Synthesis and Characterization of Polyaniline/Carbon Nanodots: Electrochemical Sensing of Alcohols for Freshness Monitoring for Application as Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CNDs
2.2. Synthesis of PANI
2.3. Synthesis of CND/PANI Nanocomposites
2.4. Characterization
2.4.1. Spectral Studies
2.4.2. Morphological Analysis
2.4.3. Electrochemical Measurements
3. Results
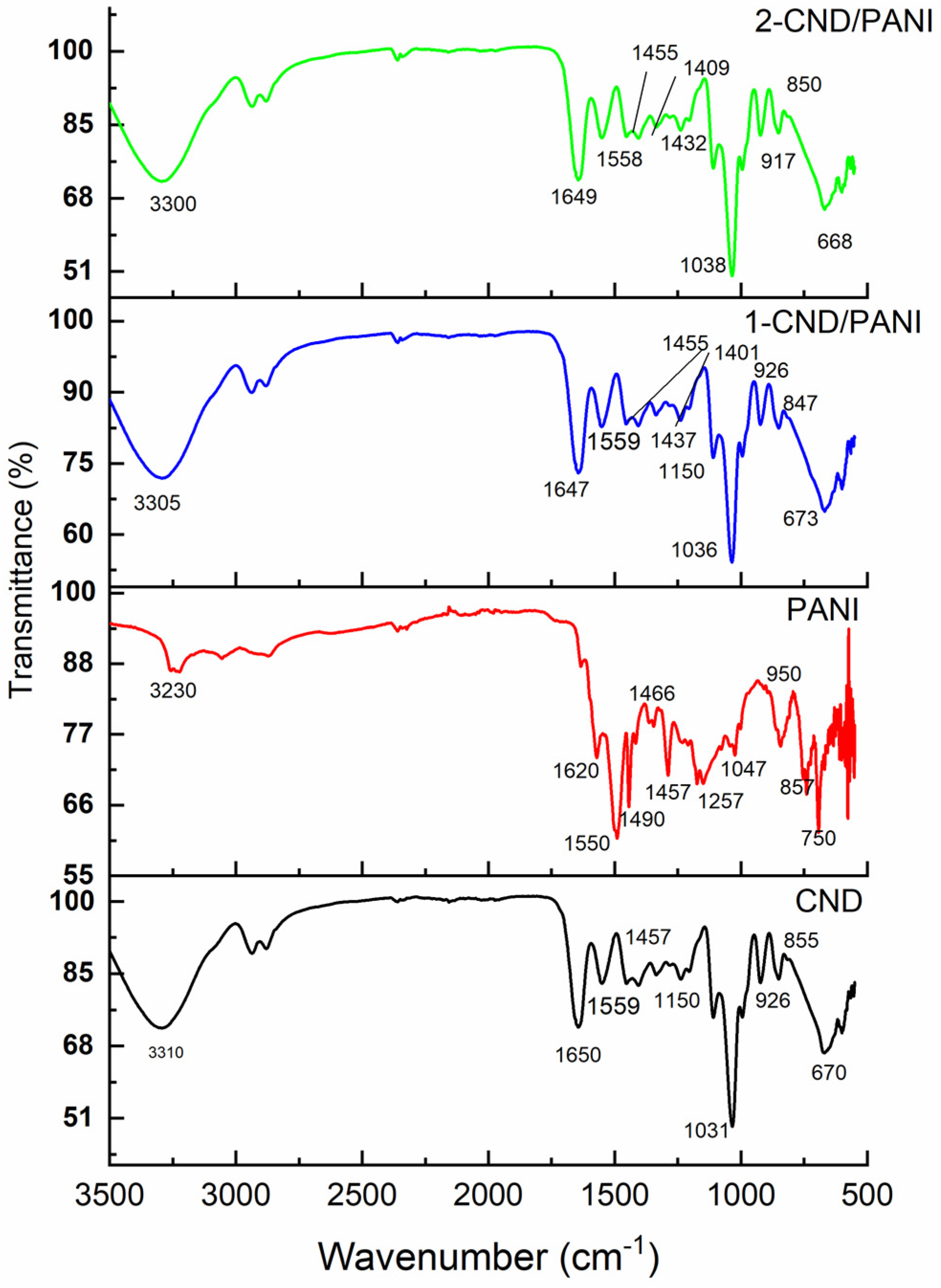
3.1. FTIR Analysis
3.2. UV-Visible Analysis
3.3. XRD Analysis
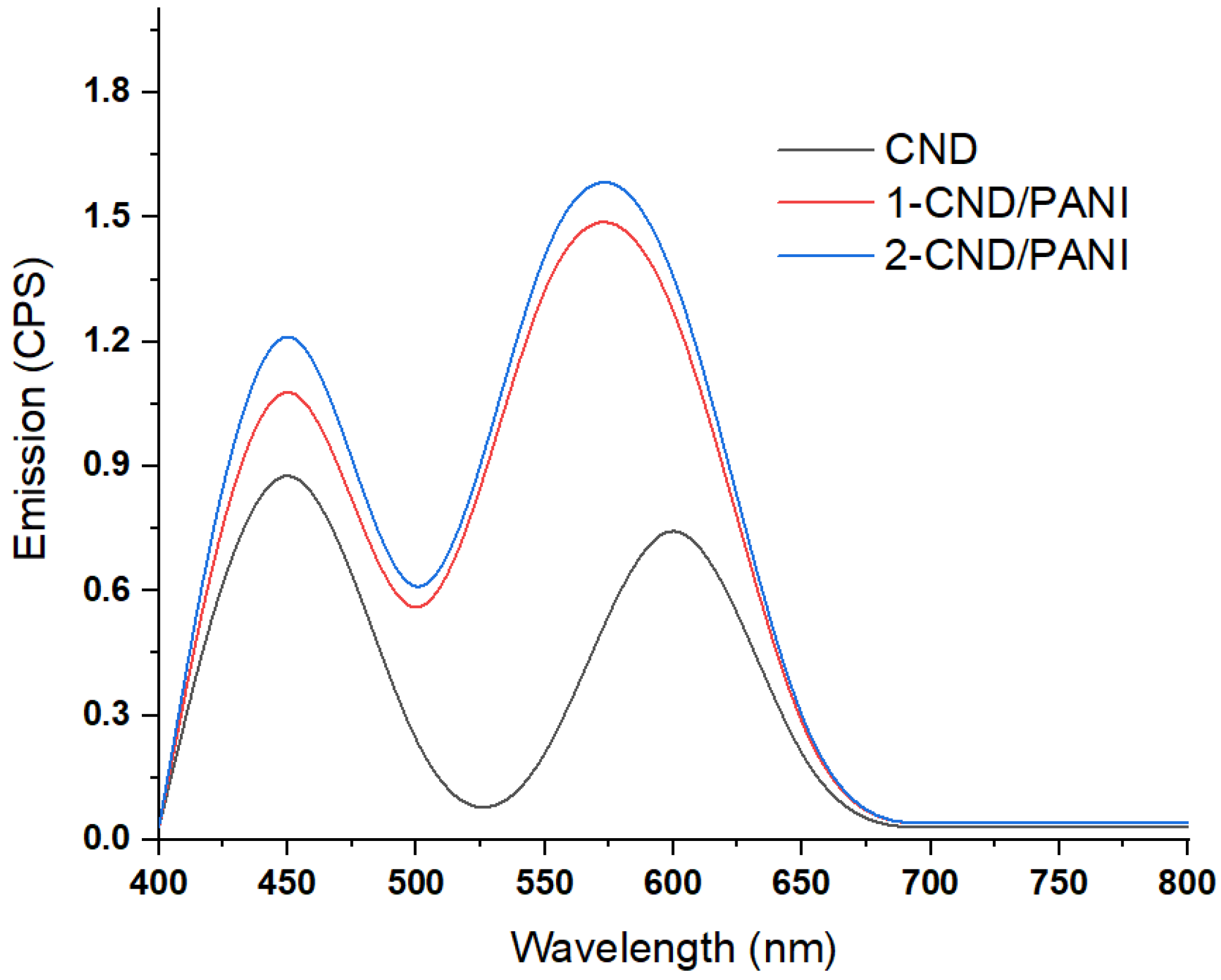
3.4. Fluorescence Studies
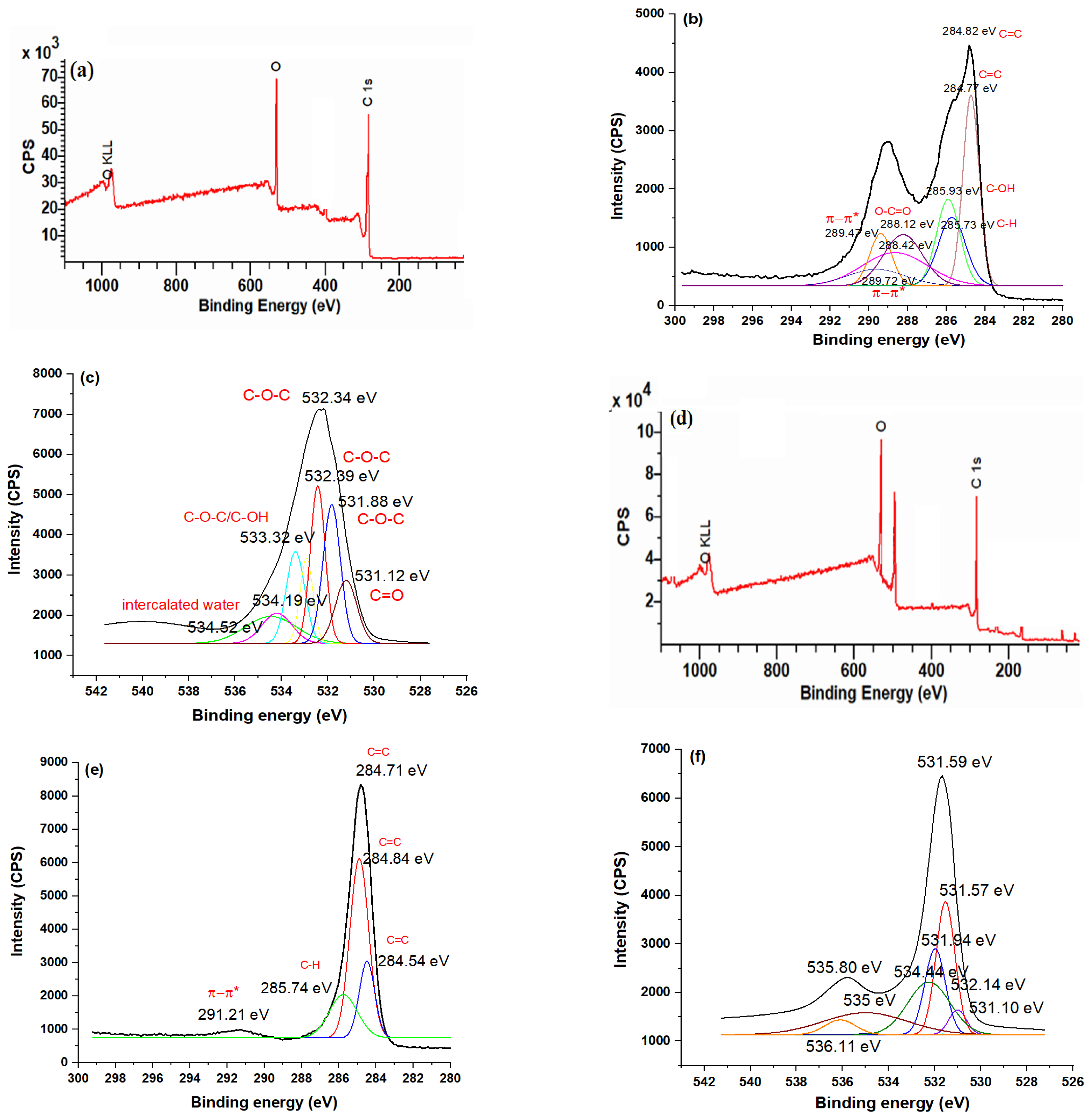
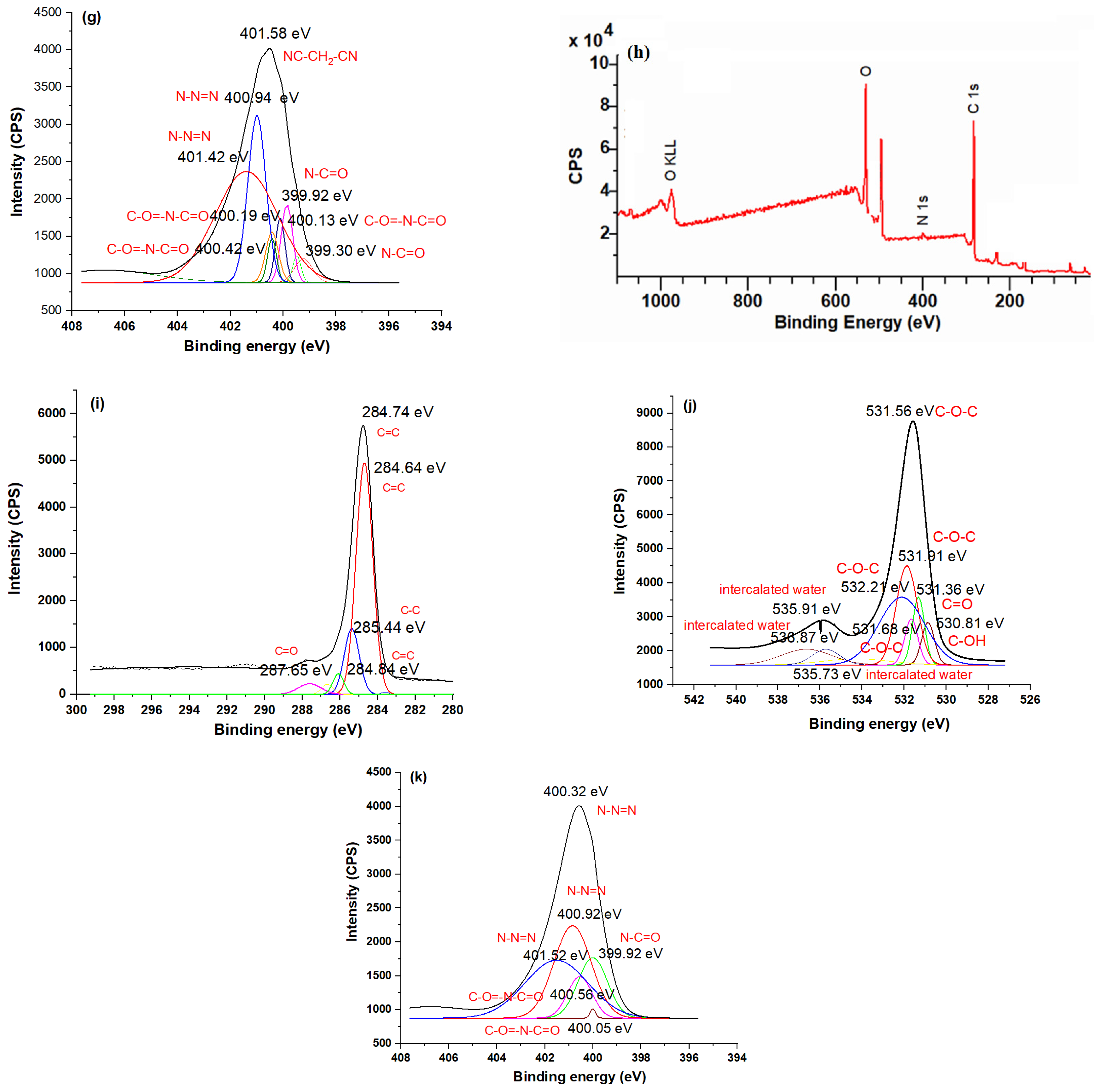
3.5. XPS Studies
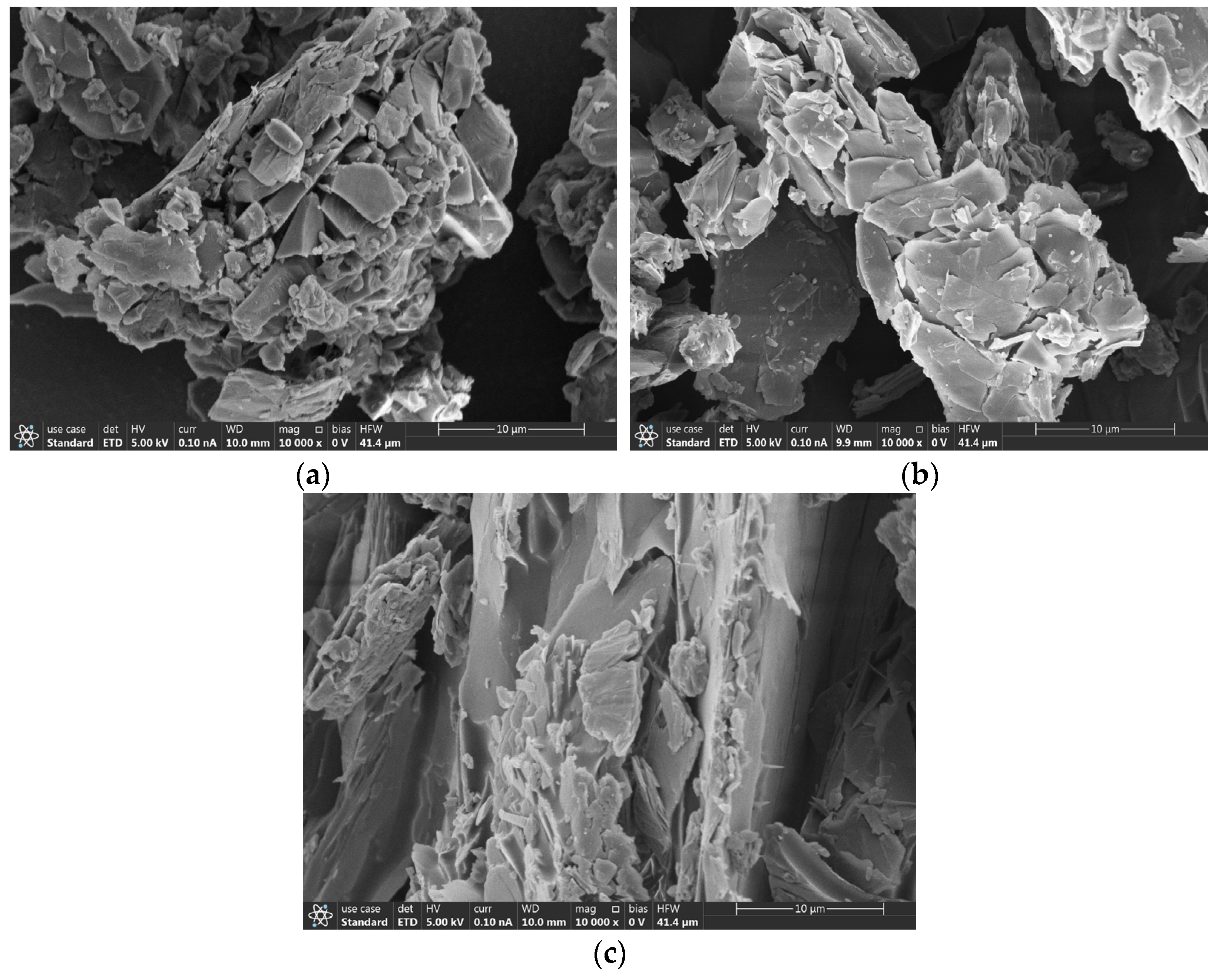
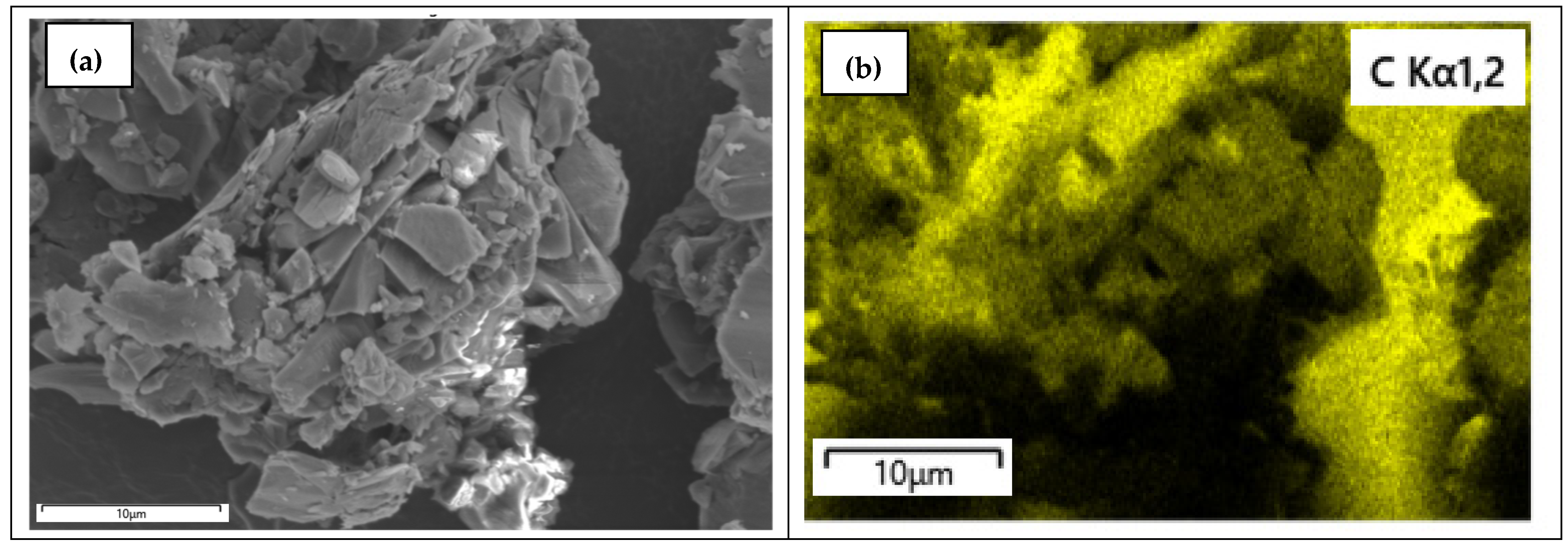
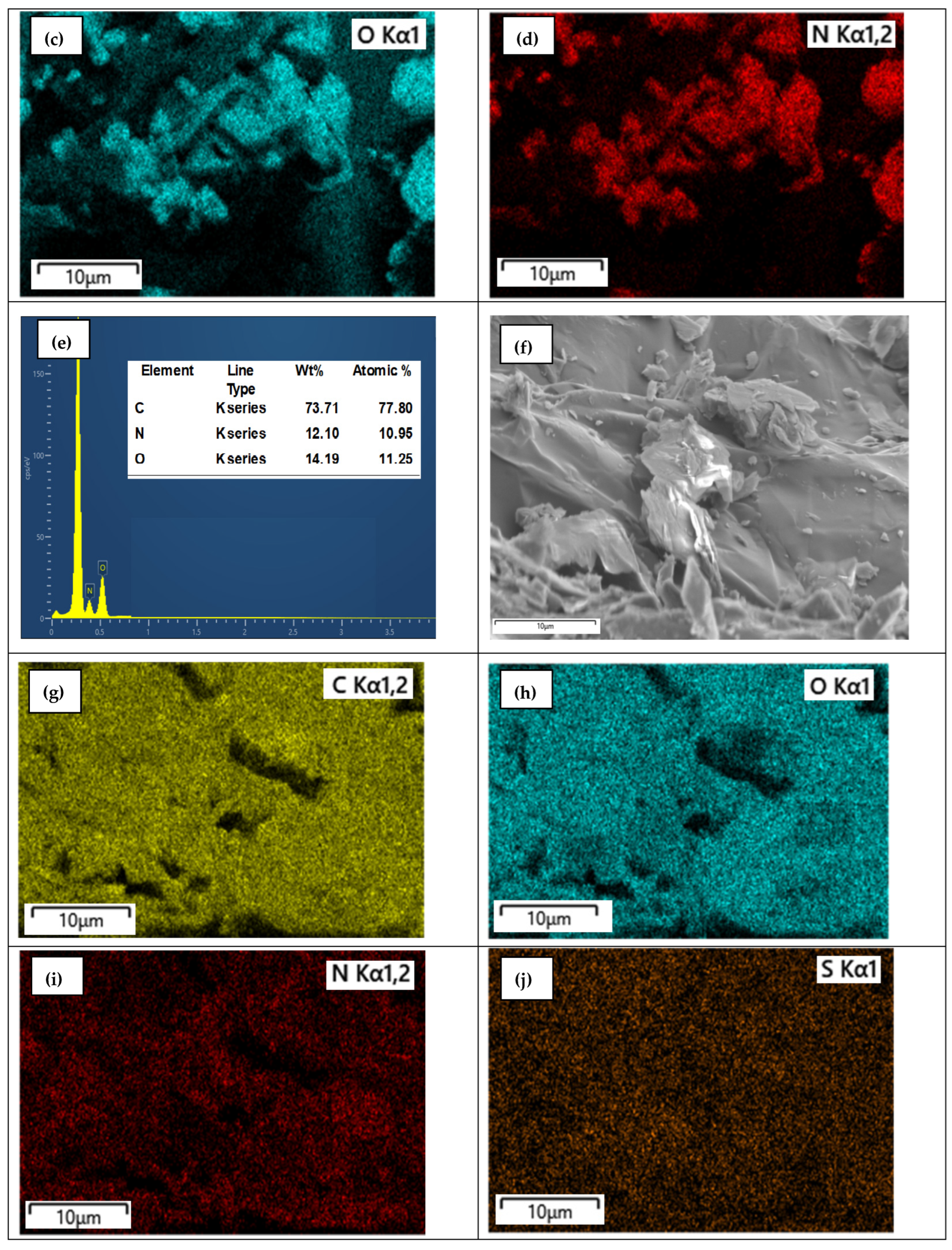
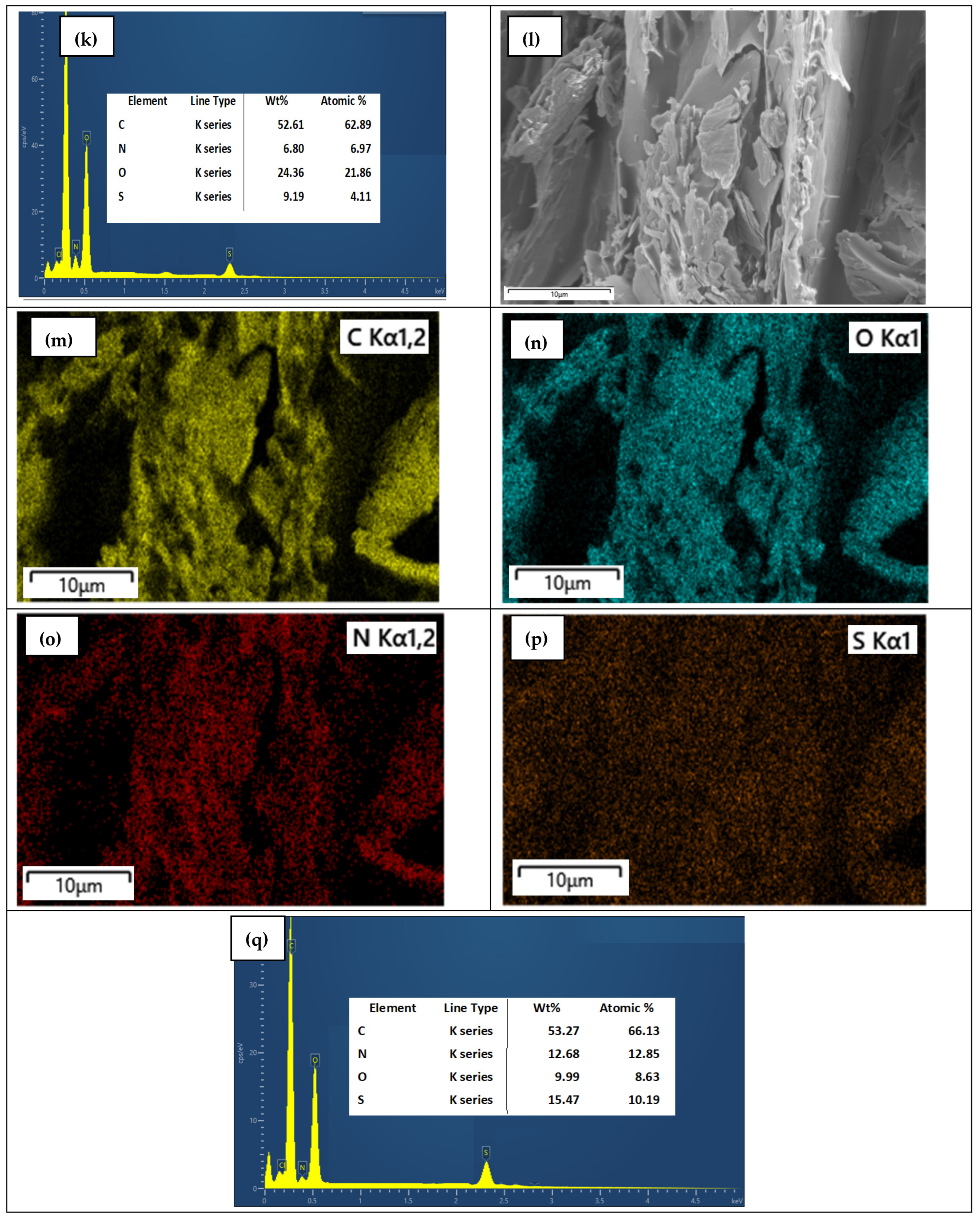
3.6. SEM with Elemental Mapping Analysis
3.7. Cyclic Voltammetry and Electrochemical Sensing of CND/PANI Composites
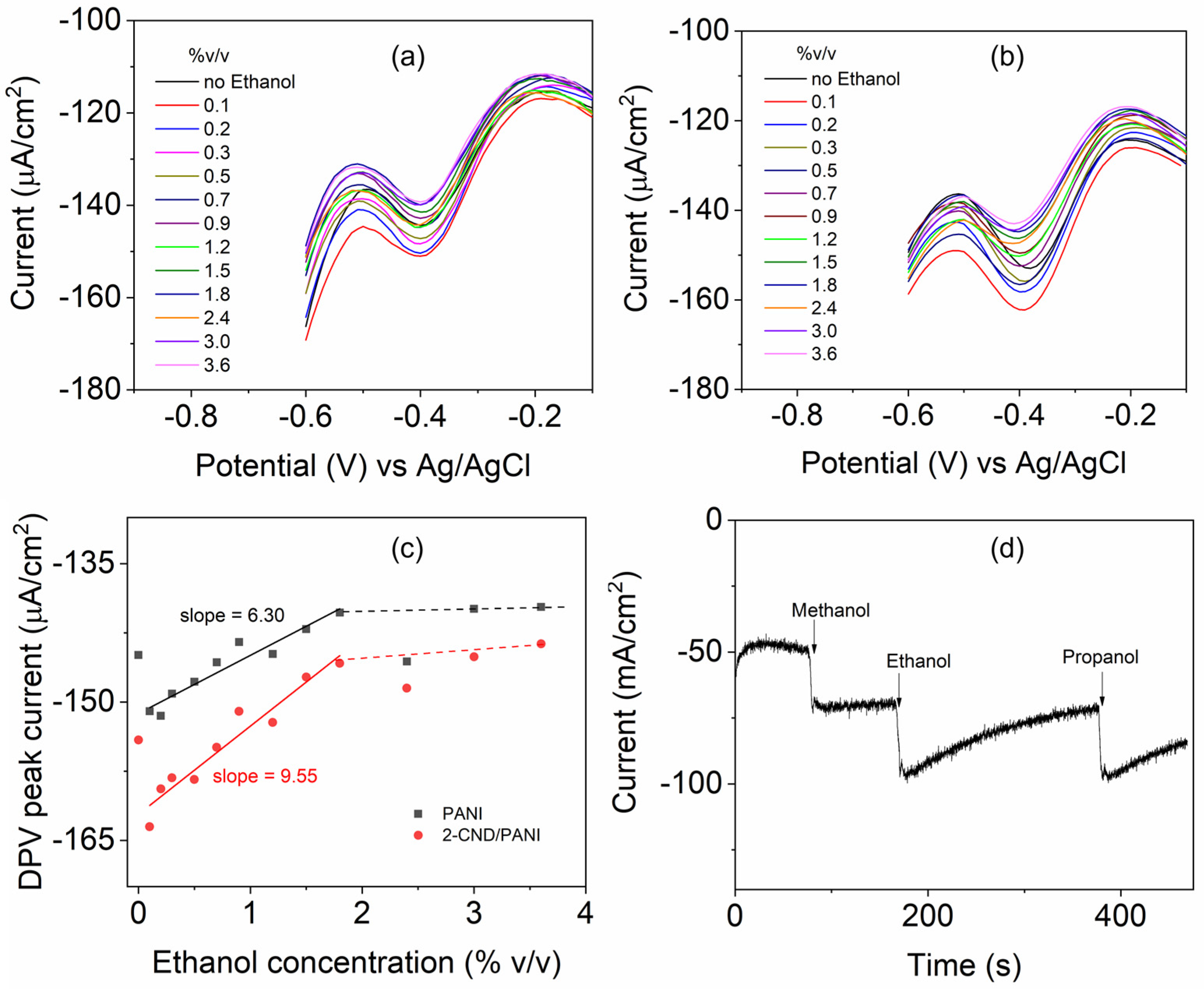
3.8. Electrochemical Sensing of Ethanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chia, M.-R.; Ahmad, I.; Phang, S.-W. Starch/Polyaniline Biopolymer Film as Potential Intelligent Food Packaging with Colourimetric Ammonia Sensor. Polymers 2022, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, T.; Gao, X.; Tang, L.; Li, X.; Li, J. Dual-mode smart packaging based on tetraphenylethylene-functionalized polyaniline sensing label for monitoring the freshness of fish. Sens. Actuators B Chem. 2020, 323, 128694. [Google Scholar] [CrossRef]
- Abdolsattari, P.; Rezazadeh-Bari, M.; Pirsa, S. Smart Film Based on Polylactic Acid, Modified with Polyaniline/ZnO/CuO: Investigation of Physicochemical Properties and Its Use of Intelligent Packaging of Orange Juice. Food Bioprocess Technol. 2022, 15, 2803–2825. [Google Scholar] [CrossRef]
- de Oliveira, A.C.S.; Ugucioni, J.C.; da Rocha, R.A.; Santos, T.A.; Borges, S.V. Chitosan/Polyaniline Conductive Blends for Developing Packaging: Electrical, Morphological, Structural and Thermal Properties. J. Polym. Environ. 2019, 27, 2250–2258. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Rajeh, A. Preparation and characterization of polyaniline/sodium alginate doped TiO2 nanoparticles with promising mechanical and electrical properties and antimicrobial activity for food packaging applications. J. Mater. Sci. Mater. Electron. 2020, 31, 9430–9442. [Google Scholar] [CrossRef]
- Rehim, M.H.A.; Yassin, M.A.; Zahran, H.; Kamel, S.; Turky, M.E.M.G. Rational design of active packaging films based on polyaniline-coated polymethyl methacrylate/nanocellulose composites. Polym. Bull. 2020, 77, 2485–2499. [Google Scholar] [CrossRef]
- Chia, M.-R.; Phang, S.-W.; Ahmad, I. Emerging Applications of Versatile Polyaniline-Based Polymers in the Food Industry. Polymers 2022, 14, 5168. [Google Scholar] [CrossRef]
- Beygisangchin, M. Recent progress in polyaniline and its composites; Synthesis, properties, and applications. Eur. Polym. J. 2024, 210, 112948. [Google Scholar] [CrossRef]
- Riaz, U.; Nwaoha, C.; Ashraf, S.M. Recent advances in corrosion protective composite coatings based on conducting polymers and natural resource derived polymers. Prog. Org. Coat. 2014, 77, 743–756. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Ahmad, S. High performance corrosion protective DGEBA/polypyrrole composite coatings. Prog. Org. Coat. 2007, 59, 138–145. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Ahmad, S.; Riaz, U. Development of novel conducting composites of linseed-oil-based poly (urethane amide) with nanostructured poly (1-naphthylamine). Polym. Int. 2007, 56, 1173–1181. [Google Scholar] [CrossRef]
- Singh, N.; Riaz, U. Recent trends on synthetic approaches and application studies of conducting polymers and copolymers: A review. Polym. Bull. 2022, 79, 10377–10408. [Google Scholar] [CrossRef]
- Zia, J.; Riaz, U. Photocatalytic degradation of anti-inflammatory drug using POPD/Sb2O3 organic-inorganic nanohybrid under solar light. J. Mater. Res. Technol. 2019, 8, 4079–4093. [Google Scholar] [CrossRef]
- Zia, J.; Aazam, E.S.; Riaz, U. Facile synthesis of MnO2 nanorods and ZnMn2O4 nano-hexagons: A comparison of microwave-assisted catalytic activity against 4-nitrophenol degradation. J. Mater. Res. Technol. 2020, 9, 9709–9719. [Google Scholar] [CrossRef]
- Riaz, U.; Nabi, N.; Nwanze, F.R.; Yan, F. Experimental and biophysical interaction studies of alanine modified polyaniline with bovine serum albumin and human serum albumin: Influence of alanine modification on the spectral, morphological and electronic properties. Synth. Met. 2023, 292, 117248. [Google Scholar] [CrossRef]
- Mir, A.; Fletcher, W.J.; Taylor, D.K.; Alam, J.; Riaz, U. Sustained Release Studies of Metformin Hydrochloride Drug Using Conducting Polymer/Gelatin-Based Composite Hydrogels. ACS Omega 2024, 9, 18766–18776. [Google Scholar] [CrossRef]
- Mir, A.; Aazam, E.S.; Riaz, U. Design of Phytic Acid Crosslinked Xerogels as Organic Photocatalysts for Visible Light-Assisted Degradation of Dyes. Adv. Mater. Interf. 2024, 11, 2400046. [Google Scholar] [CrossRef]
- Wang, L.; Fu, H.; Liu, H.; Yu, K.; Wang, Y.; Ma, J. In-situ packaging ultra-uniform 3D hematite nanotubes by polyaniline and their improved gas sensing properties. Mater. Res. Bull. 2018, 107, 46–53. [Google Scholar] [CrossRef]
- Taj, M.N.; Prasad, B.D.; Nagabhushana, H.; Reddy, A.; Ashwini, K.R.; Vinuta, K. Dielectric and structural properties of polyaniline-tungsten trioxide nanocomposites: For the packing of nanoelectronic devices and EMI shielding. Nano-Struct. Nano-Objects 2024, 39, 101219. [Google Scholar]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.-R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Kim, M.; Kim, J.; Ryu, J.; Shina, K.-Y.; Jang, J. Highly porous nanostructured polyaniline/carbon nanodots as efficient counter electrodes for Pt-free dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 19018–19026. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Qu, S.N. Supra-(carbon nanodots) with a strong visible to near-infrared absorption band and efficient photothermal conversion. Light. Sci. Appl. 2016, 5, e16120. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue light-emitting carbon dots (CDs) from a milk protein and their interaction with Spinacia oleracea leaf cells. Int. Nano. Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef]
- Sukhorukov, G.B. Carbon nanodots: Mechanisms of photoluminescence and principles of application. Trac Trends Anal. Chem. 2017, 90, 27–37. [Google Scholar]
- Gu, X.; Chen, Z.; Li, Y.; Wu, J.; Wang, X.; Huang, H.; Liu, Y.; Dong, B.; Shao, M.; Kang, Z. Polyaniline/Carbon Dots Composite as a Highly Efficient Metal-Free Dual-Functional Photoassisted Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 24814–24823. [Google Scholar] [CrossRef]
- Strauss, V.; Wang, H.; Delacroix, S.; Ledendecker, M.; Wessig, P. Carbon nanodots revised: The thermal citric acid/urea reaction†. Chem. Sci. 2020, 11, 8256. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for a toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowicz, R. Highly occupied surface states at deuterium-grown boron-doped diamond interfaces for efficient photoelectrochemistry. Small 2023, 19, 2208265. [Google Scholar] [CrossRef]
- Shringi, A.K.; Kumar, R.; Dennis, N.F.; Yan, F. Two-Dimensional Tellurium Nanosheets for the Efficient Nonenzymatic Electrochemical Detection of H2O2. Chemosensors 2024, 12, 17. [Google Scholar] [CrossRef]
- Walimbe, P.D.; Kumar, R.; Shringi, A.K.; Keelson, O.; Ouma, H.A.; Yan, F. Electrochemical Detection of H2O2 Using Bi2O3/Bi2O2Se Nanocomposites. Nanomaterials 2024, 14, 1592. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, S.; Taylor, M.; Kumar, R.; Shringi, A.K.; Leung, T.; Riaz, U. Synthesis and Characterization of Polyaniline/Carbon Nanodots: Electrochemical Sensing of Alcohols for Freshness Monitoring for Application as Packaging Materials. Nanomaterials 2025, 15, 593. https://doi.org/10.3390/nano15080593
Jackson S, Taylor M, Kumar R, Shringi AK, Leung T, Riaz U. Synthesis and Characterization of Polyaniline/Carbon Nanodots: Electrochemical Sensing of Alcohols for Freshness Monitoring for Application as Packaging Materials. Nanomaterials. 2025; 15(8):593. https://doi.org/10.3390/nano15080593
Chicago/Turabian StyleJackson, Shaila, Mary Taylor, Rajeev Kumar, Amit Kumar Shringi, TinChung Leung, and Ufana Riaz. 2025. "Synthesis and Characterization of Polyaniline/Carbon Nanodots: Electrochemical Sensing of Alcohols for Freshness Monitoring for Application as Packaging Materials" Nanomaterials 15, no. 8: 593. https://doi.org/10.3390/nano15080593
APA StyleJackson, S., Taylor, M., Kumar, R., Shringi, A. K., Leung, T., & Riaz, U. (2025). Synthesis and Characterization of Polyaniline/Carbon Nanodots: Electrochemical Sensing of Alcohols for Freshness Monitoring for Application as Packaging Materials. Nanomaterials, 15(8), 593. https://doi.org/10.3390/nano15080593






