Ultramicroporous Ionic Liquid-Supported Aerogel Composites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of HAILs and UILACs
2.3. Characterization Methods of HAILs and UILACs
2.4. NH3 Adsorption and Desorption Methods of HAILs and UILACs
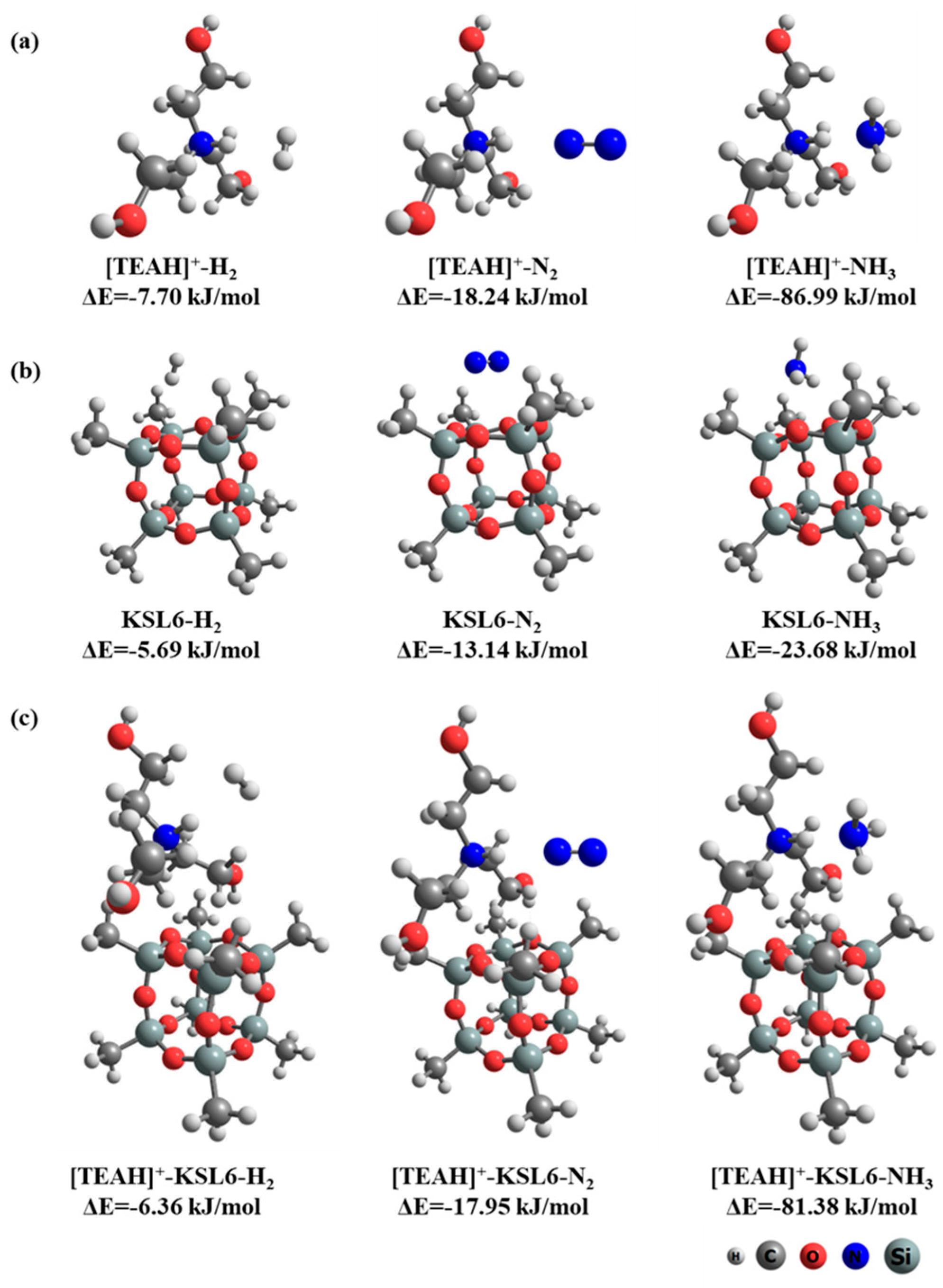
2.5. Simulated Calculations
3. Results and Discussion
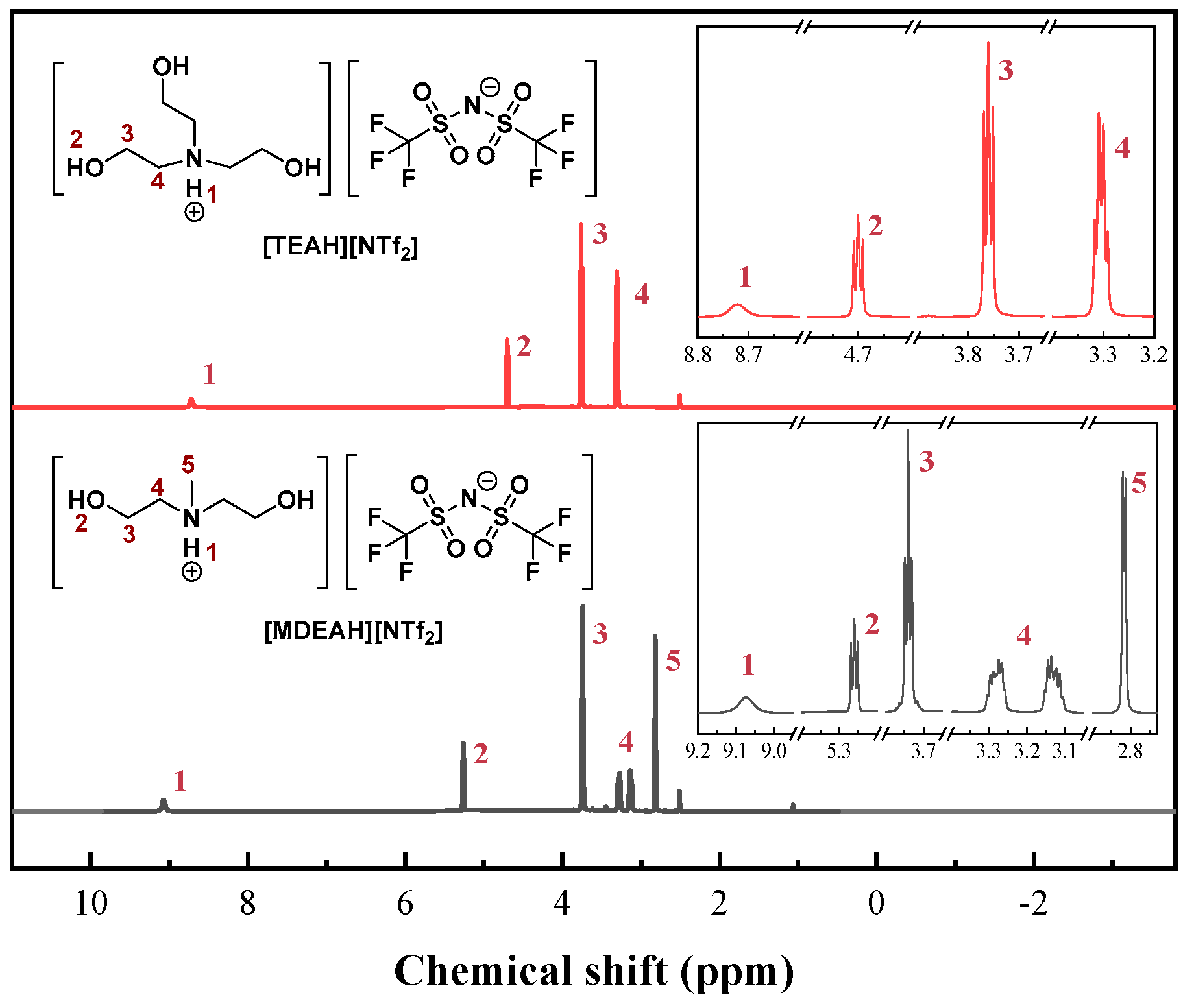
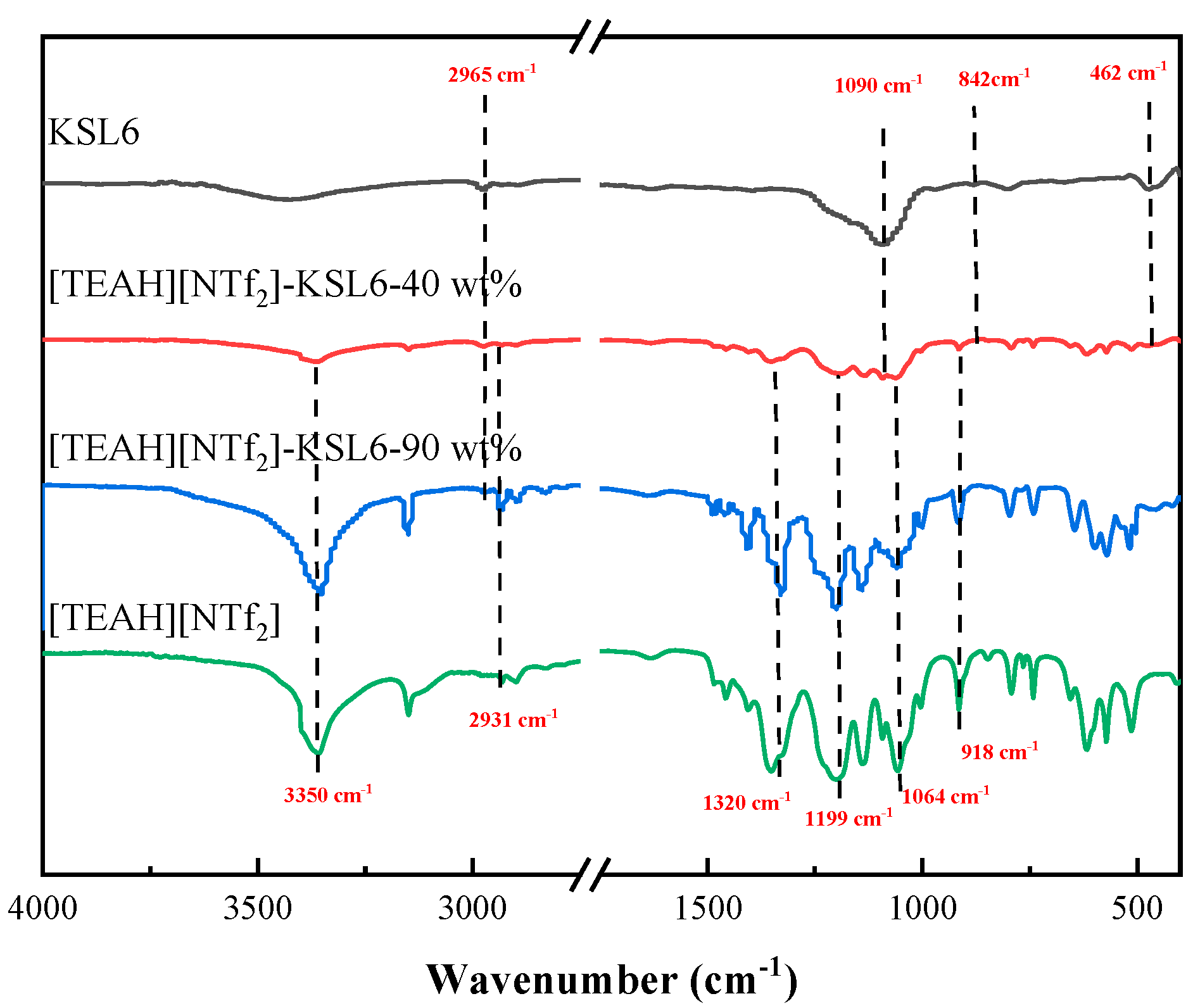
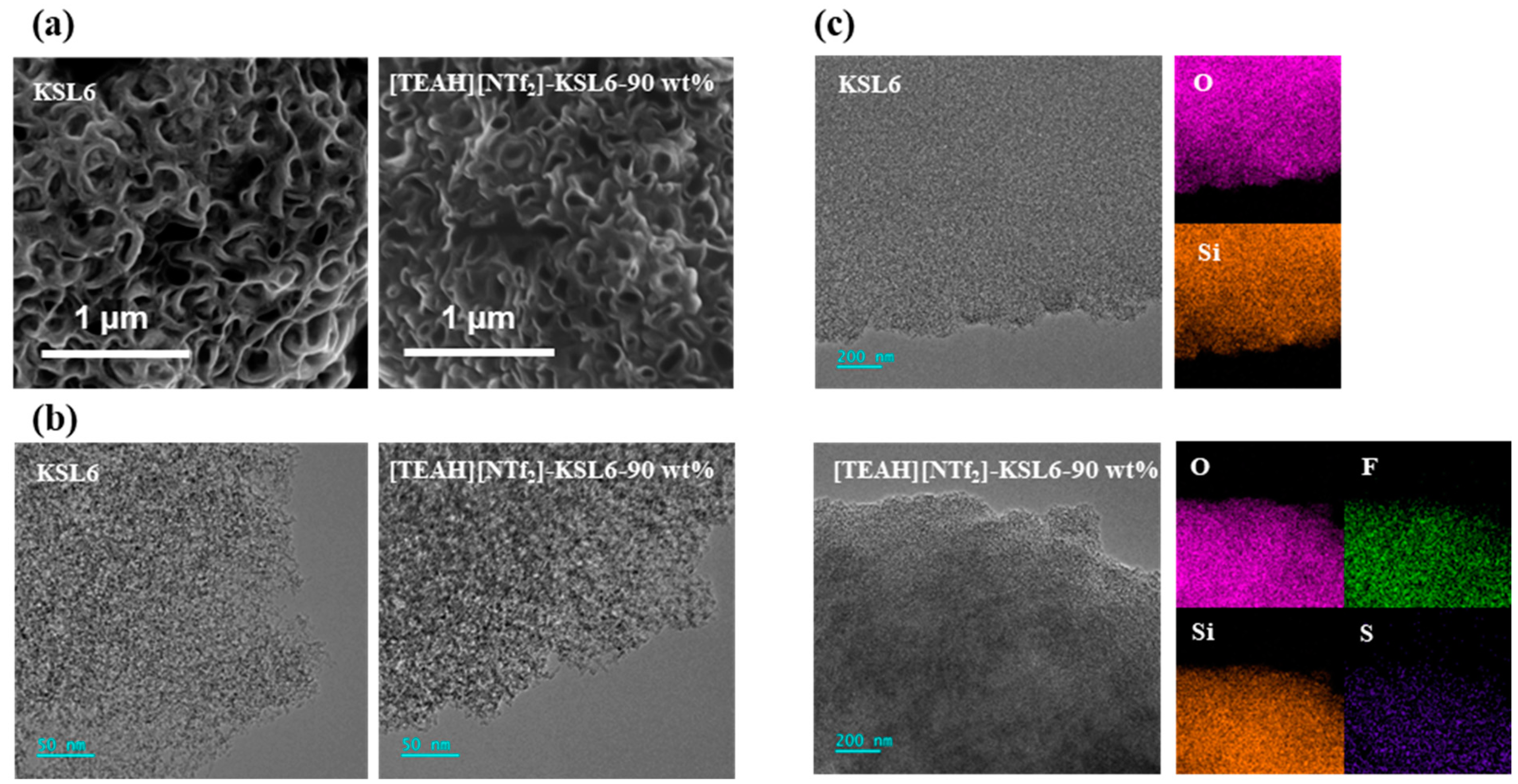
3.1. Characterizations of HAILs and UILACs
3.2. Properties of HAILs and UILACs
3.3. NH3 Adsorption Performance of UILACs
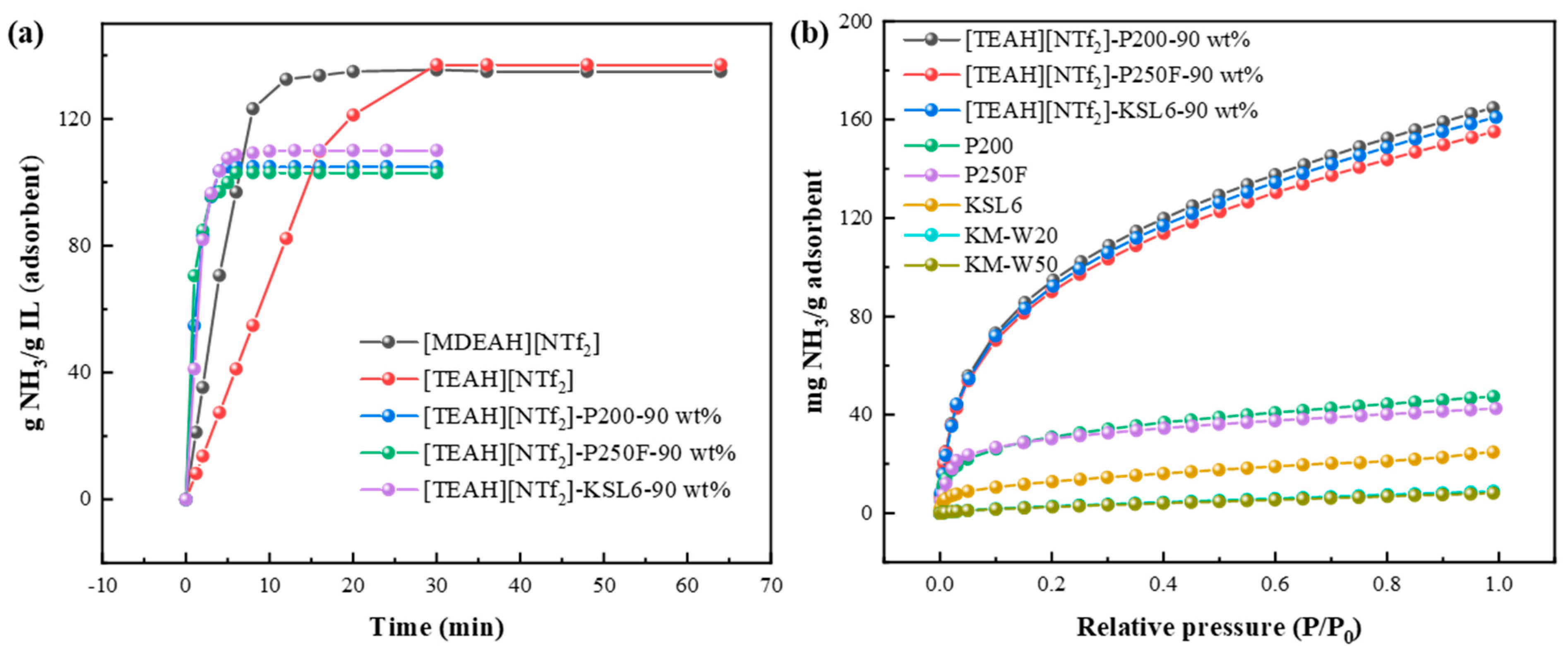
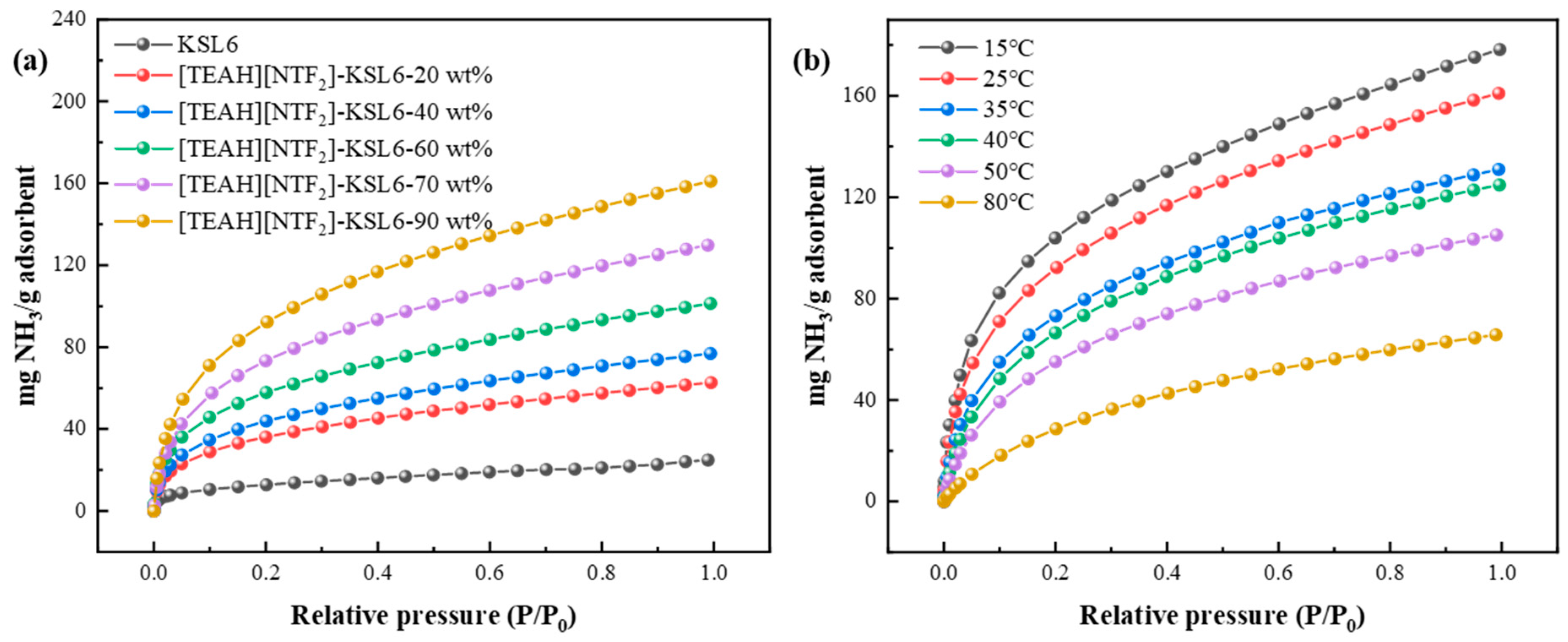
3.3.1. Effect of HAILs and Aerogel Supports
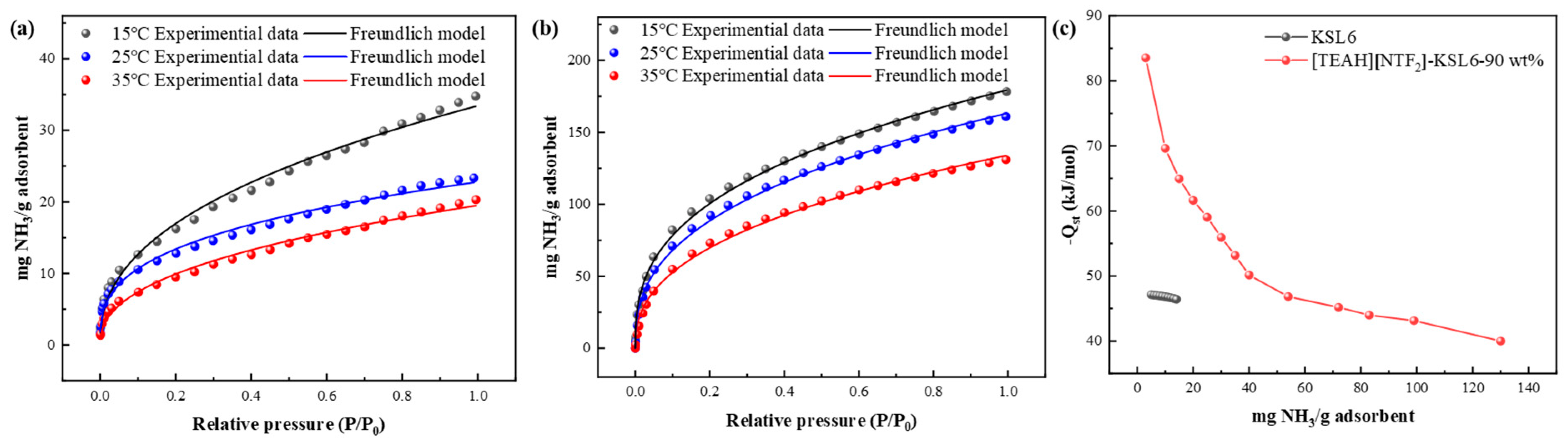
3.3.2. Effect of HAIL Loading and Temperature on NH3 Adsorption
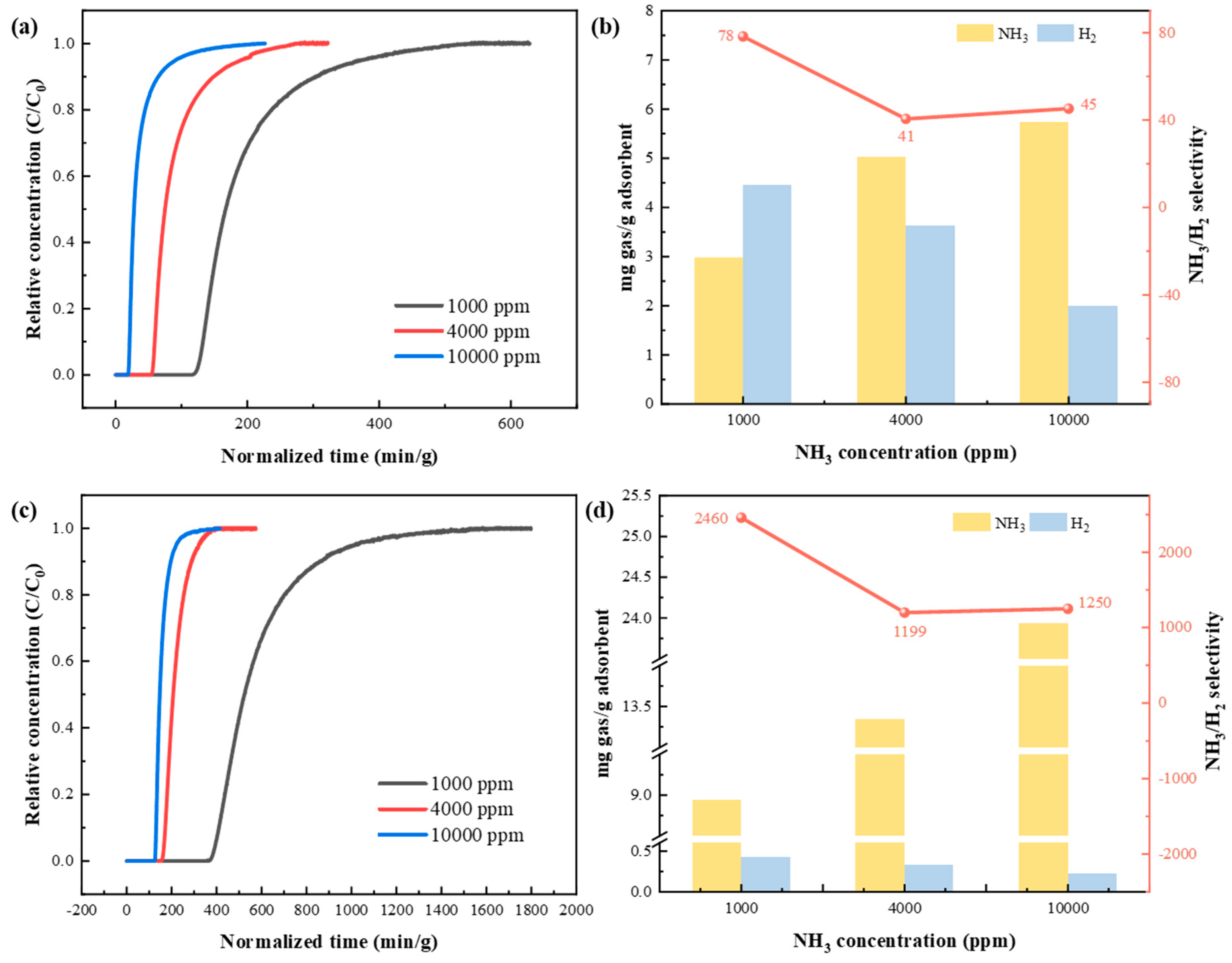
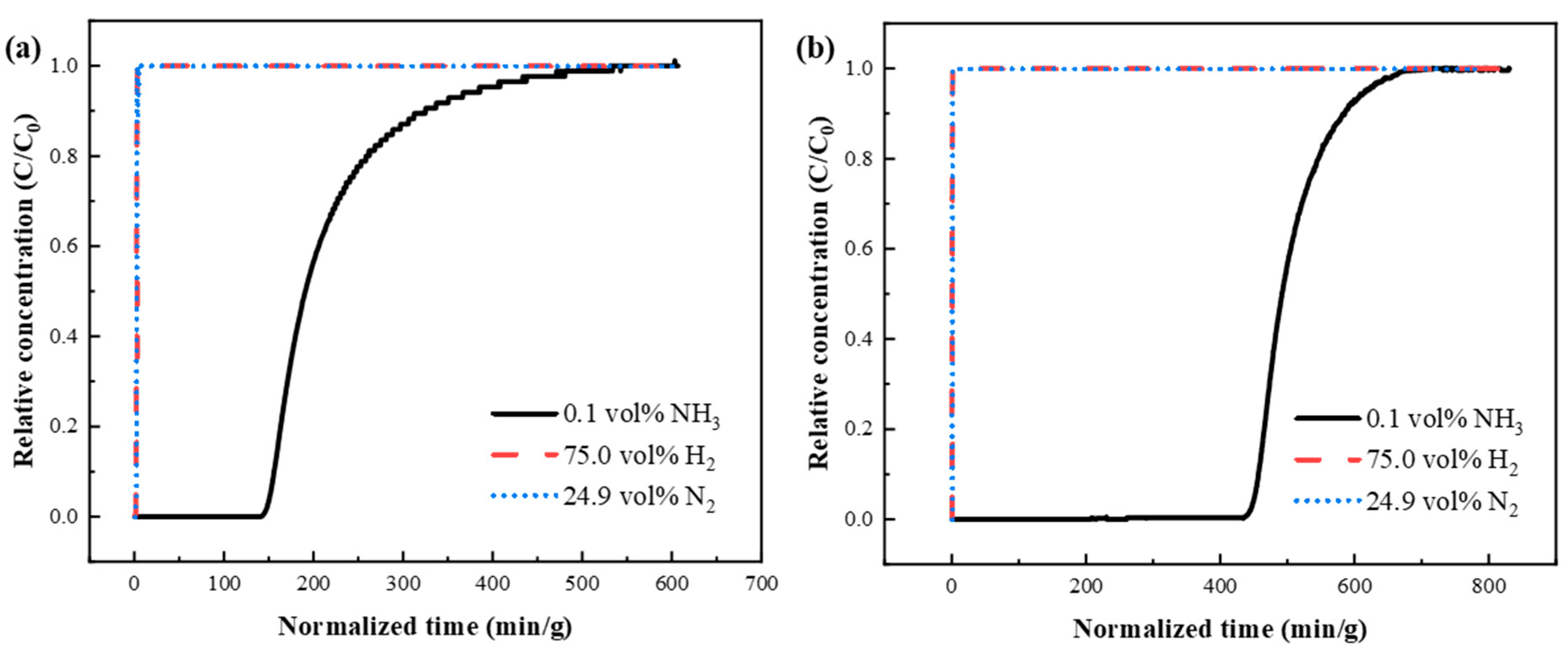
3.3.3. Breakthrough Experiments of Binary and Ternary Mixed Gases
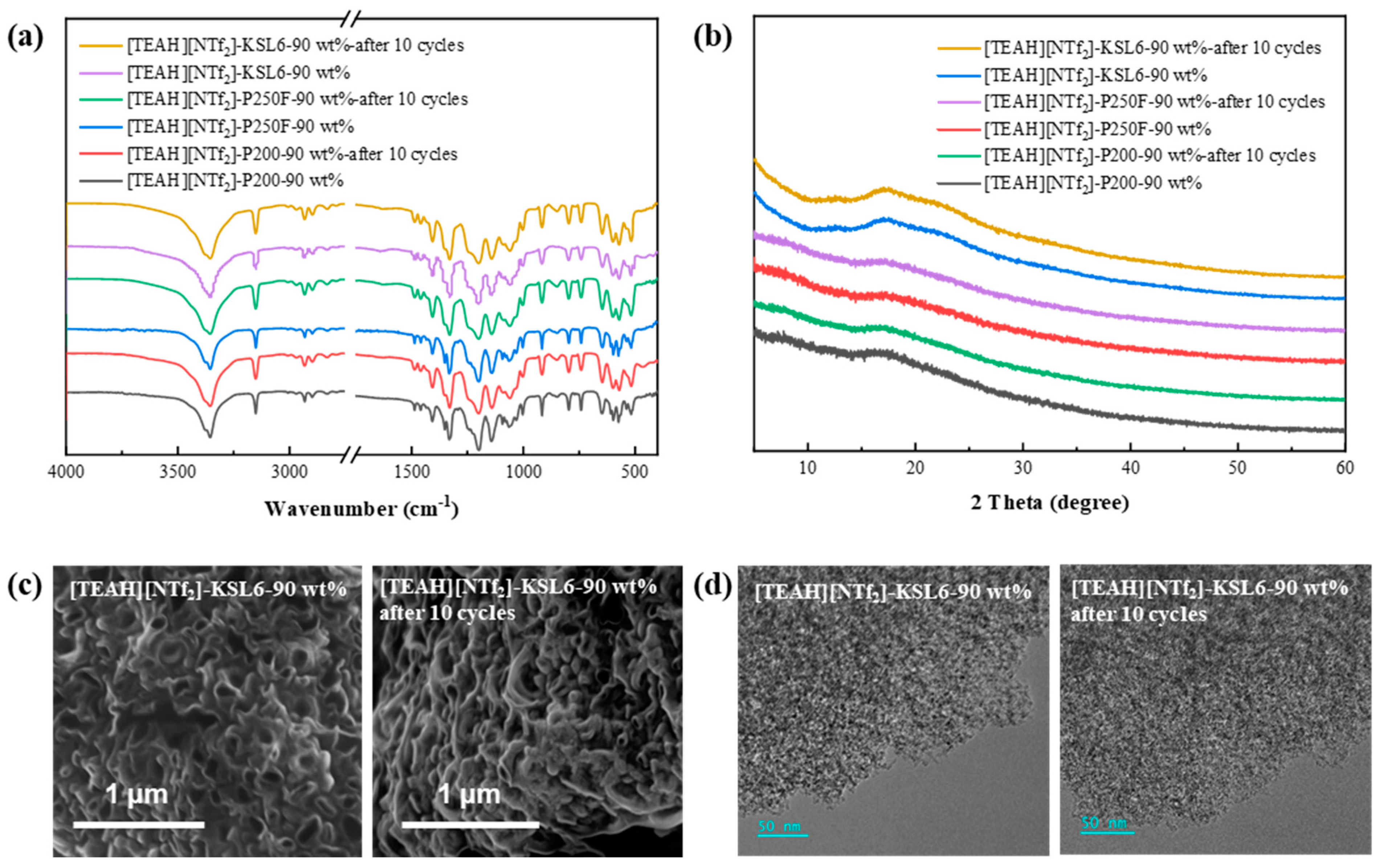
3.3.4. Regeneration and Recycling of UILACs
3.3.5. Comparison of NH3 Adsorption Performance
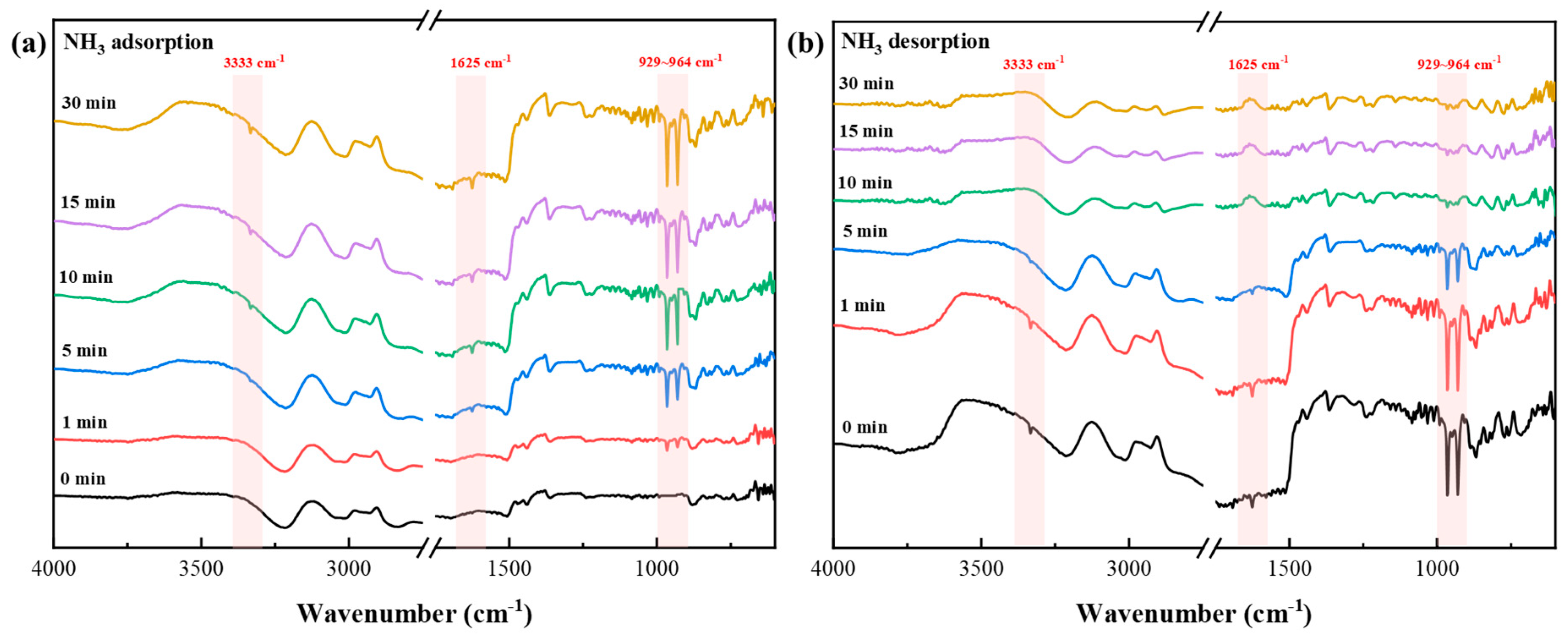
3.4. NH3 Adsorption Mechanisms by UILACs
4. Conclusions
- The ultra-low density of aerogels enables high HAIL loading;
- The multifunctional sites in HAIL enhance NH3 molecular adsorption through hydrogen bond interactions;
- UILACs demonstrated excellent durability and stability over multiple adsorption–desorption cycles.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of Electrochemical Ammonia Production Technologies and Materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Yan, W. Tin Diselinide a Stable Co-Catalyst Coupled with Branched TiO2 Fiber and g-C3N4 Quantum Dots for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2020, 270, 118900. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for Hydrogen Storage: Challenges and Opportunities. J. Mater. Chem. 2008, 18, 2304. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen Storage Materials for Hydrogen and Energy Carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Wang, P.; Chang, F.; Gao, W.; Guo, J.; Wu, G.; He, T.; Chen, P. Breaking Scaling Relations to Achieve Low-Temperature Ammonia Synthesis through LiH-Mediated Nitrogen Transfer and Hydrogenation. Nat. Chem. 2017, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia Combustion Properties and Performance in Gas-Turbine Burners. Symp. Int. Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Fu, S.; Teng, P.; Peng, Q.; Zhang, L.; Yin, R.; Tu, Y. Investigation on Combustion Characteristics and Energy Efficiency Improvement of H2/NH3 Fueled Micro Power Generator with Bluff Body and Porous Media. Int. J. Hydrogen Energy 2024, 80, 1356–1367. [Google Scholar]
- Gu, B.; Sutton, M.A.; Chang, S.X.; Ge, Y.; Chang, J. Agricultural Ammonia Emissions Contribute to China’s Urban Air Pollution. Front. Ecol. Environ. 2014, 12, 265–266. [Google Scholar] [CrossRef]
- Kang, D.W.; Holbrook, J.H. Use of NH3 Fuel to Achieve Deep Greenhouse Gas Reductions from US Transportation. Energy Rep. 2015, 1, 164–168. [Google Scholar] [CrossRef]
- Mondal, S.; Malakar, S. Synthesis of Sulfonamide and Their Synthetic and Therapeutic Applications: Recent Advances. Tetrahedron 2020, 76, 131662. [Google Scholar] [CrossRef]
- Engels, H.; Pirkl, H.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Soujoudi, R.; Manteufel, R. Thermodynamic, Economic and Environmental Analyses of Ammonia-Based Mixed Refrigerant for Liquefied Natural Gas Pre-Cooling Cycle. Processes 2021, 9, 1298. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A Review on Clean Ammonia as a Potential Fuel for Power Generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Zhai, L.; Shek Wong, C.; Zhang, H.; Xiong, P.; Xue, X.; Lun Ho, Y.; Xu, C.; Chi Fong, Y.; Mei, J.; Wa Chan, W.; et al. From Lab to Practical: An Ammonia-Powered Fuel Cell Electric Golf Cart System. Chem. Eng. J. 2023, 452, 139390. [Google Scholar] [CrossRef]
- Cai, P.; Momen, R.; Li, M.; Tian, Y.; Yang, L.; Zou, K.; Deng, X.; Wang, B.; Hou, H.; Zou, G.; et al. Functional Carbon Materials Processed by NH3 Plasma for Advanced Full-Carbon Sodium-Ion Capacitors. Chem. Eng. J. 2021, 420, 129647. [Google Scholar] [CrossRef]
- Yang, B.; Bai, L.; Zeng, S.; Luo, S.; Liu, L.; Han, J.; Nie, Y.; Zhang, X.; Zhang, S. NH3 Separation Membranes with Self-Assembled Gas Highways Induced by Protic Ionic Liquids. Chem. Eng. J. 2021, 421, 127876. [Google Scholar] [CrossRef]
- Jiang, W.; Zhong, F.; Liu, Y.; Huang, K. Effective and Reversible Capture of NH3 by Ethylamine Hydrochloride Plus Glycerol Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 10552–10560. [Google Scholar] [CrossRef]
- Uribe, F.A.; Gottesfeld, S.; Zawodzinski, T.A. Effect of Ammonia as Potential Fuel Impurity on Proton Exchange Membrane Fuel Cell Performance. J. Electrochem. Soc. 2002, 149, A293. [Google Scholar] [CrossRef]
- Omata, K.; Sato, K.; Nagaoka, K.; Yukawa, H.; Matsumoto, Y.; Nambu, T. Direct High-Purity Hydrogen Production from Ammonia by Using a Membrane Reactor Combining V-10mol%Fe Hydrogen Permeable Alloy Membrane with Ru/Cs2O/Pr6O11 Ammonia Decomposition Catalyst. Int. J. Hydrogen Energy 2022, 47, 8372–8381. [Google Scholar] [CrossRef]
- Vikrant, K.; Kumar, V.; Kim, K.-H.; Kukkar, D. Metal–Organic Frameworks (MOFs): Potential and Challenges for Capture and Abatement of Ammonia. J. Mater. Chem. A 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Tian, J.; Liu, B. Ammonia Capture with Ionic Liquid Systems: A Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 767–809. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, L.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. A Mini-Review on NH3 Separation Technologies: Recent Advances and Future Directions. Energy Fuels 2022, 36, 14516–14533. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Sun, X.; Zeng, S.; Guo, Y.; Bai, L.; Yao, M.-S.; Zhang, X.-P. Advanced Materials for NH3 Capture: Interaction Sites and Transport Pathways. Nano-Micro Lett. 2024, 16, 228. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Zeng, S.; Hu, Z.; Hussain, S.; Zhang, X. An Overview of Ammonia Separation by Ionic Liquids. Ind. Eng. Chem. Res. 2021, 60, 6908–6924. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Zeng, S.; Zhang, X.; Liang, X.; Gani, R.; Kontogeorgis, G.M. Separation of NH3/CO2 from Melamine Tail Gas with Ionic Liquid: Process Evaluation and Thermodynamic Properties Modelling. Sep. Purif. Technol. 2021, 274, 119007. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, X.; Zeng, S.; Jiang, K.; Gao, H.; Dong, H.; Yang, Q.; Zhang, S. Protic Ionic Liquid [Bim][NTf2] with Strong Hydrogen Bond Donating Ability for Highly Efficient Ammonia Absorption. Green Chem. 2017, 19, 937–945. [Google Scholar] [CrossRef]
- Sun, X.; Li, G.; Zeng, S.; Yuan, L.; Bai, L.; Zhang, X. Ultra-High NH3 Absorption by Triazole Cation-Functionalized Ionic Liquids through Multiple Hydrogen Bonding. Sep. Purif. Technol. 2023, 307, 122825. [Google Scholar] [CrossRef]
- Biswas, S.; Ahnfeldt, T.; Stock, N. New Functionalized Flexible Al-MIL-53-X (X = -Cl, -Br, -CH3, -NO2, -(OH)2) Solids: Syntheses, Characterization, Sorption, and Breathing Behavior. Inorg. Chem. 2011, 50, 9518–9526. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, S.; Huo, F.; Shang, D.; He, H.; Bai, L.; Zhang, X.; Li, J. Metal Chloride Anion-Based Ionic Liquids for Efficient Separation of NH3. J. Clean. Prod. 2019, 206, 661–669. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, L.; Shang, D.; Feng, J.; Dong, H.; Xu, Q.; Zhang, X.; Zhang, S. Efficient and Reversible Absorption of Ammonia by Cobalt Ionic Liquids through Lewis Acid–Base and Cooperative Hydrogen Bond Interactions. Green Chem. 2018, 20, 2075–2083. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Li, P.; Dong, H.; Wang, H.; Zhang, X.; Zhang, X. Efficient Adsorption of Ammonia by Incorporation of Metal Ionic Liquids into Silica Gels as Mesoporous Composites. Chem. Eng. J. 2019, 370, 81–88. [Google Scholar] [CrossRef]
- Ruckart, K.N.; Zhang, Y.; Reichert, W.M.; Peterson, G.W.; Glover, T.G. Sorption of Ammonia in Mesoporous-Silica Ionic Liquid Composites. Ind. Eng. Chem. Res. 2016, 55, 12191–12204. [Google Scholar] [CrossRef]
- Cao, D.; Li, Z.; Wang, Z.; Wang, H.; Gao, S.; Wang, Y.; Shi, Y.; Qiu, J.; Zhao, Y.; Wang, J. Highly Dispersed Ionic Liquids in Mesoporous Molecular Sieves Enable a Record NH3 Absorption. ACS Sustain. Chem. Eng. 2021, 9, 16363–16372. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Martin-Calvo, A.; Ania, C.O.; Parra, J.B.; Vicent-Luna, J.M.; Calero, S. Role of Hydrogen Bonding in the Capture and Storage of Ammonia in Zeolites. Chem. Eng. J. 2020, 387, 124062. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, Q.; Zeng, S.; Li, G.; Jiang, H.; Sun, X.; Zhang, X. Porous Multi-Site Ionic Liquid Composites for Superior Selective and Reversible Adsorption of Ammonia. Sep. Purif. Technol. 2023, 310, 123161. [Google Scholar] [CrossRef]
- Yu, M.; Zeng, S.; Wang, Z.; Hu, Z.; Dong, H.; Nie, Y.; Ren, B.; Zhang, X. Protic Ionic-Liquid-Supported Activated Carbon with Hierarchical Pores for Efficient NH3 Adsorption. ACS Sustain. Chem. Eng. 2019, 7, 11769–11777. [Google Scholar] [CrossRef]
- Qajar, A.; Peer, M.; Andalibi, M.R.; Rajagopalan, R.; Foley, H.C. Enhanced Ammonia Adsorption on Functionalized Nanoporous Carbons. Microporous Mesoporous Mater. 2015, 218, 15–23. [Google Scholar] [CrossRef]
- Milescu, R.A.; Dennis, M.R.; McElroy, C.R.; Macquarrie, D.J.; Matharu, A.S.; Smith, M.W.; Clark, J.H.; Budarin, V.L. The Role of Surface Functionality of Sustainable Mesoporous Materials Starbon® on the Adsorption of Toxic Ammonia and Sulphur Gasses. Sustain. Chem. Pharm. 2020, 15, 100230. [Google Scholar] [CrossRef]
- Dhahir, S.A.; Braihi, A.J.; Habeeb, S.A. Comparative Analysis of Hydrogel Adsorption/Desorption with and without Surfactants. Gels 2024, 10, 251. [Google Scholar] [CrossRef]
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.; Argüelles-Monal, W.M. N-(Furfural) Chitosan Hydrogels Based on Diels–Alder Cycloadditions and Application as Microspheres for Controlled Drug Release. Carbohydr. Polym. 2015, 128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Olivito, F.; Jagdale, P.; Oza, G. Direct Production of Furfural from Fructose Catalyzed by Iron(III) Sulfate Using a Simple Distillation Apparatus. ACS Sustain. Chem. Eng. 2023, 11, 17595–17599. [Google Scholar] [CrossRef]
- Kohler, F.T.U.; Popp, S.; Klefer, H.; Eckle, I.; Schrage, C.; Böhringer, B.; Roth, D.; Haumann, M.; Wasserscheid, P. Supported Ionic Liquid Phase (SILP) Materials for Removal of Hazardous Gas Compounds–Efficient and Irreversible NH3 Adsorption. Green Chem. 2014, 16, 3560. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, S.; Zheng, S.; Zhao, T.; Sun, X.; Bai, L.; Deng, C.; Zhang, X. Mesoporous Multiproton Ionic Liquid Hybrid Adsorbents for Facilitating NH3 Separation. Ind. Eng. Chem. Res. 2023, 62, 2829–2842. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Z.; Zhang, M.; He, G.; Yuan, S. Two Isostructural Aluminum-Based Metal—Organic Frameworks with Multiple Polar Sites for Reversible NH3 Capture. Chem. Eng. J. 2025, 510, 161602. [Google Scholar] [CrossRef]
- Zheng, S. Superior Selective Adsorption of Trace CO2 Induced by Chemical Interaction and Created Ultra-Micropores of Ionic Liquid Composites. Chem. Eng. J. 2023, 451, 138736. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Dichiara, A.B.; Jiang, W.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hou, Y.; Liu, Z.; Yuan, R.; Du, Y.; Ji, X.; Sheng, Z.; Zhang, X. Liquid-in-Aerogel Porous Composite Allows Efficient CO2 Capture and CO2/N2 Separation. Small 2023, 19, 2302627. [Google Scholar] [CrossRef]
- Ridha, F.N.; Yang, Y.; Webley, P.A. Adsorption Characteristics of a Fully Exchanged Potassium Chabazite Zeolite Prepared from Decomposition of Zeolite Y. Microporous Mesoporous Mater. 2009, 117, 497–507. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Zeng, S.; Liu, Y.; Dong, H.; Deng, C. Protic Ionic Liquid-Based Deep Eutectic Solvents with Multiple Hydrogen Bonding Sites for Efficient Absorption of NH3. AIChE J. 2020, 66, e16253. [Google Scholar] [CrossRef]
- Shang, D.; Zeng, S.; Zhang, X.; Zhang, X.; Bai, L.; Dong, H. Highly Efficient and Reversible Absorption of NH3 by Dual Functionalised Ionic Liquids with Protic and Lewis Acidic Sites. J. Mol. Liq. 2020, 312, 113411. [Google Scholar] [CrossRef]
- Gruzdev, M.S.; Shmukler, L.E.; Kudryakova, N.O.; Kolker, A.M.; Sergeeva, Y.A.; Safonova, L.P. Triethanolamine-Based Protic Ionic Liquids with Various Sulfonic Acids: Synthesis and Properties. J. Mol. Liq. 2017, 242, 838–844. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Albanov, A.I.; Aksamentova, T.N.; Adamovich, S.N.; Chipanina, N.N.; Mirskov, R.G.; Kochina, T.A.; Vrazhnov, D.V.; Litvinov, M.Y. Tris(2-Hydroxyethyl)Ammonium Salts: 2,8,9-Trihydroprotatranes. Russ. J. Gen. Chem. 2009, 79, 2339–2346. [Google Scholar] [CrossRef]
- Li, P.; Shang, D.; Tu, W.; Zeng, S.; Nie, Y.; Bai, L.; Dong, H.; Zhang, X. NH3 Absorption Performance and Reversible Absorption Mechanisms of Protic Ionic Liquids with Six-Membered N-Heterocyclic Cations. Sep. Purif. Technol. 2020, 248, 117087. [Google Scholar] [CrossRef]
- Zhan, W.; Mo, J.; Shi, F.; Xu, Z.; Chen, L.; Li, L.; Zhou, R.; Chen, M.; Jiang, J. Preparation and Thermo-Mechanical Properties of Modified Gelatin Reinforced SiO2 Aerogels. J. Non-Cryst. Solids 2024, 646, 123222. [Google Scholar] [CrossRef]
- Li, Z.; Gadipelli, S.; Li, H.; Howard, C.A.; Brett, D.J.L.; Shearing, P.R.; Guo, Z.; Parkin, I.P.; Li, F. Tuning the Interlayer Spacing of Graphene Laminate Films for Efficient Pore Utilization towards Compact Capacitive Energy Storage. Nat. Energy 2020, 5, 160–168. [Google Scholar] [CrossRef]
- Monson, P.A. Understanding Adsorption/Desorption Hysteresis for Fluids in Mesoporous Materials Using Simple Molecular Models and Classical Density Functional Theory. Microporous Mesoporous Mater. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, T.; Chen, X.; He, Y.; Liang, X. Model Construction of Micro-Pores in Shale: A Case Study of Silurian Longmaxi Formation Shale in Dianqianbei Area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, C.; Xie, Y.; Kang, K.; Song, H.; Bai, S.; Han, H.; Li, S. Efficient Adsorption of Ammonia by Surface-Modified Activated Carbon Fiber Mesh. Nanomaterials 2023, 13, 2857. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, P.; Durães, L.; Simões, P.N. Organically-Modified Silica Aerogels: A Density Functional Theory Study. J. Supercrit. Fluids 2019, 147, 138–148. [Google Scholar] [CrossRef]




| Adsorbents | SBET | Vt | Dp | Td | Vm/Vt Ratio |
|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | (°C) | (%) | |
| KM-W20 | 446.661 | 0.6389 | 3.398 | 280.78 | - |
| KM-W50 | 443.899 | 0.6932 | 3.400 | 216.29 | - |
| P200 | 589.895 | 2.144 | 6.552 | 580.77 | - |
| P250F | 713.004 | 4.0660 | 16.715 | 591.60 | - |
| KSL6 | 716.713 | 6.1190 | 12.694 | 506.92 | - |
| [TEAH][NTf2]-P200-90 wt% | 1.461 | 1.143 × 10−3 | 0.751 | 268.92 | 46.36 |
| [TEAH][NTf2]-P250F-90 wt% | 1.798 | 1.000 × 10−3 | 0.751 | 270.11 | 14.97 |
| [TEAH][NTf2]-KSL6-90 wt% | 3.833 | 3.356 × 10−3 | 0.718 | 258.30 | 25.85 |
| Adsorbents | B Acid | L Acid | Total Acid | NH3 Capacity |
|---|---|---|---|---|
| (μmol/g) | (μmol/g) | (μmol/g) | (mg/g) | |
| P200 | - | 71.67 | 71.67 | 47.429 |
| P250F | - | 63.12 | 63.12 | 42.537 |
| KSL6 | - | 51.21 | 51.21 | 24.849 |
| [TEAH][NTf2]-P200-90 wt% | 97.30 | 90.64 | 187.94 | 164.69 |
| [TEAH][NTf2]-P250F-90 wt% | 79.88 | 75.37 | 153.55 | 155.09 |
| [TEAH][NTf2]-KSL6-90 wt% | 86.29 | 70.24 | 150.53 | 160.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Zeng, S.; Chang, J.; Li, G.; Zhang, W.; Zhang, X. Ultramicroporous Ionic Liquid-Supported Aerogel Composites. Nanomaterials 2025, 15, 526. https://doi.org/10.3390/nano15070526
Pan W, Zeng S, Chang J, Li G, Zhang W, Zhang X. Ultramicroporous Ionic Liquid-Supported Aerogel Composites. Nanomaterials. 2025; 15(7):526. https://doi.org/10.3390/nano15070526
Chicago/Turabian StylePan, Wenshuo, Shaojuan Zeng, Jiang Chang, Guilin Li, Wei Zhang, and Xiangping Zhang. 2025. "Ultramicroporous Ionic Liquid-Supported Aerogel Composites" Nanomaterials 15, no. 7: 526. https://doi.org/10.3390/nano15070526
APA StylePan, W., Zeng, S., Chang, J., Li, G., Zhang, W., & Zhang, X. (2025). Ultramicroporous Ionic Liquid-Supported Aerogel Composites. Nanomaterials, 15(7), 526. https://doi.org/10.3390/nano15070526





