Impact of Dry Chemical-Free Mechanical Pressing on Deagglomeration of Submicron-Sized Boron Carbide Particles
Abstract
1. Introduction
| Treatment Technique | Main Operational Principle | Applied Particle Primary Size/ Particle Size Distribution Method | Advantages | Disadvantages |
|---|---|---|---|---|
| Ultrasonic homogenizer | A probe emits ultrasonic waves into a liquid sample, creating cavitation for dispersion. | Porous SiO2 (d = 15–20 nm)/dynamic light scattering (DLS), employing a He–Ne laser [93]. | Fast, efficient, reliable, and capable of processing samples in volumes ranging from a few millilitres to several litres [94]. | Generates a large amount of heat, it suffers from high energy consumption, scalability issues [94], and reagglomeration [95]. |
| Al2O3 (d = 50 nm)/elemental mapping of scanning electron microscope (SEM) [96]. | ||||
| Boron nitride nanotubes with a diameter ranging from 5 nm to 10 nm/SEM, transmission electron microscope (TEM) [97]. | ||||
| Ultrasonic bath | Sample placed in a liquid bath; ultrasonic waves propagate uniformly through the medium. | TiB2 (d = 100–200 nm)/laser diffraction [98]. | It does not require physical contact with the particles, reducing contamination risks. | Some energy is lost as heat, reducing the overall efficiency compared to direct ultrasonic probes; causes reagglomeration [95]. |
| Hydrostatic pressure + ultrasonic treatment | Ultrasonic treatment using sonotrode under a hydrostatic pressure. | Multi-walled carbon nanotubes (MWCNT Ø 5 nm to 12 nm, L ≈ 50 µm)/DLS, SEM, and TEM [23]. | Non-thermal, retains material properties. | Requires specialized, high-pressure systems. |
| SiO2 (d = 12 nm)/polarization intensity differential scattering (PIDS) [99]. | ||||
| Ultrasonic jet dispersion | Water supply at a pressure of 400 MPa with nanosuspension. High-speed jet combined with ultrasonication directly impacts particle suspensions. |
| High-speed jet combined with ultrasonication directly impacts particle suspensions. | Requires specialized equipment and it is energy intensive. |
| RESS/REHPS * | High shear stress in the nozzle passing through the Mach disc in the freely expanding jet. | Al2O3 and TiO2 (d = 1–3 µm)/scanning mobility particle spectrometer, aerodynamic particle sizer, and SEM [21]. | Produces fine particles with no solvent residue. | High cost, limited scalability. |
| Shock waves | Shock-tube filled with argon. Agglomerates suspended in a gas phase by means of shock waves, pressure, and shear forces. | SiO2 (d = 40 nm), SiC (d = 1.5 µm)/laser particle size analyzer (Mie scattering principle) [91]. | Effective for hard agglomerates, non-contact method. | Equipment complexity, high energy consumption. |
| High-shear mixer | A rotor–stator assembly creates a high-speed shearing action in a suspension with particles. | SiO2 (d = 12 nm)/laser particle size analyzer (Mie scattering principle) [83]. | Suitable for low-/high-viscosity materials, scalable. | Can damage sensitive materials |
| + ultrasonication | TiO2 (d ≈ 25 µm)/laser diffraction and PIDS [22]. | + potential thermal issues. | ||
| Swirl airflow | Powder packing in a capsule, discharging to the vortex chamber for dispersion and deagglomeration through swirl airflow-induced capsule rotation. | Drug powders (d = 60–140 µm)/ numerical simulation [101]. | Simple design, low energy Requirements. | Limited control over fine particle sizes. |
| Planetary centrifugal force | Dual-axis mixing generates centrifugal and shearing forces in a container. | Graphene nanoplatelets with 8–15 nm thickness/digital optical microscope [102]. | Uniform mixing, suitable for viscous materials. | Limited throughput, high maintenance costs. |
| Ball milling | A container contains grinding balls and suspension roll on a roller–mixer. The high shear rate breaks particle clusters mechanically. | UO2–PuO2 (d = 100 nm)/dry route laser granulometer (Mie diffraction theory) [77]. | Versatility, efficiency, and safety for diverse sample processing needs are ensured by their ability to handle multiple samples, prevent cross-contamination, and reduce aerosolization [103]. | In a laboratory setting, this type of homogenization is limited in terms of sample size. A single tube will hold only a few grams or millilitres of the sample for processing [103]. |
| Zero-valent iron (d = 200 nm)/ laser diffraction and SEM [104]. | ||||
| Mechanically reducing solid particle size by intense agitation of a slurry of the material being milled and coarse milling media [105]. | Onion-like carbon ink (d = 30–300 nm, after treatment)/dynamic light scattering, Raman spectroscopy, TEM [73]. | |||
| Rotary kiln | Thermal treatment—a rotating inclined cylinder heats (600 °C) and tumbles powder continuously. | Graphite (d50 = 375 µm)/dynamic image analysis and dry sieving analysis [106]. | Handles large volumes, effective for heat-tolerant particles. | Energy-intensive, limited to thermally stable materials. |
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, Z.-Q.; Wang, Y.-B. Development and Overview of Space Docking Mechanism. Recent Patents Eng. 2024, 19, E290124226390. [Google Scholar] [CrossRef]
- Sudarsi, R.; Sankaranarayanasamy, K.; Kenned, J.J. Effect of Carburization and Temperature on the Wear Behaviour of E16ncd13 Low Alloy Steel for Aerospace Applications. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5090787 (accessed on 10 April 2025).
- Guo, X.; Zang, F.; Zhu, Y.; Cao, D. Aircraft propeller erosion wear and aerodynamic characteristics. Acta Mech. Sin. 2024, 41, 524251. [Google Scholar] [CrossRef]
- Volosova, M.A.; Okunkova, A.A.; Kropotkina, E.Y.; Mustafaev, E.S.; Gkhashim, K.I. Wear Resistance of Ceramic Cutting Inserts Using Nitride Coatings and Microtexturing by Electrical Discharge Machining. Eng 2025, 6, 11. [Google Scholar] [CrossRef]
- Hatanaka, S.; Ogawa, Y.; Okubo, H.; Hanzawa, K.; Kajiki, R.; Yamaguchi, K.; Nakano, K. Correlation between friction and wear of rubber: An experimental approach based on the disconnections of Stribeck curves. Wear 2025, 562–563, 205623. [Google Scholar] [CrossRef]
- Kumaraswamy, J.; Bharath, L.; Anil, K.; Geetha, T.; Nagesh, R. Results in mechanical properties and wear behaviour of AA6061-Si3N4 composites. Results Surf. Interfaces 2025, 18, 100376. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Zhao, Z.; Zhang, L. Fabrication of Cu-Doped Diamond-like Carbon Film for Improving Sealing Performance of Hydraulic Cylinder of Shearers. C-J. Carbon Res. 2024, 10, 93. [Google Scholar] [CrossRef]
- Oldknow, K.; Stock, R.; Vollebregt, E. Effects of rail hardness on transverse profile evolution and computed contact conditions in a full-scale wheel-rail test rig evaluation. Wear 2025, 560–561, 205589. [Google Scholar] [CrossRef]
- Kim, S.; Shin, S. Improved unsteady fluid–structure interaction analysis using the dynamic mode decomposition on a composite marine propeller. Ocean Eng. 2025, 319, 120255. [Google Scholar] [CrossRef]
- Sun, N.; Hou, Z.; Jiang, Z.; Geng, J.; Xia, L. Facile modification of sepiolite and its application in wear-resistant and superhydrophobic epoxy coatings by mimicking the structure of shark skin. Appl. Clay Sci. 2025, 264, 107642. [Google Scholar] [CrossRef]
- Nie, M.; Jiang, P.; Li, X.; Zhu, D.; Yue, T.; Zhang, Z. Directed energy deposition combined with interlayer remelting for improving NiTi wear resistance by grain refinement. Tribol. Int. 2025, 202, 110300. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, Y.; Chen, Z.; Liu, H.; Wang, X. Study on microstructure and sliding wear behavior of similar and dissimilar welded joints produced by laser-arc hybrid welding of wear-resistant steels. Wear 2025, 562–563, 205643. [Google Scholar] [CrossRef]
- Dalan, F.C.; Sobrinho, A.S.D.S.; Nishihora, R.K.; Santos, S.F.; Martins, G.V.; Cardoso, K.R. Effect of Nb and Ti additions on microstructure, hardness and wear properties of AlCoCrFeNi high-entropy alloy. J. Alloys Compd. 2025, 1010, 177117. [Google Scholar] [CrossRef]
- Chakraborti, A.; Das, S.K.; Sahoo, P. Tailoring the Tribomechanical and Corrosion Behavior of Reverse Pulse Deposited Ni–Co–P Coating Through Cobalt Content Modulation. J. Tribol. 2025, 147, 071401. [Google Scholar] [CrossRef]
- Hukumdar, O.; Kumlu, U.; Keskin, A.; Akar, M.A. Effect of TMAB and ZrC concentration on mechanical and morphological properties of Ni–B/ZrC composite electrodeposition. Mater. Test. 2024, 67, 17–35. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Oh, M.; Kobayashi, E.; Chen, C.-M.; Shen, Y.-A. Investigating microstructure and interfacial stability of Bi-enhanced Sn-9Zn alloy on electroplated Cu during aging. Mater. Sci. Semicond. Process. 2025, 186, 109046. [Google Scholar] [CrossRef]
- Ditenberg, I.A.; Smirnov, I.V.; Osipov, D.A.; Grinyaev, K.V. Structural-phase state and microhardness of the surfacing formed on a steel substrate by pulsed argon tungsten arc remelting of Cu-tube containing W-Ta-Mo-Nb-Zr-Cr-Ti powder mixture. Intermetallics 2025, 179, 108639. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, K.; Li, H.; Teng, N.; Xu, S.; Chen, Q.; Gong, X.; He, Y.; Yan, S. A Ni-Cu/CuPP composite coating with good wear resistance and long-term corrosion resistance for seawater applications. Tribol. Int. 2025, 202, 110393. [Google Scholar] [CrossRef]
- Eiler, K.; Alcaide, F.; García-Lecina, E.; Sort, J.; Pellicer, E. Mesoporous Ni–Pt nanoparticles on Ni foam by electrodeposition: Dual porosity for efficient alkaline hydrogen evolution. Int. J. Hydrogen Energy 2025, 99, 448–457. [Google Scholar] [CrossRef]
- Pietraccini, M.; Glaude, P.-A.; Dufour, A.; Marmo, L.; Danzi, E.; Dufaud, O. A journey through space and time in the Godbert-Greenwald furnace: The evolution of a dust cloud particle size distribution. Process. Saf. Environ. Prot. 2024, 182, 509–526. [Google Scholar] [CrossRef]
- To, D.; Dave, R.; Yin, X.; Sundaresan, S. Deagglomeration of nanoparticle aggregates via rapid expansion of supercritical or high-pressure suspensions. AIChE J. 2009, 55, 2807–2826. [Google Scholar] [CrossRef]
- Krzosa, R.; Makowski, Ł.; Orciuch, W.; Adamek, R. Population Balance Application in TiO2 Particle Deagglomeration Process Modeling. Energies 2021, 14, 3523. [Google Scholar] [CrossRef]
- Yu, L.; Lin, Y.; Li, L.; Zong, H.; Zhou, Y.; Zhao, S.; Zhang, Z.; Grobert, N.; Maciejewska, B.M.; Qin, L. Understanding interfacial dynamics: Hydrostatic pressure-induced sono-dispersion of carbon nanotubes. Surf. Interfaces 2024, 51, 104740. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S. Mild oxidizing synthesis of non-agglomerated Fe2O3 nanoparticles for H2S gas sensing. Results Chem. 2024, 7, 101395. [Google Scholar] [CrossRef]
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A review on the production of metal matrix composites through stir casting—Furnace design, properties, challenges, and research opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Wang, Z.; Xing, X.; Cui, G. Enhancement of superhydrophobicity in Ni-WS2-PTFE composite coatings via magnetic coupling electrodeposition. Surf. Coat. Technol. 2025, 497, 131731. [Google Scholar] [CrossRef]
- Chakraborty, S.; Langford, N.; Kohl, Y.; Varsou, D.-D.; Stokes, W.; Papaioannou, E.; Wien, S.; Berkesi, K.; Britton, A.; Ibrahim, B.; et al. Are Ni–SiC nanoparticle electroplated coatings a safer alternative to hard chromium? A comprehensive aging, toxicity, and in silico study to assess safety by design. Environ. Sci. Nano 2025, 12, 894–908. [Google Scholar] [CrossRef]
- Ebrahimian-Hosseinabadi, M.; Azari-Dorcheh, K.; Vaghefi, S.M. Wear behavior of electroless Ni–P–B4C composite coatings. Wear 2006, 260, 123–127. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron Carbide: Structure, Properties, and Stability under Stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Niihara, K.; Nakahira, A.; Hirai, T. The Effect of Stoichiometry on Mechanical Properties of Boron Carbide. J. Am. Ceram. Soc. 1984, 67, C-13. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Walther, G. The Elastic Modulus and Fracture of Boron Carbide. J. Am. Ceram. Soc. 1980, 63, 610–613. [Google Scholar] [CrossRef]
- Rey, J.; Kapsa, P.; Male, G. Dry friction and wear of chemically vapour deposited boron carbide coatings. Surf. Coat. Technol. 1988, 36, 375–386. [Google Scholar] [CrossRef]
- Venkitachalapathy, K.; Manivannan, I.; Periandy, S.; Suresh, S.; Priya, M. (Eds.) Nano Boron Carbide Reinforcement in Al6061 Metal Matrix Composite; CRC Press: Boca Raton, FL, USA, 2025; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003606611-20/nano-boron-carbide-reinforcement-al6061-metal-matrix-composite-venkitachalapathy-manivannan-periandy-suresh-priya (accessed on 1 February 2025).
- Li, X.; Jiang, D.; Zhang, J.; Lin, Q.; Chen, Z.; Huang, Z. The dispersion of boron carbide powder in aqueous media. J. Eur. Ceram. Soc. 2013, 33, 1655–1663. [Google Scholar] [CrossRef]
- Costakis, W.J.; Rueschhoff, L.M.; Diaz-Cano, A.I.; Youngblood, J.P.; Trice, R.W. Additive manufacturing of boron carbide via continuous filament direct ink writing of aqueous ceramic suspensions. J. Eur. Ceram. Soc. 2016, 36, 3249–3256. [Google Scholar] [CrossRef]
- Kumar, R.V.; Reddappa, H.N.; Chandrashekar, A.; Paruti, B. Stir-cast Al2024-based composite reinforced with boron carbide and graphene particles: Mechanical and dry sliding wear characteristics. Eng. Rep. 2024, 6, e12850. [Google Scholar] [CrossRef]
- Ravi, B.; Naik, B.B.; Prakash, J.U. Characterization of Aluminium Matrix Composites (AA6061/B4C) Fabricated by Stir Casting Technique. Mater. Today Proc. 2015, 2, 2984–2990. [Google Scholar] [CrossRef]
- Kumar, D.; Seetharam, R.; Ponappa, K. Effects of graphene nano particles on interfacial microstructure and mechanical properties of Al7150/B4C hybrid nanocomposite fabricated by novel double ultrasonic two stage stir casting technique. J. Alloys Compd. 2024, 1008, 176686. [Google Scholar] [CrossRef]
- Krishnan, B.R.; Vellaichamy, R.; Sanjeevi, R.; Ramakrishnan, H.; Rajkumar, M.K. Experimental investigation of mechanical and metallurgical properties of LM25 aluminium alloy with boron carbide composite. Interactions 2025, 246, 23. [Google Scholar] [CrossRef]
- Vegari, A.; Abdisaray, A.; Mostafanejad, K.; Jabbari, N. High-density polyethylene (HDPE)-incorporated boron carbide and boric acid nanoparticles as a nanoshield of photoneutrons from medical linear accelerators. Int. J. Radiat. Biol. 2024, 100, 609–618. [Google Scholar] [CrossRef]
- Park, J.; Her, S.; Cho, S.; Woo, S.M.; Bae, S. Synthesis and characterization of Polyethylene/B4C composite, and its neutron shielding performance in cementitious materials: Experimental and simulation studies. Cem. Concr. Compos. 2022, 129, 104458. [Google Scholar] [CrossRef]
- Özcan, M.; Avcıoğlu, S.; Kaya, C.; Kaya, F. Boron carbide reinforced electrospun nanocomposite fiber mats for radiation shielding. Polym. Compos. 2023, 44, 4155–4167. [Google Scholar] [CrossRef]
- Magarajan, U.; Kumar, S.S.; Prabhu, L. Mechanical and ballistic studies of boron carbide filler reinforced glass fiber composites. Polym. Compos. 2024, 45, 14953–14965. [Google Scholar] [CrossRef]
- Dai, J.; Singh, J.; Yamamoto, N. Fabrication and characterization of FAST sintered micro/nano boron carbide composites with enhanced fracture toughness. J. Eur. Ceram. Soc. 2020, 40, 5272–5285. [Google Scholar] [CrossRef]
- Demir, E.; Mirzayev, M.; Abdurakhimov, B.; Mauyey, B.; Jabarov, S. Characterization of lattice parameter variations, defect dynamics, and surface morphology in Al2O3-B4C coatings on 321 stainless steel under swift heavy ion irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2025, 165, 116103. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Zhu, T.; Xu, Y.; Li, Y.; Fu, Z. Exceptional strength-toughness-hardness integrated B4C ceramics with synergistic reinforcement of nano-BN and in-situ ceramic phases. Compos. Part B Eng. 2025, 288, 111921. [Google Scholar] [CrossRef]
- Additive Manufacturing Process for Producing Aluminum-Boron Carbide Metal Matrix Composites. U.S. Patent US11898226B2, 13 February 2024. Available online: https://patents.google.com/patent/US11898226B2/en?oq=Additive+manufacturing+process+for+producing+aluminum-boron+carbide+metal+matrix+composites+(US11898226) (accessed on 14 March 2025).
- Stone, M.; Kolesnikov, A.; Fanelli, V.; May, A.; Bai, S.; Liu, J. Characterization of aluminum and boron carbide based additive manufactured material for thermal neutron shielding. Mater. Des. 2024, 237, 112463. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Argyros, A.; Papadakis, V.; Moutsopoulou, A.; Rogdakis, K.; Kymakis, E. Optimization course of hexagonal boron carbide ceramic nanofiller content in polypropylene for material extrusion additive manufacturing: Engineering response, nanostructure, and rheology insights. Next Nanotechnol. 2024, 5, 100054. [Google Scholar] [CrossRef]
- Kosedag, E.; Ekici, R. Low-velocity impact behaviors of B4C/SiC hybrid ceramic reinforced Al6061 based composites: An experimental and numerical study. J. Alloys Compd. 2025, 1010, 177525. [Google Scholar] [CrossRef]
- He, T.; He, Y.; Li, H.; Su, Z.; Fan, Y.; He, Z. Fabrication of Ni-W-B4C composite coatings and evaluation of its micro-hardness and corrosion resistance properties. Ceram. Int. 2018, 44, 9188–9193. [Google Scholar] [CrossRef]
- McCrea, J.L.; Marcoccia, M. Electroformed Nanocrystalline Coatings: An Advanced Alternative to Hard Chromium Electroplating. Available online: https://apps.dtic.mil/sti/pdfs/ADA637483.pdf (accessed on 5 June 2024).
- Zhang, Y.; Zhang, S.; He, Y.; Li, H.; He, T.; Fan, Y.; Zhang, H. Mechanical properties and corrosion resistance of pulse electrodeposited Ni-B/B4C composite coatings. Surf. Coat. Technol. 2021, 421, 127458. [Google Scholar] [CrossRef]
- Fayyaz, O.; Yusuf, M.M.; Bagherifard, S.; Montemor, M.; Shakoor, R. Probing into the properties of B4C reinforced nickel phosphorus-based nanocomposite coating. J. Mater. Res. Technol. 2022, 20, 2323–2334. [Google Scholar] [CrossRef]
- Jiang, J.B.; Liu, W.D.; Zhang, L.; Zhong, Q.D.; Wang, Y.; Zhou, Q.Y. Electrodeposition and Hardness and Corrosion Resistance Propertie of Ni/Nano-B4C Composite Coatings. Adv. Mater. Res. 2012, 399–401, 2055–2060. [Google Scholar] [CrossRef]
- Bestetti, M.; Lecis, N.; Magagnin, L.; Pirovano, R.; Cavallotti, P.L. Alternatives to Coatings from Chromium VI Baths. 2002. Available online: https://sterc.org/pdf/sf2002/sf02r03.pdf (accessed on 18 November 2024).
- Bayatlı, A.; Şahin, E.F.; Kocabaş, M. Effect of boron carbide reinforcement on surface properties of electroless Ni–B and Ni–B–W coatings. Mater. Chem. Phys. 2023, 305, 127899. [Google Scholar] [CrossRef]
- Functionally Graded Abrasive Structure and Methods of Using and Making Same. U.S. Patent WO2024118120A1, 6 June 2024. Available online: https://patents.google.com/patent/WO2024118120A1/en (accessed on 18 November 2024).
- Araghi, A.; Paydar, M. Electroless deposition of Ni–P–B4C composite coating on AZ91D magnesium alloy and investigation on its wear and corrosion resistance. Mater. Des. 2010, 31, 3095–3099. [Google Scholar] [CrossRef]
- Paydar, S.; Jafari, A.; Bahrololoom, M.E.; Mozafari, V. Influence of BN and B4C particulates on wear and corrosion resistance of electroplated nickel matrix composite coatings. Tribol. Mater. Surfaces Interfaces 2015, 9, 105–110. [Google Scholar] [CrossRef]
- Vasu, C.; Andhare, A.B.; Dumpala, R. Machinability and tool wear studies on AZ91/B4C metal matrix composites using uncoated and CVD diamond coated WC-Co turning inserts. Int. J. Refract. Met. Hard Mater. 2024, 119, 106538. [Google Scholar] [CrossRef]
- Zheng, R.; Hao, X.; Yuan, Y.; Wang, Z.; Ameyama, K.; Ma, C. Effect of high volume fraction of B4C particles on the microstructure and mechanical properties of aluminum alloy based composites. J. Alloys Compd. 2013, 576, 291–298. [Google Scholar] [CrossRef]
- Rachmaniar, S.; Nugraha, D.A.; Santjojo, D.J.D.H.; Tjahjanto, R.T.; Mufti, N.; Masruroh. Prevention of particle agglomeration in sol–gel synthesis of TiO2 nanoparticles via addition of surfactant. J. Nanopart. Res. 2024, 26, 45. [Google Scholar] [CrossRef]
- Kunjomana, A.G.; Bibin, J.; Athira, R.C.; Teena, M. Planetary Ball Milling and Tailoring of the Optoelectronic Properties of Monophase SnSe Nanoparticles. J. Electron. Mater. 2024, 53, 298–311. [Google Scholar] [CrossRef]
- Bhavesh, G.; Hariharan, P.; Dhinasekaran, D. Comparative study on the synthesis and characterization of Gd2Zr2O7 nanoparticles by mechanical milling and Co-precipitation techniques. Ceram. Int. 2024, 50, 27476–27485. [Google Scholar] [CrossRef]
- Sarma, S.S.; Padya, B.; Sarada, B.V.; Akhila, V.; Gowthami, C.; Krishna, P.V.; Joardar, J. Two-dimensional hexagonal boron nitride by cryo-milling: Microstructure and oxidation behavior at elevated temperature. J. Nanopart. Res. 2024, 26, 80. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Zakani, B.; Grecov, D. Influence of buffer on colloidal stability, microstructure, and rheology of cellulose nanocrystals in hyaluronic acid suspensions. J. Colloid Interface Sci. 2025, 678, 1194–1211. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Pacek, A.W. Effect of pH on deagglomeration and rheology/morphology of aqueous suspensions of goethite nanopowder. J. Colloid Interface Sci. 2008, 325, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Chou, C.-C.; Wang, H.-T.; Cheng, C.-H.; Hou, K.-H.; Ger, M.-D. Tribocorrosion study of electrodeposited Ni W alloy/BN(h) composited coatings for piston rings. Surf. Coat. Technol. 2022, 436, 128289. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; He, T.; Qing, D.; Luo, F.; Fan, Y.; Chen, X. Ni-W/BN(h) electrodeposited nanocomposite coating with functionally graded microstructure. J. Alloys Compd. 2017, 704, 32–43. [Google Scholar] [CrossRef]
- Ünal, E.; Karahan, I. Production and characterization of electrodeposited Ni-B/hBN composite coatings. Surf. Coat. Technol. 2018, 333, 125–137. [Google Scholar] [CrossRef]
- Bauer, C.; Neff, T.; Day, A.; Krueger, A. Scalable Fabrication of Flexible Supercapacitor Electrodes Using Sustainable Water-Based Onion-like Carbon Inks. Batter. Supercaps 2024, 7, e202400203. [Google Scholar] [CrossRef]
- da Igreja, P.; Klump, D.; Bartsch, J.; Thommes, M. Reduction of submicron particle agglomeration via melt foaming in solid crystalline suspension. J. Dispers. Sci. Technol. 2024, 45, 307–316. [Google Scholar] [CrossRef]
- Diego, D.Z.; Gianluigi, S. Electroplating Bath Containing Trivalent Chromium and Process for Depositing Chromium. 2020. Available online: https://patents.google.com/patent/US20200308723A1/en (accessed on 18 November 2024).
- Zhou, Y.-M.; Liao, Y.-L.; Pan, X.-Y.; Zhao, X.; Zhang, F.-L.; Qin, H.-Q.; Zhang, Z.-J. Synthesis and lapping performance of a detonation nanodiamonds slurry by a SPS deagglomeration technology. Int. J. Refract. Met. Hard Mater. 2024, 118, 106433. [Google Scholar] [CrossRef]
- La Lumia, F.; Ramond, L.; Pagnoux, C.; Coste, P.; Lebreton, F.; Sevilla, J.; Bernard-Granger, G. Dense and homogeneous MOX fuel pellets manufactured using the freeze granulation route. J. Am. Ceram. Soc. 2020, 103, 3020–3029. [Google Scholar] [CrossRef]
- Heida, R.; Hagedoorn, P.; van Meel, M.C.; Prins, J.E.R.; Simonis, F.S.; Akkerman, R.; Huckriede, A.L.W.; Frijlink, H.W.; de Boer, A.H.; Hinrichs, W.L.J. Performance Testing of a Homemade Aerosol Generator for Pulmonary Administration of Dry Powder Formulations to Mice. Pharmaceutics 2023, 15, 1847. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-C.; Lai, C.-H.; Li, C.-C. Dispersion stabilization of carbonyl iron particles and its applications in chemical mechanical planarization. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 683, 133003. [Google Scholar] [CrossRef]
- Sukhija, M.; Coleri, E. A review on the incorporation of reclaimed asphalt pavement material in asphalt pavements: Management practices and strategic techniques. Road Mater. Pavement Des. 2025, 1–40. [Google Scholar] [CrossRef]
- Yao, G.; Chen, H.; Zhao, Z.-H.; Luo, L.-M.; Ma, Y.; Cheng, J.-G.; Zan, X.; Xu, Q.; Wu, Y.-C. The superior thermal stability and irradiation resistance capacities of tungsten composites synthesized by simple second-phase particle component modulation. J. Nucl. Mater. 2022, 561, 153522. [Google Scholar] [CrossRef]
- Bevan, R. Review of Industrial Size Reduction Equipment Used in the Processing of Coal. In Rotary Breakers, Roll Crushers, Hammer Mills, Impactors, Tumbling Mills, Roller Mills and Misc; Kennedy Van Saun Corp.: Danville, PA, USA, 1977. Available online: https://www.osti.gov/biblio/6707008 (accessed on 2 April 2025).
- Li, Z.; Xu, Z.; Wan, L.; Zhang, Z.; Liu, B. Nanoparticle deagglomeration driven by a high shear mixer and intensification of low-speed stirring in a viscous system. Chem. Eng. Process. Process. Intensif. 2025, 208, 110092. [Google Scholar] [CrossRef]
- Gerde, P.; Ewing, P.; Låstbom, L.; Ryrfeldt, Å.; Waher, J.; Lidén, G. A Novel Method to Aerosolize Powder for Short Inhalation Exposures at High Concentrations: Isolated Rat Lungs Exposed to Respirable Diesel Soot. Inhal. Toxicol. 2004, 16, 45–52. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Lam, J.K.W. Enhancing Pulmonary Drug Delivery: A Study on Mixed Particle Formulations. Available online: https://ddl-conference.com/ddl2024/conference-papers/enhancing-pulmonary-drug-delivery-a-study-on-mixed-particle-formulations/ (accessed on 1 March 2025).
- Xie, B.; Hassan-Naji, R.; Hall, D.A. Effects of process parameters on deposition behavior and mechanical properties of alumina coatings by aerosol deposition. J. Am. Ceram. Soc. 2025, 108, e20169. [Google Scholar] [CrossRef]
- Wu, Y.; Klauck, M.; Trollmann, K.; Allelein, H.-J. Influence of different thermodynamic parameters on variation across the aerosol size spectrum. Ann. Nucl. Energy 2024, 202, 110468. [Google Scholar] [CrossRef]
- Ye, Y.; Tu, C.; Zhang, Z.; Xu, R.; Bao, F.; Lin, J. Deagglomeration of airborne nanoparticles in a decelerating supersonic round jet. Adv. Powder Technol. 2021, 32, 1488–1501. [Google Scholar] [CrossRef]
- Strecker, J.J.F.; Roth, P. Particle Breakup in Shock Waves Studies by single Particle Light Scattering. Part. Part. Syst. Charact. 1994, 11, 222–226. [Google Scholar] [CrossRef]
- To, D. Deagglomeration and Mixing via the Rapid Expansion of High Pressure and Supercritical Suspensions. Ph.D. Thesis, New Jersey Institute of Technology, Newark, NJ, USA, 2011. Available online: https://digitalcommons.njit.edu/dissertations/265/ (accessed on 24 February 2025).
- Brandt, O.; Rajathurai, A.M.; Roth, P. First observations on break-up of particle agglomerates in shock waves. Exp. Fluids 1987, 5, 86–94. [Google Scholar] [CrossRef]
- Kellenberger, M.; Johansen, C.; Ciccarelli, G.; Zhang, F. Dense particle cloud dispersion by a shock wave. Shock Waves 2013, 23, 415–430. [Google Scholar] [CrossRef]
- Strach, A.; Dulski, M.; Wasilkowski, D.; Matus, K.; Dudek, K.; Podwórny, J.; Rawicka, P.; Grebnevs, V.; Waloszczyk, N.; Nowak, A.; et al. Multifaceted Assessment of Porous Silica Nanocomposites: Unraveling Physical, Structural, and Biological Transformations Induced by Microwave Field Modification. Nanomaterials 2024, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Exploring the Power and Potential of Ultrasonic Homogenizers [Online]. Available online: https://nanografi.com/blog/exploring-the-power-and-potential-of-ultrasonic-homogenizers-nanografi-/ (accessed on 3 January 2025).
- Nguyen, V.S.; Rouxel, D.; Hadji, R.; Vincent, B.; Fort, Y. Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason. Sonochem. 2011, 18, 382–388. [Google Scholar] [CrossRef]
- Javdani, A.; Najafabadi, M.A. Achieving Homogeneous Distribution and Dispersion of Al2O3np Nanoparticles within 316L Matrix for Production of Metal Matrix Nanocomposites via Blended Powder Semisolid Forming. Arab. J. Sci. Eng. 2024, 49, 10669–10685. [Google Scholar] [CrossRef]
- John, D.; Sukumaran, A.K.; Mohammed, S.M.A.K.; Orikasa, K.; Lou, L.; Nisar, A.; Paul, T.; Lama, A.; Park, C.; Chu, S.; et al. Cold-Sprayed Boron-Nitride-Nanotube-Reinforced Aluminum Matrix Composites with Improved Wear Resistance and Radiation Shielding. Adv. Eng. Mater. 2024, 26, 2401490. [Google Scholar] [CrossRef]
- Čekerevac, D.; Bajić, D.; Perković, S.; Fidanovski, B.; Marinković, J.; Pejović, V.; Rigueiro, C.; Pereira, E.; Santiago, A. Impact resistance of aramid honeycomb tandem sandwich composites reinforced with TiB2. Compos. Adv. Mater. 2024, 33, 26349833241257960. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.; Schuchmann, H.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef]
- Galinovskiy, A.L.; Htet, K.M.; Provatorov, A.S. Ultra-Jet as a Tool for Dispersing Nanosuspensions. Polym. Sci. Ser. D 2020, 13, 209–213. [Google Scholar] [CrossRef]
- Zhu, Q. Optimizing Powder Dispersion in Dry Powder Inhalers: A CFD-DEM Investigation of Drug Formulation, Device Design and Electrostatic Charging. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2024. [Google Scholar] [CrossRef]
- Bergseth, Z.; Qi, X.; Wang, X. A study on graphene nanoplatelet dispersion methods and their effects on coating abrasion resistance and surface properties. Prog. Org. Coat. 2024, 189, 108352. [Google Scholar] [CrossRef]
- O’Driscoll, A. Bead Mill Homogenizers Versus Ultrasonic Homogenizers (Sonicators). 2019. Available online: https://homogenizers.net/blogs/blog/bead-mill-homogenizers-versus-ultrasonic-homogenizers-sonicators (accessed on 27 December 2024).
- Li, L.; Shi, Y.; Zhang, S.; Wei, M.; Li, S.; Zhang, W.-X. Enhanced breakage of the aggregates of nanoscale zero-valent iron via ball milling. Sci. Total. Environ. 2024, 946, 174399. [Google Scholar] [CrossRef] [PubMed]
- Sadler, L.Y.; Stanley, D.A.; Brooks, D.R. Attrition mill operating characteristics. Powder Technol. 1975, 12, 19–28. [Google Scholar] [CrossRef]
- Martinez, G. Variation in Graphite Removal from Spent EV Black Mass. Master Thesis, Luleå University of Technology, Luleå, Sweden, 2024. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:ltu:diva-109965 (accessed on 27 December 2024).
- S3500—Laserbeuger für Partikelgrößenbestimmung—Microtrac. Available online: https://www.microtrac.de/de/produkte/partikel-groesse-form-analyse/laserbeugung/s3500/ (accessed on 15 February 2025).
- Partikelgrößenverteilung: Partikelanalysatoren: Microtrac. Available online: https://www.microtrac.de/de/wissen/partikelgroessenverteilung/ (accessed on 18 February 2025).
- ISO 9276-2:2014(en); Representation of Results of Particle Size Analysis—Part 2: Calculation of Average Particle Sizes/Diameters and Moments from Particle Size Distributions. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:9276:-2:ed-2:v1:en (accessed on 16 February 2025).
- Zhang, S.; Lu, W.; Wang, C.; Shen, Q.; Zhang, L. Synthesis and characterization of B13C2 boron carbide ceramic by pulsed electric current sintering. Ceram. Int. 2012, 38, 895–900. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, M.Y.; Goddard, W.A.; An, Q. Strengthening boron carbide by doping Si into grain boundaries. J. Am. Ceram. Soc. 2022, 105, 2978–2989. [Google Scholar] [CrossRef]
- Thévenot, F. Boron carbide—A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Rey, J.; Male, G.; Kapsa, P.; Loubet, J.L. Boron carbide coatings: Correlation between mechanical properties and LPCVD parameters values. Le J. de Phys. Colloq. 1989, 50, C5-311–C5-321. [Google Scholar] [CrossRef]
- Bouchacourt, M. Etudes sur la Phase Carbure de Bore. Corrélations Propriétés-Composition. Ph.D. Thesis, Institut Polytechnique de Grenoble, Saint-Martin-d’Hères, France, 1982. Available online: https://theses.hal.science/tel-01177071 (accessed on 2 April 2025).
- Bouchacourt, M.; Thevenot, F. The properties and structure of the boron carbide phase. J. Less Common Met. 1981, 82, 227–235. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Wang, T.; Wang, F.; Li, J.; Gong, Y.; Zhang, Y.; Wu, J.; Sun, M.; Han, L. Study on the Correlation Between Mechanical Properties, Water Absorption, and Bulk Density of PVA Fiber-Reinforced Cement Matrix Composites. Buildings 2024, 14, 3580. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, J.; Méndez-Lagunas, L.; López-Ortiz, A.; Torres, S.S. True Density and Apparent Density During the Drying Process for Vegetables and Fruits: A Review. J. Food Sci. 2012, 77, R146–R154. [Google Scholar] [CrossRef]
- Plantz, P.E. Explanation of Data Reported be Microtrac Instruments: Provided by: Microtrac Inc. Particle Size Measuring. Available online: http://www.vahitech.com/Assets/MicrotracDataExplinationSheet.pdf (accessed on 20 February 2025).
- Ayan, U.; Mohoppu, M.; Sebastian, J.A.; Shreyan, B.R.; Toragall, V.B.; Maniruzzaman, M.; Al-Ostaz, A.; Werfel, T.; Villacorta, B.S. Polylactic Acid/Polybutylene Adipate Terephthalate-Carbon Nanotube Nanobiocomposites with a Segregated Toughening Morphology Yielding Large Ductility for Biocompatible Materials. ACS Appl. Nano Mater. 2025, 8, 1537–1556. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Lin, Q. Aqueous processing of boron carbide powders. J. Ceram. Soc. Jpn. 2008, 116, 681–684. [Google Scholar] [CrossRef]
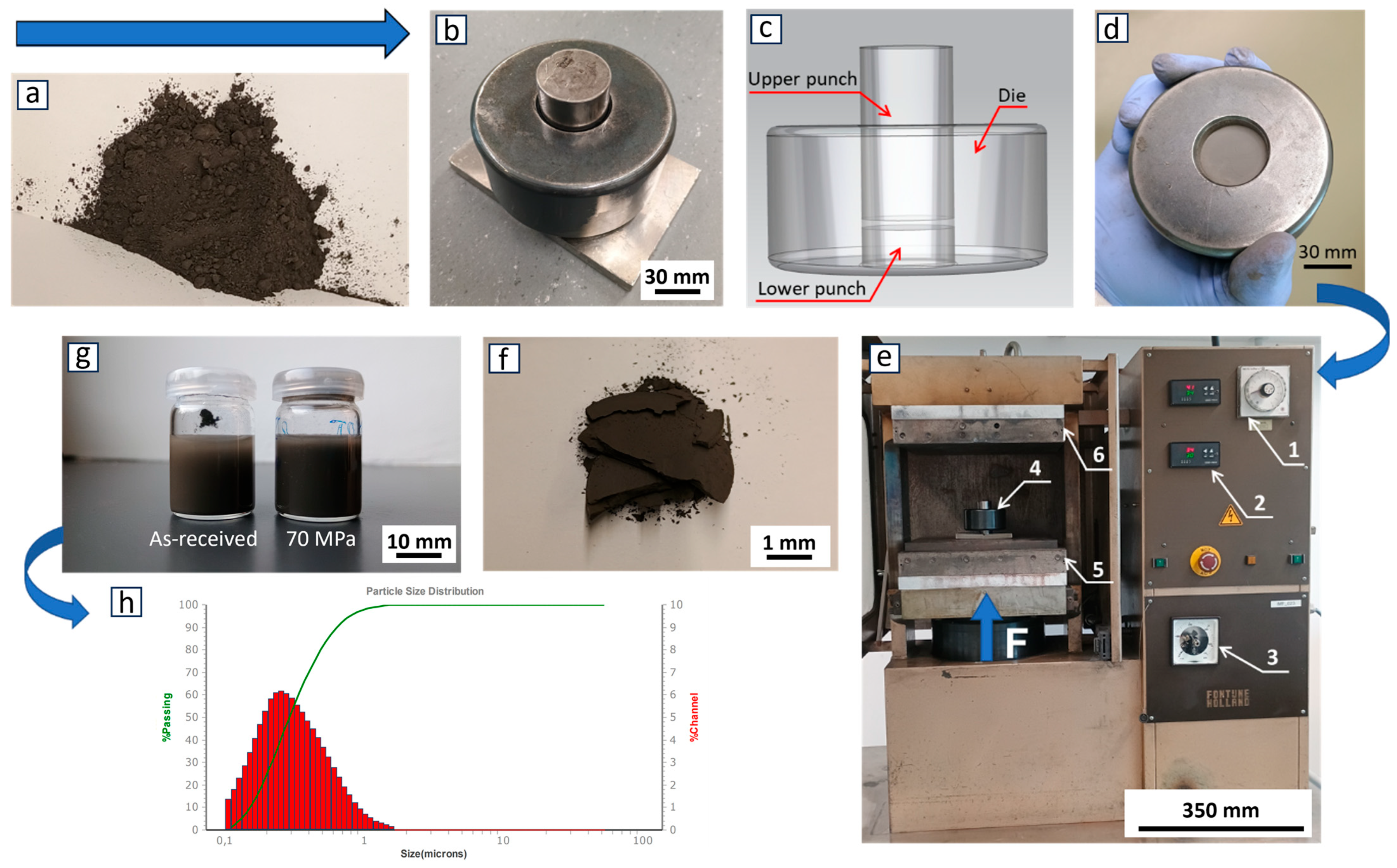
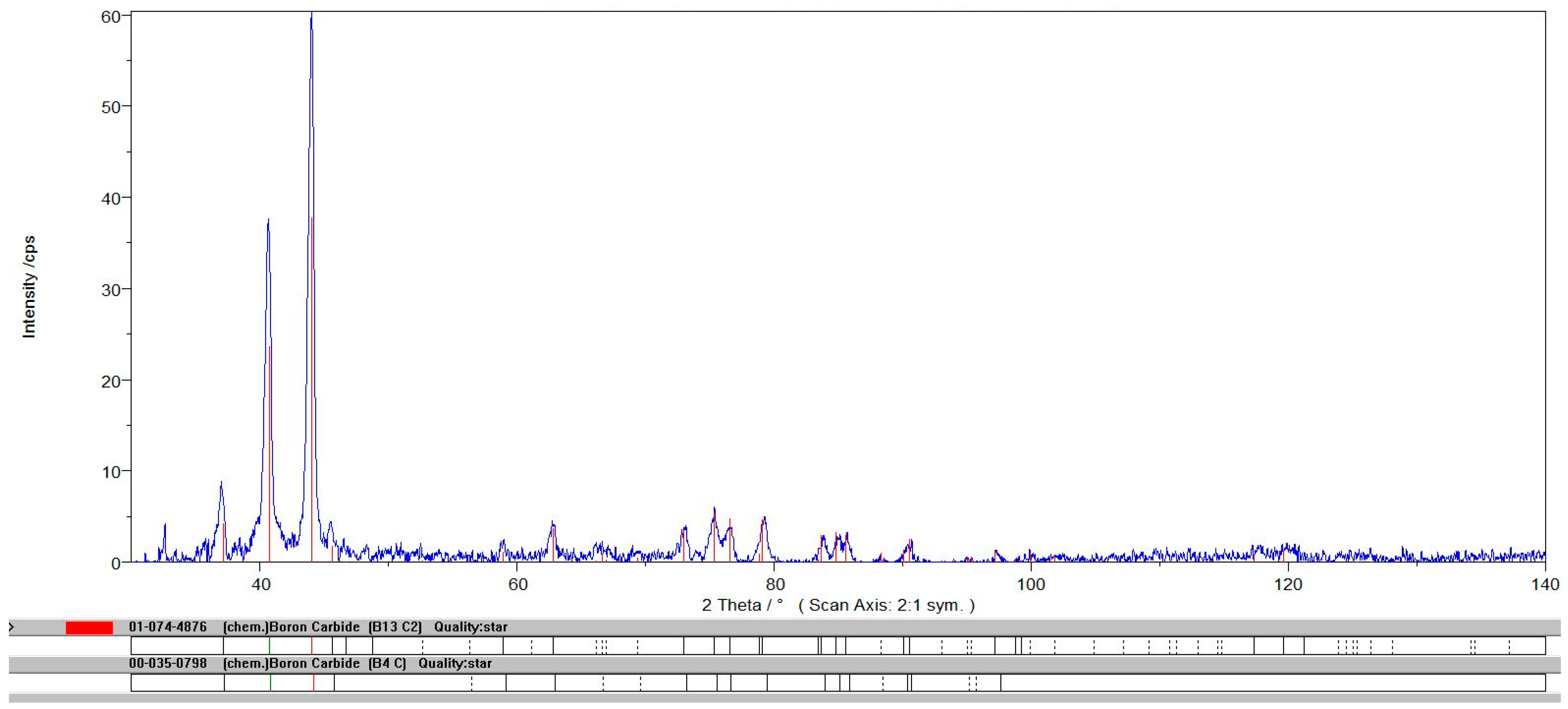
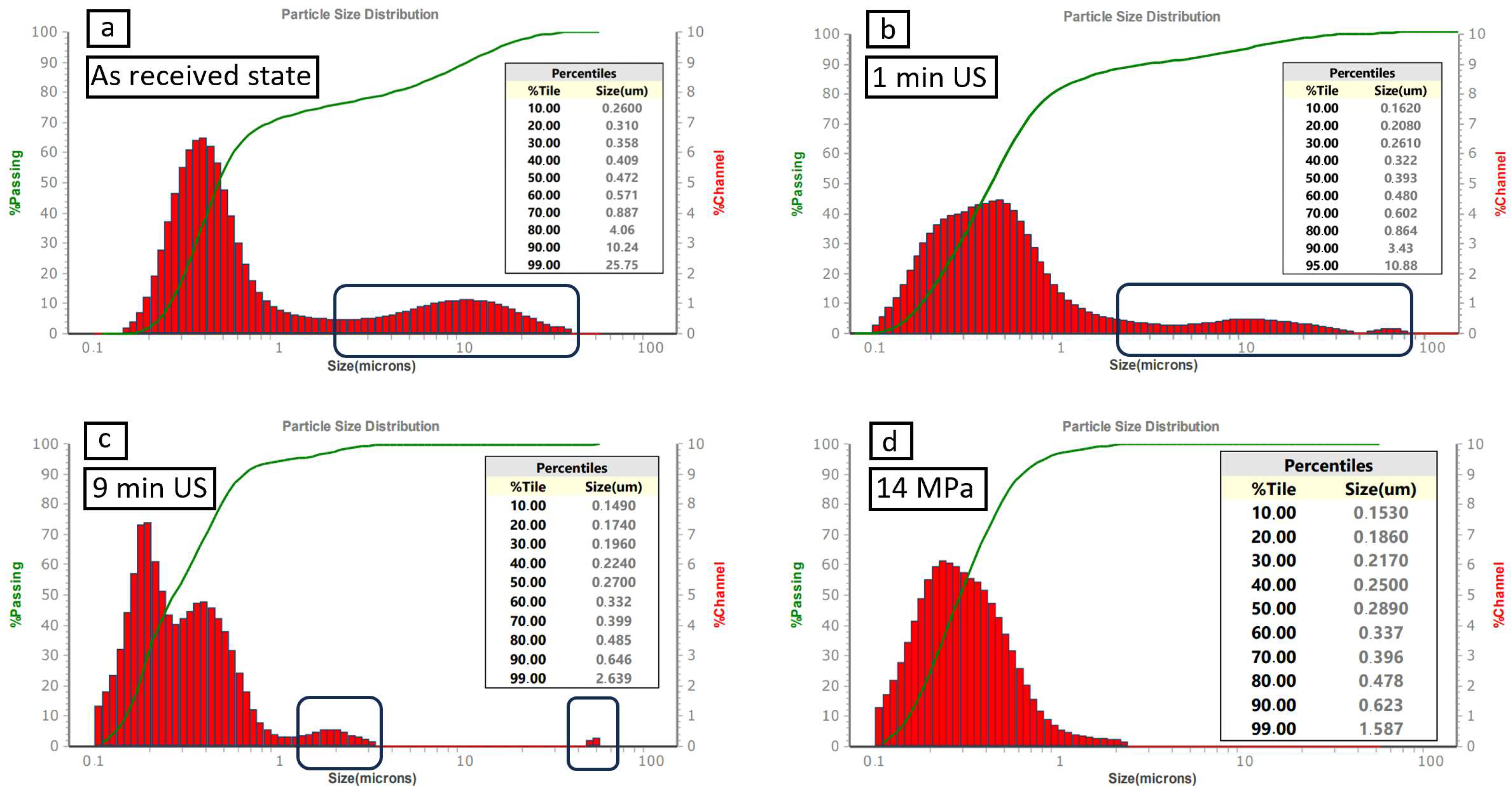
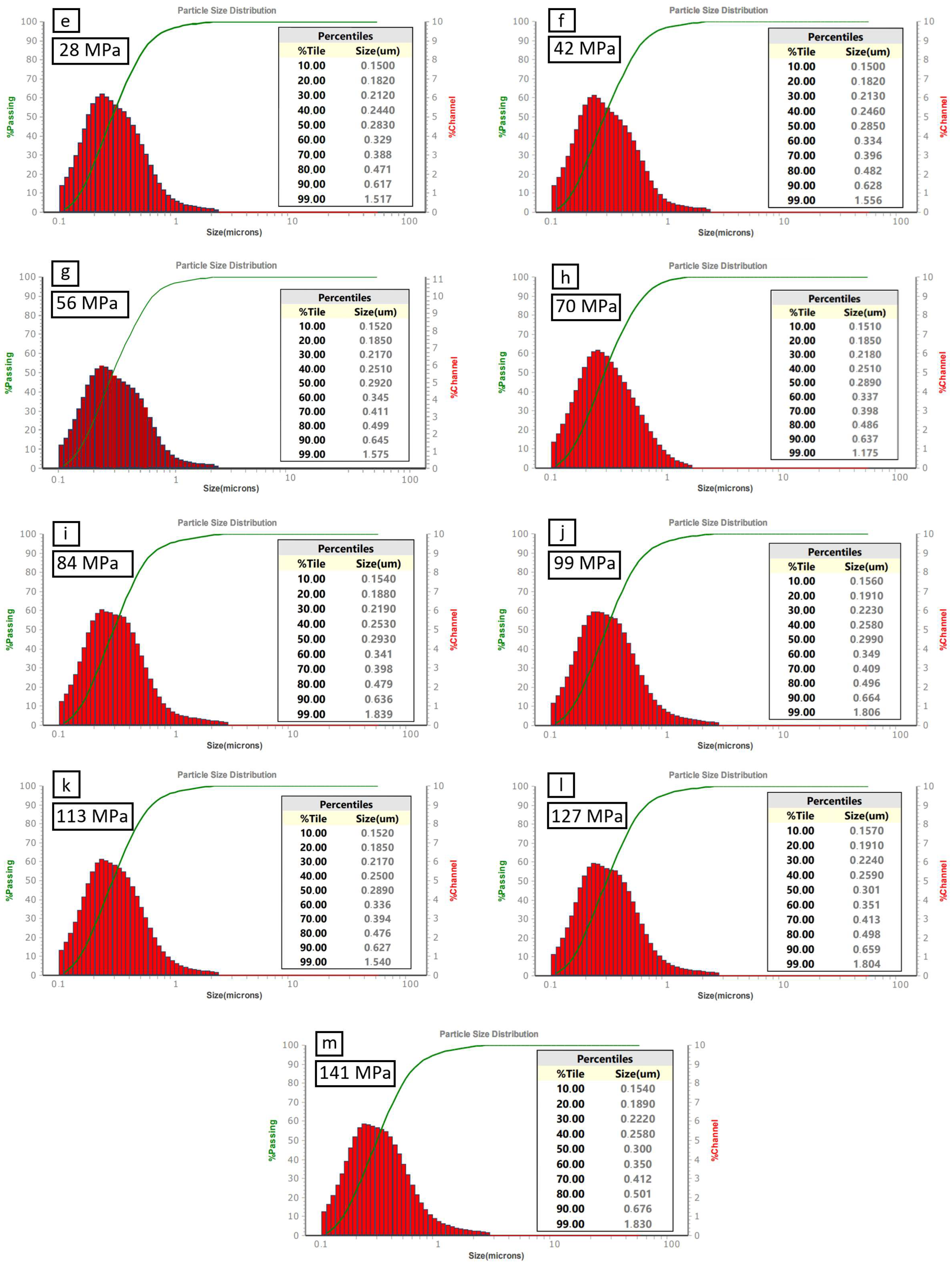
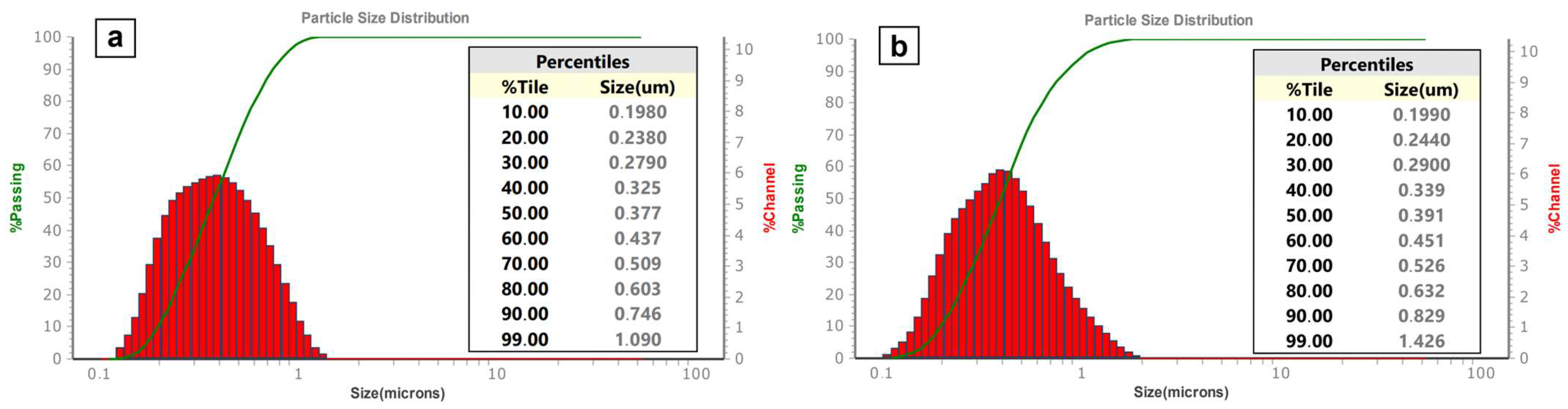
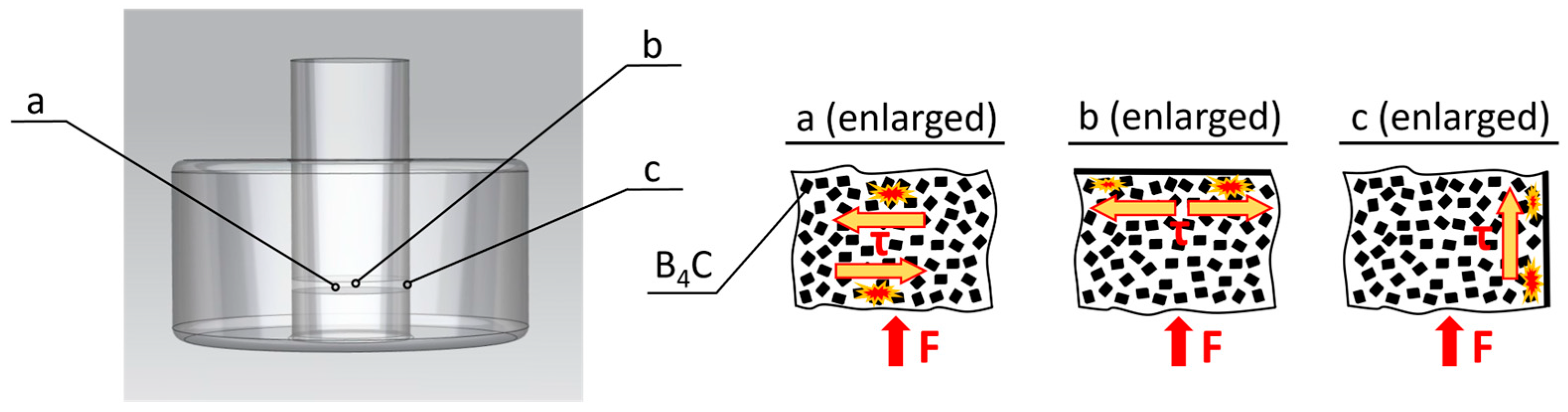

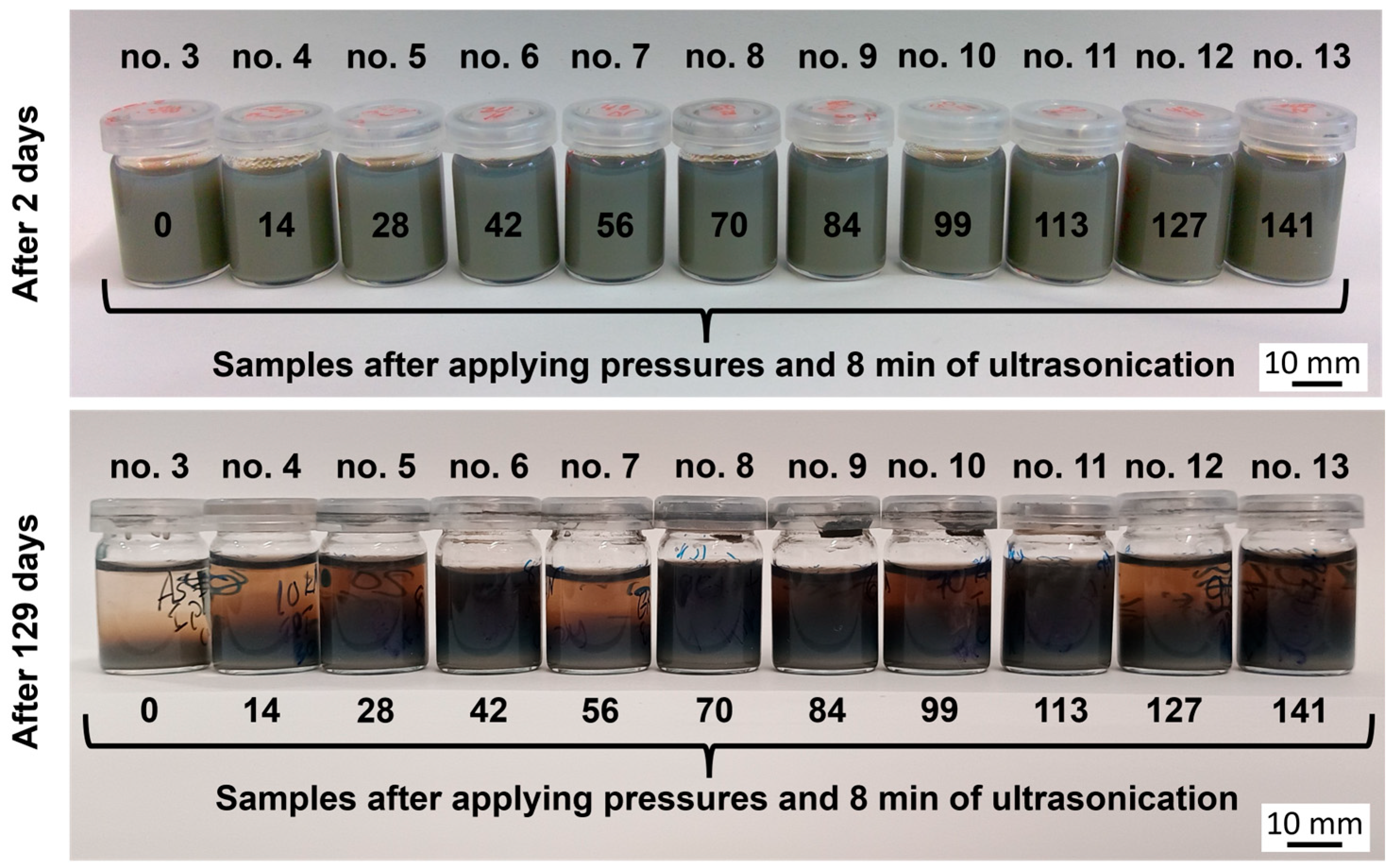
| Impurities | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | B4C |
|---|---|---|---|---|---|---|
| Percentage | <1.2 | <0.1 | <0.4 | <0.05 | <0.1 | Base |
| Parameter | Value |
|---|---|
| Theoretical density [g/cm3] | 2.51 |
| Melting point [°C] | 2350 |
| Hardness [HV10] | 30–40 |
| Thermal conductivity [W/(m·K)] | 30 |
| Color | Dark grey |
| Sample Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||||||||
| Applied pressure [MPa] | 0 | 0 | 0 | 14 | 28 | 42 | 56 | 70 | 84 | 99 | 113 | 127 | 141 | |
| Holding time [s] | - | - | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| External US treatment * | - | - | + | + | + | + | + | + | + | + | + | + | + | |
| Internal US treatment ** | - | + | + | + | + | + | + | + | + | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkady, M.; Sörgel, T. Impact of Dry Chemical-Free Mechanical Pressing on Deagglomeration of Submicron-Sized Boron Carbide Particles. Nanomaterials 2025, 15, 611. https://doi.org/10.3390/nano15080611
Elkady M, Sörgel T. Impact of Dry Chemical-Free Mechanical Pressing on Deagglomeration of Submicron-Sized Boron Carbide Particles. Nanomaterials. 2025; 15(8):611. https://doi.org/10.3390/nano15080611
Chicago/Turabian StyleElkady, Mahmoud, and Timo Sörgel. 2025. "Impact of Dry Chemical-Free Mechanical Pressing on Deagglomeration of Submicron-Sized Boron Carbide Particles" Nanomaterials 15, no. 8: 611. https://doi.org/10.3390/nano15080611
APA StyleElkady, M., & Sörgel, T. (2025). Impact of Dry Chemical-Free Mechanical Pressing on Deagglomeration of Submicron-Sized Boron Carbide Particles. Nanomaterials, 15(8), 611. https://doi.org/10.3390/nano15080611







