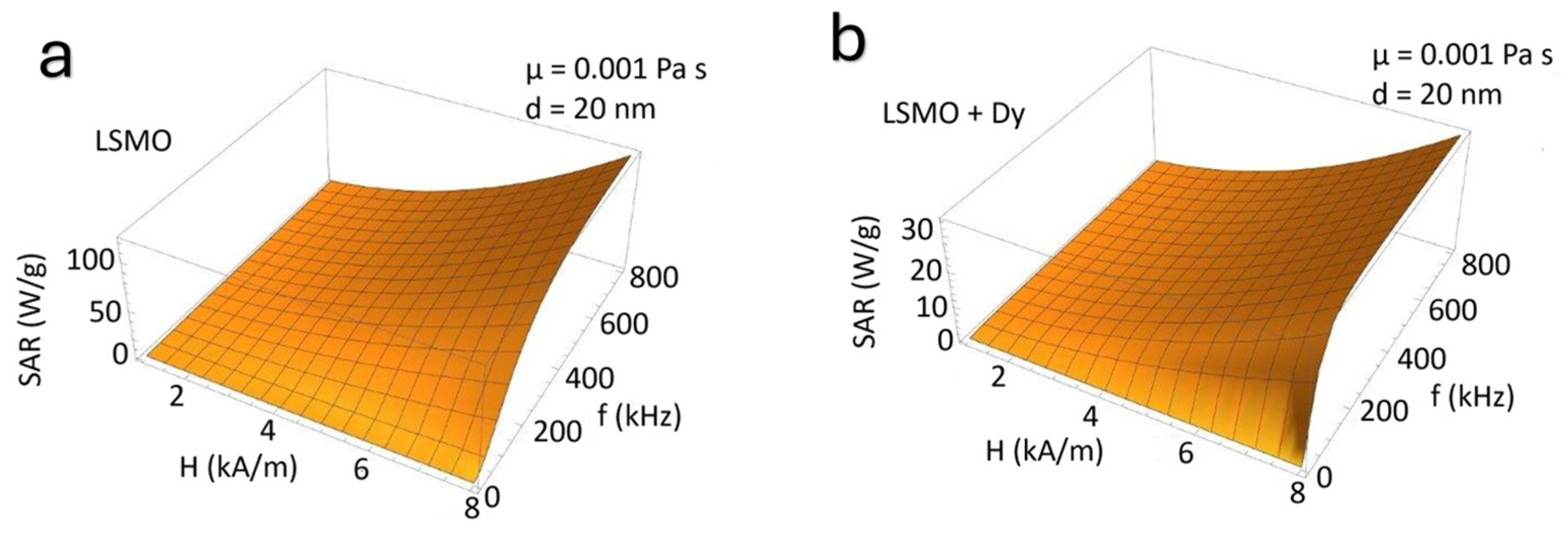
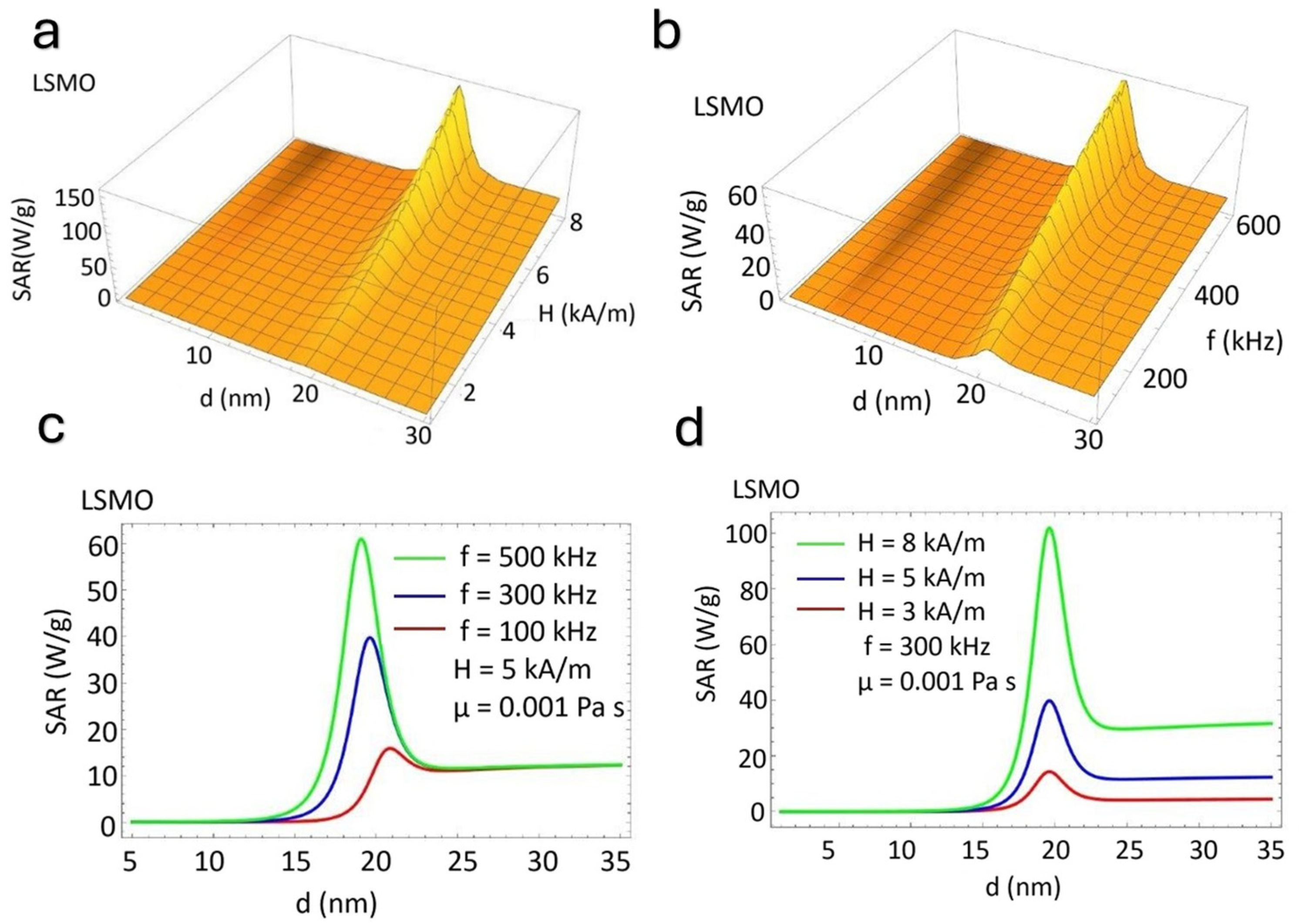
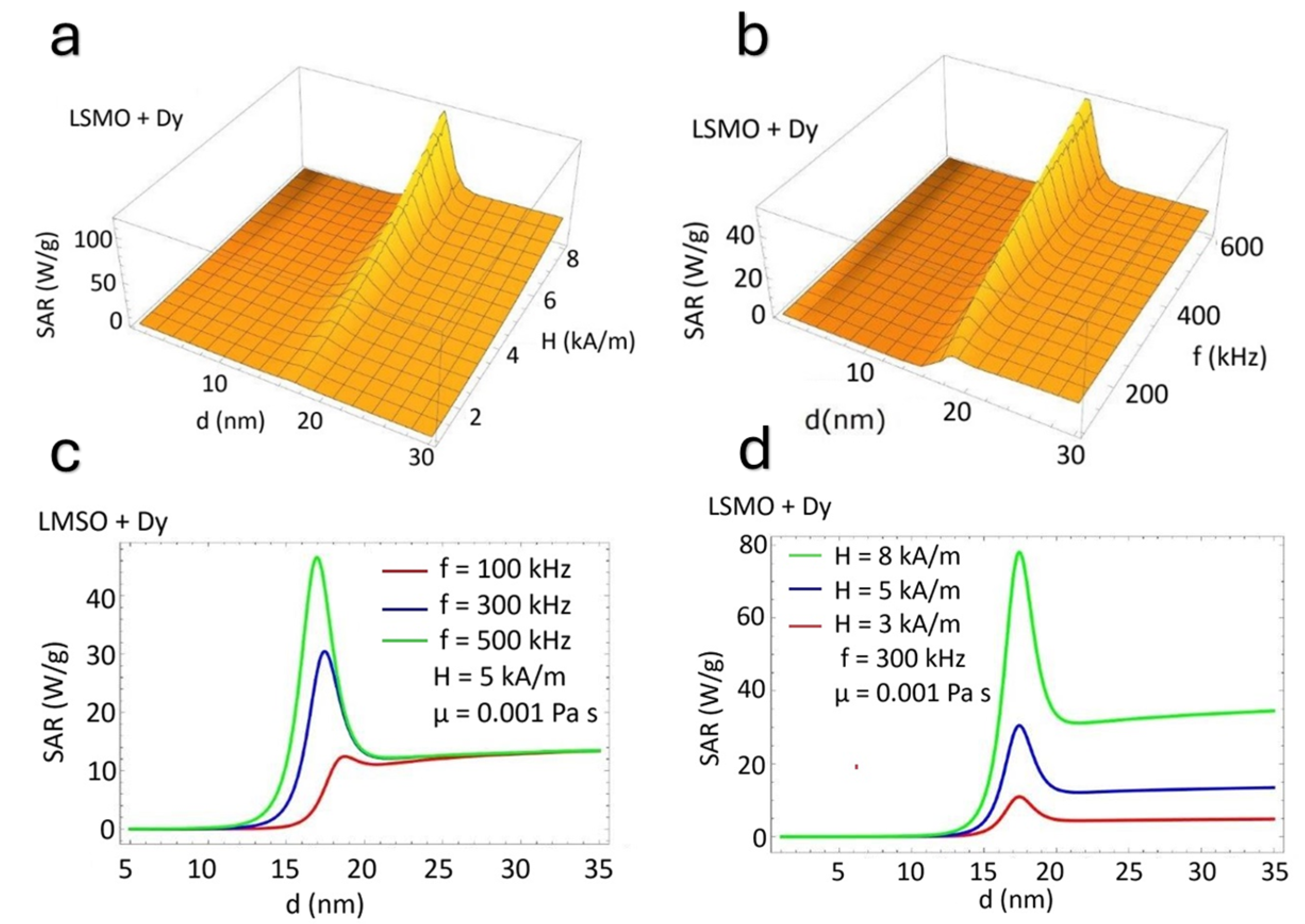
5. Theoretical Estimates of the SAR
- The frequency and amplitude of the applied magnetic field;
- The nanoparticle size;
- The viscosity of the ferrofluid.
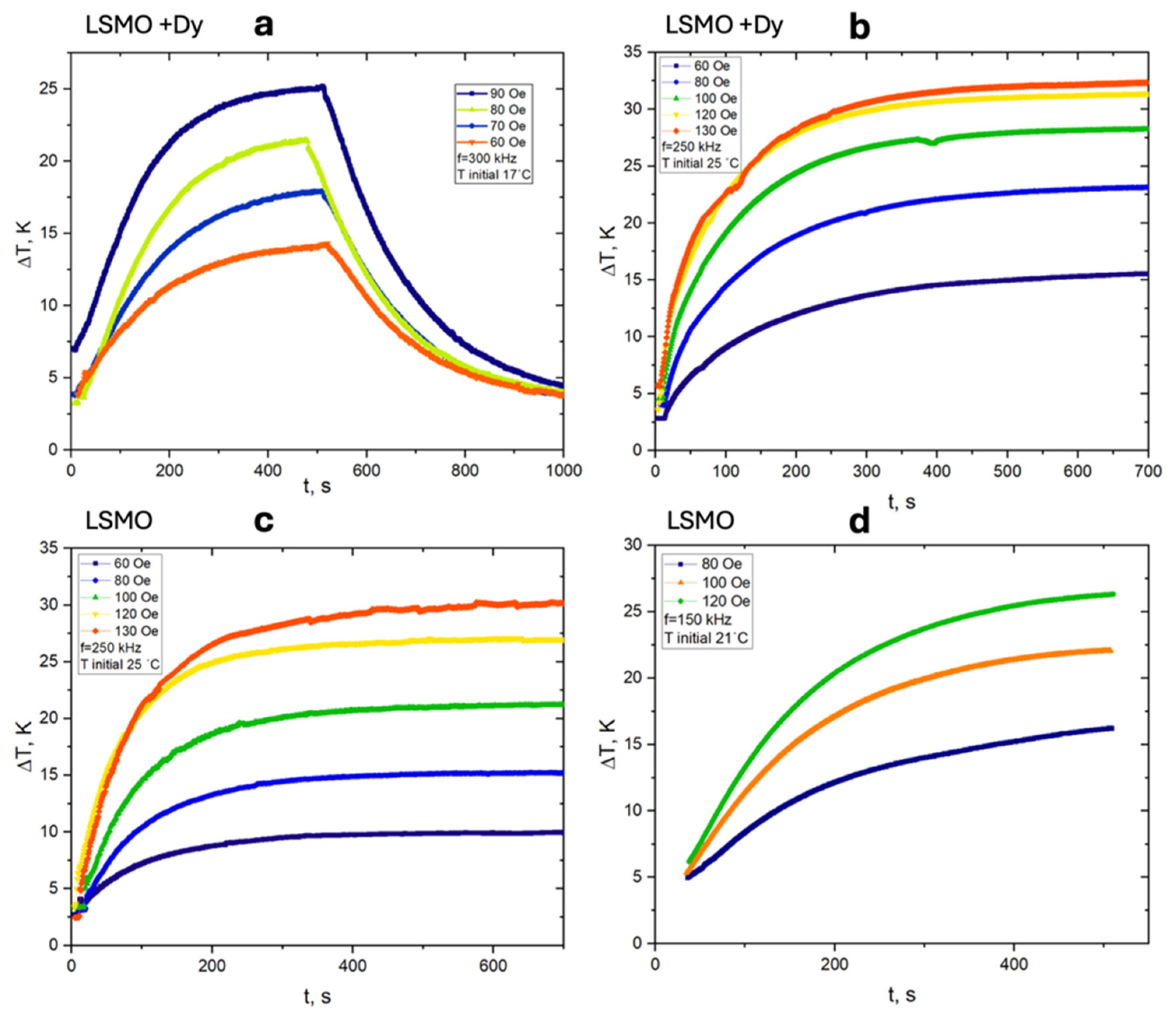
6. Experimental Setup and SAR Measurement Method
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.J.; Agliardi, G.; Lin, F.-Y.; Ellis, M.; Jones, C.; Robson, M.; Richard-Londt, A.; Southern, P.; Lythgoe, M.; Thin, M.Z.; et al. Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation. Small 2021, 17, 2005241. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano J. 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 2019, 138, 233–246. [Google Scholar] [CrossRef]
- Brezovich, I.A.; Meredith, R.F. Practical aspects of ferromagnetic thermoseed hyperthermia. Radiol. Clin. N. Am. 1989, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 2023, 123, 3904–3943. [Google Scholar] [CrossRef]
- Barrera, G.; Allia, P.; Tiberto, P. Temperature-dependent heating efficiency of magnetic nanoparticles for applications in precision nanomedicine. Nanoscale 2020, 12, 6360–6377. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.-H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zong, Y.; Tan, G.; Sun, Y.; Lan, Y.; He, M.; Ren, Z.; Zheng, X. Solvothermal synthesis of nitrogen-doped graphene decorated by superparamagnetic Fe3O4 nanoparticles and their applications as enhanced synergistic microwave absorbers. Carbon 2017, 115, 493–502. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Sun, Z.; Zhang, Q.; Wei, P.; Mu, X.; Zhou, H.; Li, C.; Ma, S.; He, D.; et al. Superparamagnetic enhancement of thermoelectric performance. Nature 2017, 549, 247–251. [Google Scholar] [CrossRef]
- Faílde, D.; Ocampo-Zalvide, V.; Serantes, D.; Iglesias, Ò. Understanding magnetic hyperthermia performance within the “Brezovich criterion”: Beyond the uniaxial anisotropy description. Nanoscale 2024, 16, 14319–14329. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Y.; You, J.; Shen, K.; Chen, W.; Li, L. Morphology-dependent magnetic hyperthermia characteristics of Fe3O4 nanoparticles. Mater. Chem. Phys. 2025, 329, 130045. [Google Scholar] [CrossRef]
- Dizajyekan, B.S.; Jafari, A.; Vafaie-Sefti, M.; Saber, R.; Fakhroueian, Z. Preparation of stable colloidal dispersion of surface modified Fe3O4 nanoparticles for magnetic heating applications. Sci. Rep. 2024, 14, 1296. [Google Scholar] [CrossRef] [PubMed]
- Getahun, Y.; Habib, A.; Erives-Sedano, V.; Lee, W.-Y.; Poon, W.; El-Gendy, A.A. Superparamagnetic iron oxide nanoparticles functionalized by biocompatible ligands with enhanced high specific absorption rate for magnetic hyperthermia. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134036. [Google Scholar] [CrossRef]
- Pimentel, B.; Caraballo-Vivas, R.J.; Checca, N.R.; Zverev, V.I.; Salakhova, R.T.; Makarova, L.A.; Pyatakov, A.P.; Perov, N.S.; Tishin, A.M.; Shtil, A.A.; et al. Threshold heating temperature for magnetic hyperthermia: Controlling the heat exchange with the blocking temperature of magnetic nanoparticles. J. Solid State Chem. 2018, 260, 34–38. [Google Scholar] [CrossRef]
- Mamiya, H.; Jeyadevan, B. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Sci. Rep. 2011, 1, 157. [Google Scholar] [CrossRef]
- Mille, N.; Faure, S.; Estrader, M.; Yi, D.; Marbaix, J.; De Masi, D.; Soulantica, K.; Millán, A.; Chaudret, B.; Carrey, J. A setup to measure the temperature-dependent heating power of magnetically heated nanoparticles up to high temperature. Rev. Sci. Instrum. 2021, 92, 54905. [Google Scholar] [CrossRef]
- Xu, X.; Xie, W.; Li, F.; Niu, C.; Li, M.; Wang, H. General approach for efficient prediction of refrigeration performance in caloric materials. Phys. Rev. Appl. 2024, 22, 14036. [Google Scholar] [CrossRef]
- Vajtai, L.; Nemes, N.M.; Morales, M.D.; Molnár, K.; Pinke, B.G.; Simon, F. Incidence of the Brownian Relaxation Process on the Magnetic Properties of Ferrofluids. Nanomaterials 2024, 14, 634. [Google Scholar] [CrossRef]
- Mansour, M.; Sedky, A.; Alshammari, A.S.; Khan, Z.R.; Bouzidi, M.; Alshammari, M.S. Structural, Optical, Magnetic, and Dielectric Investigations of Pure and Co-Doped La0.67Sr0.33Mn1−x−yZnxCoyO3 Manganites with (0.00 < x + y < 0.20). Crystals 2024, 14, 981. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, P.; Anil, A. Insights into structural and magnetic properties of Lanthanum Manganite ceramic co-doped with calcium and cobalt. Preprints 2024. [Google Scholar] [CrossRef]
- Ramlan, F.H.; Setiawan, J.; Susetyo, F.B.; Akbar, H. Preparation, Synthesis and Characterization of La (1−x) Sr (x) MnO3 Alloy. J. Appl. Eng. Technol. Sci. 2024, 5, 1232–1241. [Google Scholar] [CrossRef]
- Brahem, R. LSNMTix Contribution to the examination of the effect of Ti–substitution on the crystallographic and transport characteristics of. J. Qassim Univ. Sci. 2024, 3. [Google Scholar] [CrossRef]
- Zaidi, N.; Mnefgui, S.; Dhahri, A.; Dhahri, J.; Hlil, E.K. The effect of Dy doped on structural, magnetic and magnetocaloric properties of La0.67−xDyxPb0.33MnO3 (x = 0.00, 0.15 and 0.20) compounds. Phys. B Condens. Matter 2014, 450, 155–161. [Google Scholar] [CrossRef]
- Manna, P.; Kanthal, S.; Aquilanti, G.; Banerjee, A.; Bandyopadhyay, S. Correlated temperature and field dependent magnetization: Enhanced magnetic hysteresis upon Dy doping in La-based francisite Cu3La(SeO3)2O2Cl. J. Magn. Magn. Mater. 2022, 564, 170196. [Google Scholar] [CrossRef]
- Ribeiro, J.L.; Vieira, L.G. Landau model for the phase diagrams of the orthorhombic rare-earth manganites RMnO3 (R = Eu, Gd, Tb, Dy, Ho). Phys. Rev. B 2010, 82, 64410. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Zheng, T.; Zhang, Z.; Liu, W.; Zhao, X.; Liu, X. Synthesis of perovskite-type manganites Yb1−xDyxMnO3 (0.1 ≤ x ≤ 0.5) via solid-state reaction and high-pressure flux methods followed by structural characterization and magnetic property studies. New J. Chem. 2015, 39, 2596–2601. [Google Scholar] [CrossRef]
- Chakraborty, A.R.; Toma, F.T.Z.; Alam, K.; Yousuf, S.B.; Hossain, K.S. Influence of annealing temperature on Fe2O3 nanoparticles: Synthesis optimization and structural, optical, morphological, and magnetic properties characterization for advanced technological applications. Heliyon 2024, 10, e40000. [Google Scholar] [CrossRef]
- Attanayake, S.B.; Chanda, A.; Das, R.; Phan, M.H.; Srikanth, H. Effects of annealing temperature on the magnetic properties of highly crystalline biphase iron oxide nanorods. AIP Adv. 2023, 13, 25333. [Google Scholar] [CrossRef]
- Smari, M.; Hamdi, R.; Mansour, S.A.; Al-Haik, M.Y.; Zakaria, Y.; Haik, Y. Dy-Doped La0.51Sr0.49MnO3 nanoparticles: Tuning structural and magnetocaloric properties via Sol-Gel synthesis for energy-efficient applications. Nano Trends 2025, 9, 100069. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Gimaev, R.; Zverev, V.; Tishin, A.; Stancu, A. Cubic and Sphere Magnetic Nanoparticles for Magnetic Hyperthermia Therapy: Computational Results. Nanomaterials 2023, 13, 2383. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L.; et al. Understanding the Dependence of Nanoparticles Magnetothermal Properties on Their Size for Hyperthermia Applications: A Case Study for La-Sr Manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Astefanoaei, I.; Gimaev, R.; Zverev, V.; Stancu, A. Modelling of working parameters of Gd and FeRh nanoparticles for magnetic hyperthermia. Mater. Res. Express 2019, 6, 125089. [Google Scholar] [CrossRef]
- Kahil, H.; Faramawy, A.; El-Sayed, H.; Abdel-Sattar, A. Magnetic Properties and SAR for Gadolinium-Doped Iron Oxide Nanoparticles Prepared by Hydrothermal Method. Crystals 2021, 11, 1153. [Google Scholar] [CrossRef]
- Shayestefar, M.; Mirahmadi-Zare, S.Z.; Mashreghi, A.; Hasani, S. Investigation of magnetic and structural properties of Dy-substituted Mn-Zn ferrite nanoparticles for hyperthermia applications. J. Sol-Gel Sci. Technol. 2025, 1–11. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Sun, J.; Shi, Z.; Li, R.; Liu, X.; Wang, N.; Weaver, J.B.; Wu, K. Study and optimization on hyperthermia performance of magnetic fluids modeled by coupled Brownian–Néel rotations. J. Appl. Phys. 2025, 137, 54702. [Google Scholar] [CrossRef]
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.D.; Barber, D.F. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers 2021, 13, 4583. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Pathak, S.; Jain, K.; Garg, P.; Pant, M.; Mahapatro, A.K.; Rath, D.; Wang, L.; Kim, S.-K.; et al. A threefold increase in SAR performance for magnetic hyperthermia by compositional tuning in zinc-substituted iron oxide superparamagnetic nanoparticles with superior biocompatibility. J. Alloys Compd. 2023, 968, 171868. [Google Scholar] [CrossRef]
- Thorat, N.D.; Khot, V.M.; Salunkhe, A.B.; Prasad, A.I.; Ningthoujam, R.S.; Pawar, S.H. Surface functionalized LSMO nanoparticles with improved colloidal stability for hyperthermia applications. J. Phys. D Appl. Phys. 2013, 46, 105003. [Google Scholar] [CrossRef]
- McBride, K.; Cook, J.; Gray, S.; Felton, S.; Stella, L.; Poulidi, D. Evaluation of La1−xSrxMnO3 (0 ≤ x < 0.4) synthesised via a modified sol–gel method as mediators for magnetic fluid hyperthermia. CrystEngComm 2016, 18, 407–416. [Google Scholar] [CrossRef]
- Asghar, M.S.; Ghazanfar, U.; Rizwan, M.; Manan, M.Q.; Baig, A.; Qaiser, M.A.; Haq, Z.; Wang, L.; Duta, L. Potential Molecular Interactions and In Vitro Hyperthermia, Thermal, and Magnetic Studies of Bioactive Nickel-Doped Hydroxyapatite Thin Films. Int. J. Mol. Sci. 2025, 26, 1095. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Maekawa, Y.; Wada, H.; Yamauchi, K.; Oguchi, T.; Harima, H. Hall effect of itinerant electron metamagnet Co(S1-xSex)2. J. Magn. Magn. Mater. 2022, 557, 169460. [Google Scholar] [CrossRef]
- Aqra, F. The cohesive energy density and the isothermal compressibility: Their relationships with the surface tension. Phys. B Condens. Matter. 2014, 446, 28–31. [Google Scholar] [CrossRef]
- Devi, Y.H.; Singh, L.H.; Wareppam, B. Effects of Viscosity on the Magnetic-Induced Heat Generation BT—Advances in Nanostructured Materials. In Advances in Nanostructured Materials; Swain, B.P., Ed.; Springer: Singapore, 2022; pp. 145–161. [Google Scholar] [CrossRef]
- Viktorov, V.; Nimafar, M. A novel generation of 3D SAR-based passive micromixer: Efficient mixing and low pressure drop at a low Reynolds number. J. Micromechanics Microengineering 2013, 23, 55023. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, L.; Li, Y.; Li, S.; Tian, Y.; Ma, L.; Luo, J. Magnetic field effect on apparent viscosity reducing of different crude oils at low temperature. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127372. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, S.; Sun, M.; Wang, S.; Huang, B.; Du, Y. Heteroatom-Driven Coordination Fields Altering Single Cerium Atom Sites for Efficient Oxygen Reduction Reaction. Adv. Mater. 2023, 35, 2302485. [Google Scholar] [CrossRef]
- Laha, S.S.; Thorat, N.D.; Singh, G.; Sathish, C.I.; Yi, J.; Dixit, A.; Vinu, A. Rare-Earth Doped Iron Oxide Nanostructures for Cancer Theranostics: Magnetic Hyperthermia and Magnetic Resonance Imaging. Small 2022, 18, 2104855. [Google Scholar] [CrossRef]
- Sibanda, E.T.; Prinsloo, A.R.E.; Sheppard, C.J.; Mohanty, P. Structural and magnetic properties of DyCrO3. AIP Adv. 2022, 12, 35342. [Google Scholar] [CrossRef]

| f, kHz | H, Oe | 70 | 80 | 90 | 100 | 120 |
|---|---|---|---|---|---|---|
| 150 | 5.85 | 7.23 | 9.0 | |||
| 250 | 7.01 | 10.48 | 12.8 | |||
| 300 | 4.87 | 6.88 | 8.49 | |||
| f, kHz | H, Oe | 70 | 80 | 90 | 100 | 120 |
|---|---|---|---|---|---|---|
| 150 | 4.0 | 6.78 | 7.85 | |||
| 250 | 6.71 | 9.76 | 14.36 | |||
| 300 | 2.93 | 3.73 | 4.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smari, M.; Moisiuc, M.V.; Al-Haik, M.Y.; Astefanoaei, I.; Stancu, A.; Shelkovyi, F.; Gimaev, R.; Piashova, J.; Zverev, V.; Haik, Y. Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior. Nanomaterials 2025, 15, 642. https://doi.org/10.3390/nano15090642
Smari M, Moisiuc MV, Al-Haik MY, Astefanoaei I, Stancu A, Shelkovyi F, Gimaev R, Piashova J, Zverev V, Haik Y. Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior. Nanomaterials. 2025; 15(9):642. https://doi.org/10.3390/nano15090642
Chicago/Turabian StyleSmari, Mourad, Monica Viorica Moisiuc, Mohammad Y. Al-Haik, Iordana Astefanoaei, Alexandru Stancu, Fedor Shelkovyi, Radel Gimaev, Julia Piashova, Vladimir Zverev, and Yousef Haik. 2025. "Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior" Nanomaterials 15, no. 9: 642. https://doi.org/10.3390/nano15090642
APA StyleSmari, M., Moisiuc, M. V., Al-Haik, M. Y., Astefanoaei, I., Stancu, A., Shelkovyi, F., Gimaev, R., Piashova, J., Zverev, V., & Haik, Y. (2025). Tunable Magnetic Heating in La0.51Sr0.49MnO3 and La0.51Dy0.045Sr0.445MnO3 Nanoparticles: Frequency- and Amplitude-Dependent Behavior. Nanomaterials, 15(9), 642. https://doi.org/10.3390/nano15090642







