Aging Characteristics of ZSM-5 Zeolite on Low-Frequency Acoustic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.1.1. Materials
2.1.2. Synthesis of Zeolite
2.1.3. Characterization
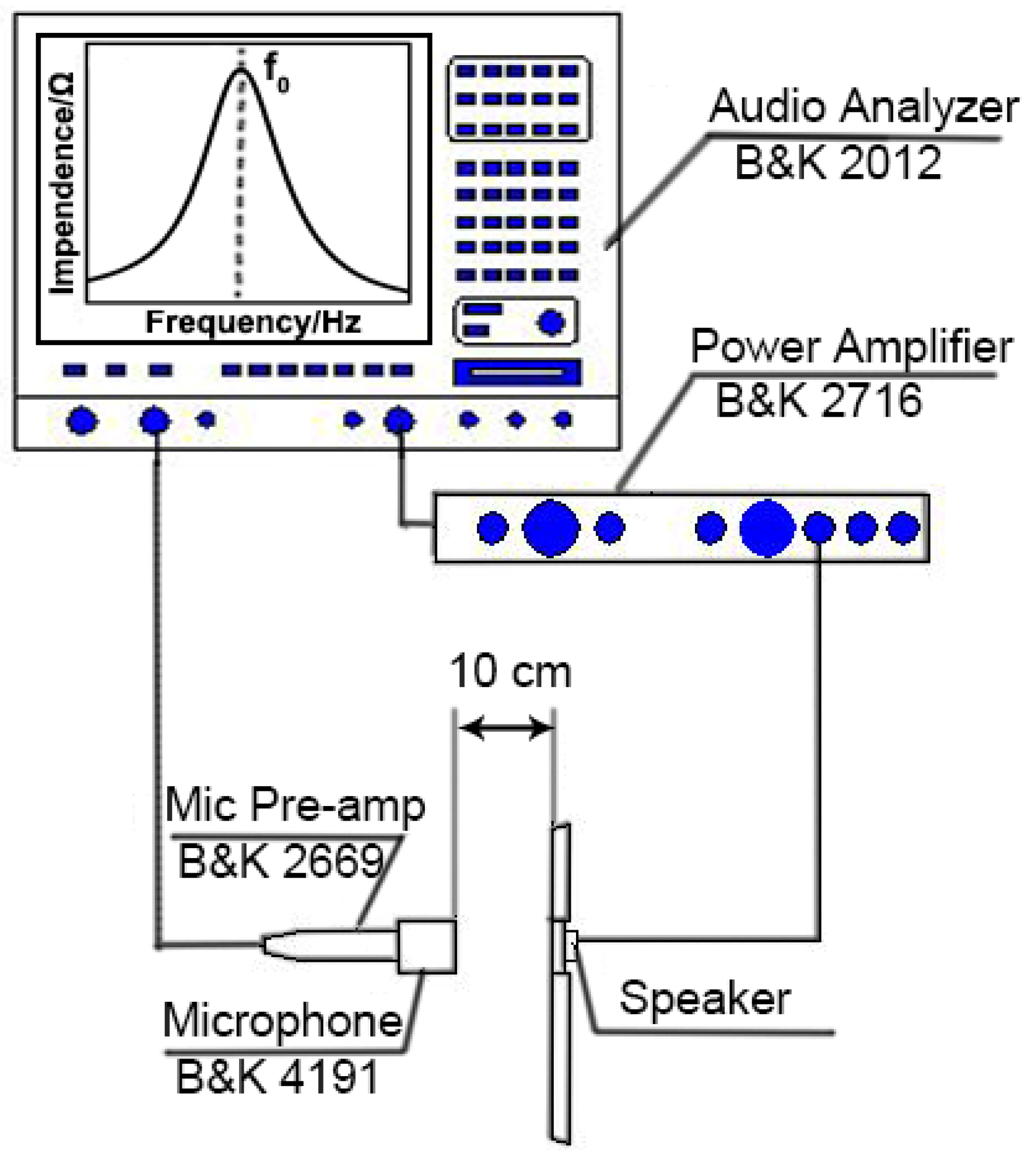
2.1.4. Acoustic Performance Measurement
2.1.5. Aging Experiments
3. Results and Discussion
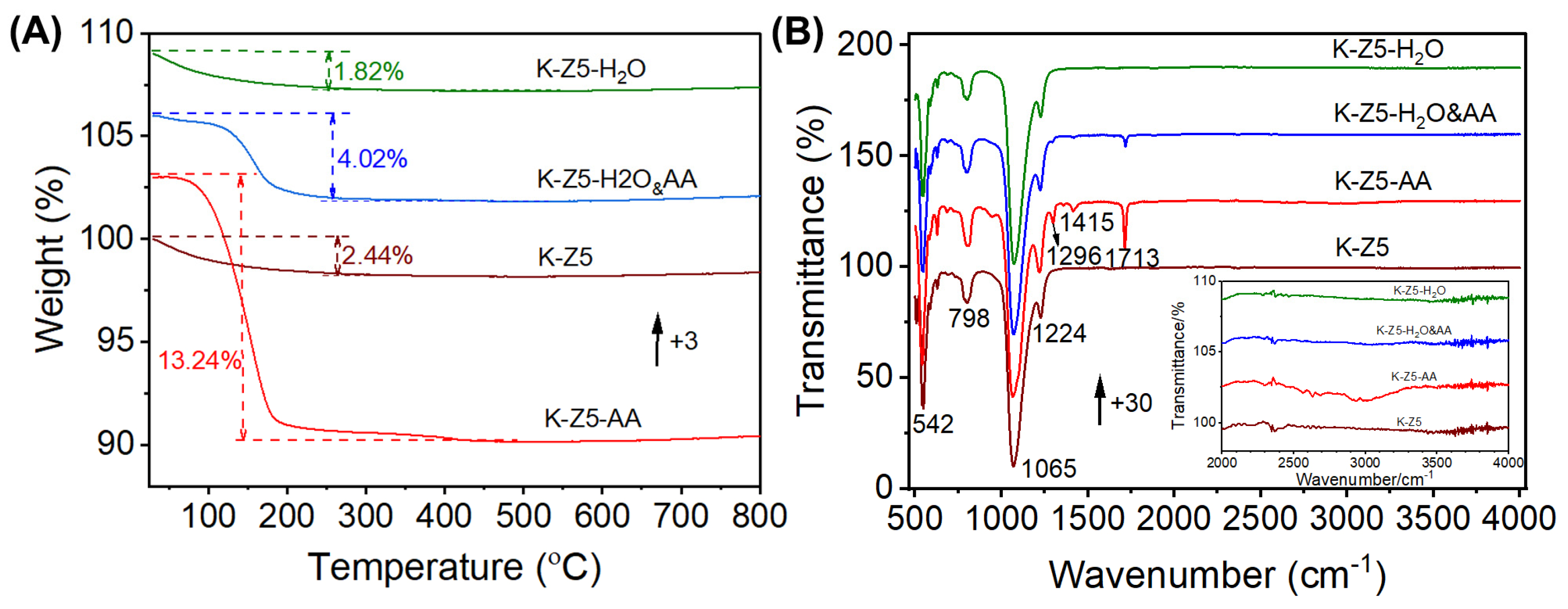
3.1. Characterization Analysis
3.2. Acoustic Enhancement Performance
3.3. Adsorption–Desorption Behavior Studied by In Situ DRIFTS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Ma, Y.; Zheng, Q.; Cao, K.; Lu, Y.; Xie, H. Review of Recent Development of MEMS Speakers. Micromachines 2021, 12, 1257. [Google Scholar] [CrossRef]
- Er, H.-C.; Huang, J.-H. Acoustical analysis for a microspeaker used in cellular phones. Int. Conf. Comput. Exp. Eng. Sci. 2009, 10, 55–56. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Lo, S.-C.; Huang, Z.-R.; Wang, Y.-J.; Wu, M.; Fang, W. On the design of piezoelectric MEMS microspeaker for the sound pressure level enhancement. Sens. Actuators A 2020, 306, 111960. [Google Scholar] [CrossRef]
- Ma, R.; Liu, G.; Hao, Q.; Wang, C. Smart microphone array design for speech enhancement in financial VR and AR. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Shiah, Y.C.; Her, H.-C.; Huang, J.H.; Huang, B. Parametric analysis for a miniature loudspeaker used in cellular phones. J. Appl. Phys. 2008, 104, 104905. [Google Scholar] [CrossRef]
- Shuji, S.; Toshiyuki, M.; Akiko, F. Speaker System. EP2003924B1, 2 April 2007. [Google Scholar]
- Wright, J.R. The virtual loudspeaker cabinet. J. Audio Eng. Soc. 2003, 51, 244–247. [Google Scholar]
- Jiang, Y.-W.; Xu, D.-P.; Kwon, J.-H.; Jiang, Z.-X.; Kim, J.-H.; Hwang, S.-M. Analysis and application of zeolite in microspeaker box. J. Mech. Sci. Technol. 2019, 33, 3679–3683. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Che, S.A. Mesoporous Silica Microspheres Composited with SBA-15s for Resonance Frequency Reduction in a Miniature Loudspeaker. Chem. Res. Chin. Univ. 2020, 36, 760–767. [Google Scholar] [CrossRef]
- Fan, S.; Wang, H.; Wang, P.; Jiao, W.; Wang, S.; Qin, Z.; Dong, M.; Wang, J.; Fan, W. Formation and evolution of the coke precursors on the zeolite catalyst in the conversion of methanol to olefins. Chem Catal. 2024, 4, 100927. [Google Scholar] [CrossRef]
- T/CECA 78-2022; Porous Sound-Adsorbing Particles for Micrspeakers. China Electronic Components Association: Beijing, China, 2022.
- Cruz, A.J.; Pires, J.; Carvalho, A.P.; Brotas de Carvalho, M. Adsorption of Acetic Acid by Activated Carbons, Zeolites, and Other Adsorbent Materials Related with the Preventive Conservation of Lead Objects in Museum Showcases. J. Chem. Eng. Data 2004, 49, 725–731. [Google Scholar] [CrossRef]
- Yan, T.; Yang, L.; Dai, W.; Wu, G.; Guan, N.; Hunger, M.; Li, L. Cascade Conversion of Acetic Acid to Isobutene over Yttrium-Modified Siliceous Beta Zeolites. ACS Catal. 2019, 9, 9726–9738. [Google Scholar] [CrossRef]
- Konsue, A.; Gleeson, M.P.; Gleeson, D. DFT Investigation of the catalytic conversion of acetic acid to acetone on the zeolite H-ZSM5. Mol. Catal. 2022, 533, 112782. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Zhang, Q.; Li, Y.; Sun, N.; Liu, W.; Liu, Y.; Yi, H.; Tang, X. Silica-alumina zeolite adsorbents for oxygen generation via pressure swing adsorption: Mechanisms and challenge. Chem. Eng. J. 2024, 481, 148788. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ryu, K.-H.; Bae, W.B.; Min, H.; Kweon, S.; Park, M.B.; Kang, D.; Kim, Y.J.; Kang, S.B. Active metal cation exchanged in ZSM-5 for enhanced direct air capture of CO2. Chem. Eng. J. 2025, 503, 158380. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, L.; Xu, B.; Yang, L.; Dai, Z.; Jiang, W. Recent advances in zeolite-based materials for volatile organic compounds adsorption. Sep. Purif. Technol. 2024, 350, 127742. [Google Scholar] [CrossRef]
- IEC60268-5-2007; Sound System Equipment—Part 5: Loudspeakers. IEC: Geneva, Switzerland, 2007.
- Meng, X.; Lian, Z.; Wang, X.; Shi, L.; Liu, N. Effect of dealumination of HZSM-5 by acid treatment on catalytic properties in non-hydrocracking of diesel. Fuel 2020, 270, 117426. [Google Scholar] [CrossRef]
- Zhang, J.X.; Ren, L.M.; Zhou, A.J.; Li, W.H.; Shang, S.J.; Liu, Y.; Jia, Z.H.; Liu, W.; Zhang, A.F.; Guo, X.W.; et al. Tailored Synthesis of ZSM-5 Nanosheets with Controllable b-Axis Thickness and Aspect Ratio: Strategy and Growth Mechanism. Chem. Mater. 2022, 34, 3217–3226. [Google Scholar] [CrossRef]
- Precisvalle, N.; Mancinelli, M.; Ardit, M.; Beltrami, G.; Gigli, L.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G.; Guidi, V.; et al. Temperature Induced Monoclinic to Orthorhombic Phase Transition in Protonated ZSM-5 Zeolites with Different Si/Al Ratios: An In-Situ Synchrotron X-ray Powder Diffraction Study. Crystals 2023, 13, 979. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, H.K.; Lv, E.J.; Ren, P.J.; Ren, J. Synthesis and characterization of an unusual snowflake-shaped ZSM-5 zeolite with high catalytic performance in the methanol to olefin reaction. Chin. J. Catal. 2016, 37, 308–315. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, N.; Dai, C.N.; Xu, R.N.; Yu, G.Q.; Chen, B.H.; Wang, N. Recent advances in shape selectivity of MFI zeolite and its effect on the catalytic performance. Chem. Synth. 2023, 3, 2. [Google Scholar] [CrossRef]
- Wang, Q.H.; Zhang, L.C.; Yao, Z.J.; Guo, Y.N.; Gao, Z.H.; Zheng, J.J.; Li, W.L.; Fan, B.B.; Wang, Y.; Chen, S.W.; et al. Synthesis of loosely aggregating polycrystalline ZSM-5 with luxuriant mesopore structure and its hierarchically cracking for bulky reactants. Mater. Chem. Phys. 2020, 243, 122610. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Q.; Wang, J.; Ding, C.; Zhang, K. Synthesis, characterization, and catalytic application of hierarchical nano-ZSM-5 zeolite. RSC Adv. 2020, 10, 29618–29626. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Meng, T.; Guo, Q.; Xue, Z.; Mao, D. Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene. Nanomaterials 2024, 14, 1558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Xia, Y.D.; Mokaya, R. Zeolite ZSM-5 with Unique Supermicropores Synthesized Using Mesoporous Carbon as a Template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Jonscher, C.; Seifert, M.; Kretzschmar, N.; Marschall, M.S.; Le Anh, M.; Doert, T.; Busse, O.; Weigand, J.J. Origin of Morphology Change and Effect of Crystallization Time and Si/Al Ratio during Synthesis of Zeolite ZSM-5. ChemCatChem 2022, 14, e202101248. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Feng, W.; Lin, Y.; Wang, S. Effect of triethylamine treatment of titanium silicalite-1 on propylene epoxidation. Front. Chem. Sci. Eng. 2014, 8, 478–487. [Google Scholar] [CrossRef]
- Kukulska-Zając, E.; Góra-Marek, K.; Datka, J. IR and TPD studies of the reaction of acetic acid in zeolites NaHY. Microporous Mesoporous Mater. 2006, 96, 216–221. [Google Scholar] [CrossRef]
- Gadermann, M.; Vollmar, D.; Signorell, R. Infrared spectroscopy of acetic acid and formic acid aerosols: Pure and compound acid/ice particles. Phys. Chem. Chem. Phys. 2007, 9, 4535–4544. [Google Scholar] [CrossRef]
- Mihaylov, M.; Ivanova, E.; Zdravkova, V.; Andonova, S.; Drenchev, N.; Chakarova, K.; Kefirov, R.; Kukeva, R.; Stoyanova, R.; Hadjiivanov, K. Purification of Hydrogen from CO with Cu/ZSM-5 Adsorbents. Molecules 2022, 27, 96. [Google Scholar] [CrossRef]
- Wu, W.; Weitz, E. Modification of acid sites in ZSM-5 by ion-exchange: An in-situ FTIR study. Appl. Surf. Sci. 2014, 316, 405–415. [Google Scholar] [CrossRef]
- Kolyagin, Y.G.; Ivanova, I.I.; Ordomsky, V.V.; Gedeon, A.; Pirogov, Y.A. Methane Activation over Zn-Modified MFI Zeolite: NMR Evidence for Zn−Methyl Surface Species Formation. J. Phys. Chem. C 2008, 112, 20065–20069. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv. Catal. 2014, 57, 99–318. [Google Scholar] [CrossRef]
- Tahraoui, Z.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Yoon, S.; Kim, T.W.; Hong, S.K.; Cho, K. Significant improvement of adsorption thermal energy storage of zeolite by simple cation exchange, inducing strong polarization of water. J. Energy Storage 2024, 89, 111805. [Google Scholar] [CrossRef]
- Hunger, B.; Heuchel, M.; Matysik, S.; Beck, K.; Einicke, W.D. Adsorption of water on ZSM-5 zeolites. Thermochim. Acta 1995, 269–270, 599–611. [Google Scholar] [CrossRef]
- Randrianandraina, J.; Badawi, M.; Cardey, B.; Grivet, M.; Groetz, J.-E.; Ramseyer, C.; Anzola, F.T.; Chambelland, C.; Ducret, D. Adsorption of water in Na-LTA zeolites: An ab initio molecular dynamics investigation. Phys. Chem. Chem. Phys. 2021, 23, 19032–19042. [Google Scholar] [CrossRef]
- Takemoto, M.; Yoshihara, Y.; Ito, Y.; Yamada, H.; Iyoki, K.; Okubo, T.; Wakihara, T. Improving mechanical stability of ZSM-5 zeolite by defect-healing treatment. Microporous Mesoporous Mater. 2024, 372, 113087. [Google Scholar] [CrossRef]
- He, L.H.; Li, J.J.; Han, S.Y.; Fan, D.; Li, X.J.; Xu, S.T.; Wei, Y.X.; Liu, Z.M. Dynamic evolution of HZSM-5 zeolite framework under steam treatment. Chem. Synth. 2024, 4, 1. [Google Scholar] [CrossRef]
- Iyoki, K.; Kikumasa, K.; Onishi, T.; Yonezawa, Y.; Chokkalingam, A.; Yanaba, Y.; Matsumoto, T.; Osuga, R.; Elangovan, S.P.; Kondo, J.N.; et al. Extremely Stable Zeolites Developed via Designed Liquid-Mediated Treatment. J. Am. Chem. Soc. 2020, 142, 3931–3938. [Google Scholar] [CrossRef]
- Hunger, M.; Kärger, J.; Pfeifer, H.; Caro, J.; Zibrowius, B.; Bülow, M.; Mostowicz, R. Investigation of internal silanol groups as structural defects in ZSM-5-type zeolites. J. Chem. Soc. Faraday Trans. 1 1987, 83, 3459–3468. [Google Scholar] [CrossRef]
- Chezeau, J.M.; Delmotte, L.; Guth, J.L.; Gabelica, Z. Influence of synthesis conditions and postsynthesis treatments on the nature and quantity of structural defects in highly siliceous MFI zeolites: A high-resolution solid-state 29Si n.m.r. study. Zeolites 1991, 11, 598–606. [Google Scholar] [CrossRef]
- Oni, B.; Ikhazuangbe, P. Reaction rate and rate constant of the hydrolysis of ethyl acetate with sodium hydroxide. Am. J. Sci. Ind. Res. 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Gumidyala, A.; Sooknoi, T.; Crossley, S. Selective ketonization of acetic acid over HZSM-5: The importance of acyl species and the influence of water. J. Catal. 2016, 340, 76–84. [Google Scholar] [CrossRef]
- Lai, Z.; Tsapatsis, M.; Nicolich, J.P. Siliceous ZSM-5 Membranes by Secondary Growth of b-Oriented Seed Layers. Adv. Funct. Mater. 2004, 14, 716–729. [Google Scholar] [CrossRef]







| Sample | SAR | Stotal/(m2·g−1) | Vtotal/(cm3·g−1) | Sext/(m2·g−1) | Vmicro/(cm3·g−1) |
|---|---|---|---|---|---|
| K-Z5 | 608 | 390 | 0.25 | 84 | 0.11 |
| K-Z5-H2O | 613 | 392 | 0.25 | 84 | 0.10 |
| K-Z5-AA | 646 | 363 | 0.22 | 59 | 0.11 |
| K-Z5-H2O&AA | 617 | 381 | 0.24 | 68 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Wang, Y.; Zhang, L.; Lu, J.; Gong, C.; Zhang, W.; Fang, Y.; Zhu, X.; Che, S. Aging Characteristics of ZSM-5 Zeolite on Low-Frequency Acoustic Applications. Nanomaterials 2025, 15, 639. https://doi.org/10.3390/nano15090639
Guo M, Wang Y, Zhang L, Lu J, Gong C, Zhang W, Fang Y, Zhu X, Che S. Aging Characteristics of ZSM-5 Zeolite on Low-Frequency Acoustic Applications. Nanomaterials. 2025; 15(9):639. https://doi.org/10.3390/nano15090639
Chicago/Turabian StyleGuo, Mingbo, Yijun Wang, Lei Zhang, Junran Lu, Chang Gong, Wanning Zhang, Yuxi Fang, Xinyuan Zhu, and Shunai Che. 2025. "Aging Characteristics of ZSM-5 Zeolite on Low-Frequency Acoustic Applications" Nanomaterials 15, no. 9: 639. https://doi.org/10.3390/nano15090639
APA StyleGuo, M., Wang, Y., Zhang, L., Lu, J., Gong, C., Zhang, W., Fang, Y., Zhu, X., & Che, S. (2025). Aging Characteristics of ZSM-5 Zeolite on Low-Frequency Acoustic Applications. Nanomaterials, 15(9), 639. https://doi.org/10.3390/nano15090639








