Constructing of Ni-Nx Active Sites in Self-Supported Ni Single-Atom Catalysts for Efficient Reduction of CO2 to CO
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. CO2 Reduction Reaction Measurements
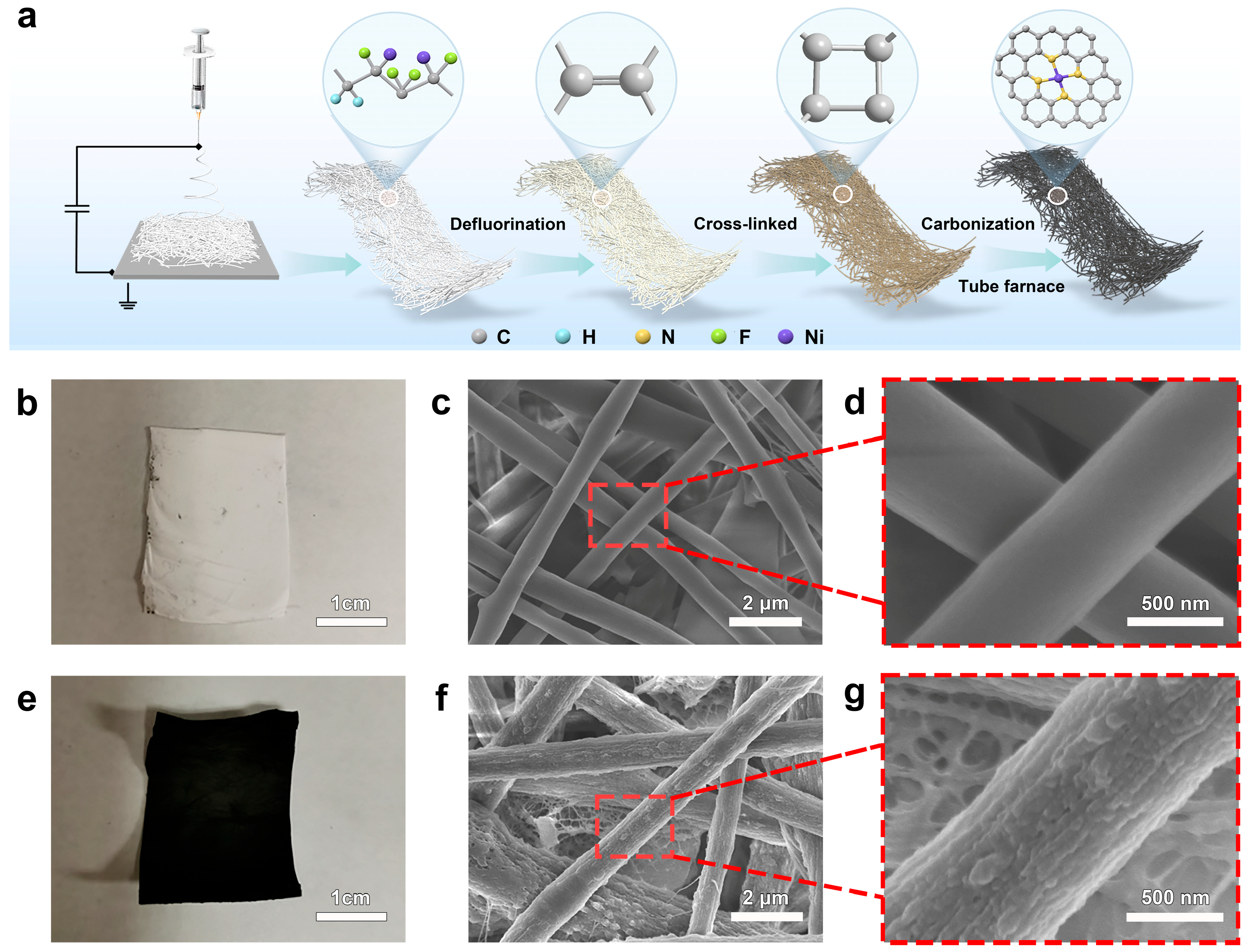
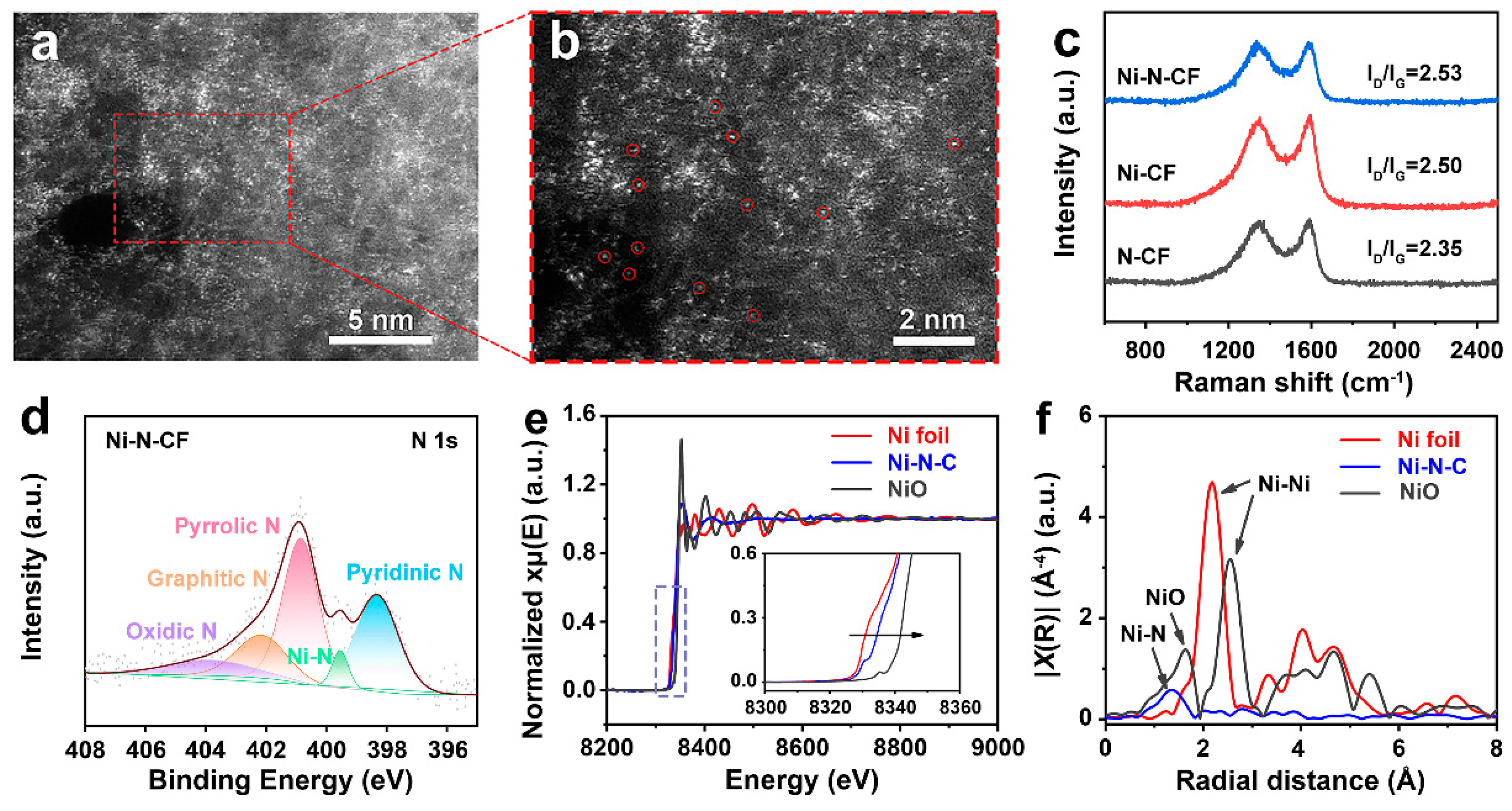
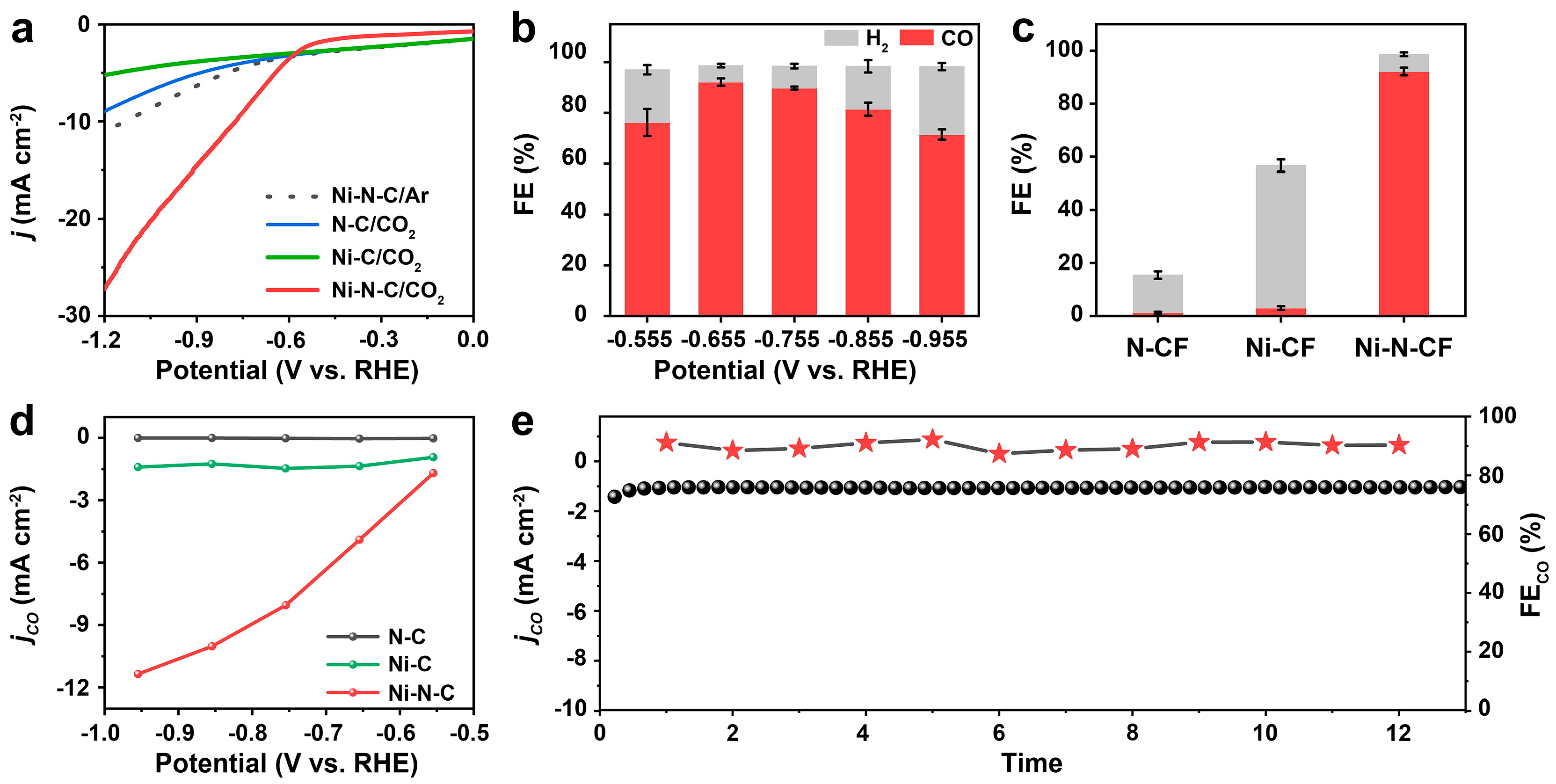
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, J.-W.; Yang, X.-F.; Wu, Z.-L.; Liang, B.-L.; Huang, Y.-Q.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Duan, X.-C.; Xu, J.-T.; Wei, Z.-X.; Ma, J.-M.; Guo, S.-J.; Wang, S.-Y.; Liu, H.-K.; Dou, S.-X. Metal-free carbon materials for CO2 electrochemical reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [PubMed]
- Liu, M.-J.; Zhan, L.-S.; Wang, Y.-C.; Zhao, X.; Wu, J.; Deng, D.-N.; Jiang, J.-B.; Zheng, X.-R.; Lei, Y.-P. Achieving integrated capture and reduction of CO2: A promising electrocatalyst. J. Mater. Sci. Technol. 2023, 165, 235–243. [Google Scholar]
- Zhang, X.-Y.; Xue, D.-P.; Jiang, S.; Xia, H.-C.; Yang, Y.-L.; Yan, W.-F.; Hu, J.-S.; Zhang, J.-N. Rational confinement engineering of MOF-derived carbon-based electrocatalysts toward CO2 reduction and O2 reduction reactions. InfoMat 2021, 4, e12257. [Google Scholar]
- Liu, J.-Z.; Wang, Y.-T.; Jiang, H.; Jiang, H.-B.; Zhou, X.-D.; Li, Y.-H.; Li, C.-Z. Ag@Au core-shell nanowires for nearly 100 % CO2-to-CO electroreduction. Chem. Asian J. 2020, 15, 425–431. [Google Scholar]
- Daiyan, R.; Chen, R.; Kumar, P.; Bedford, N.-M.; Qu, J.-T.; Cairney, J.-M.; Lu, X.; Amal, R. Tunable syngas production through CO2 electroreduction on cobalt–carbon composite electrocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 9307–9315. [Google Scholar] [CrossRef]
- Li, C.-C.; Zhang, T.-T.; Liu, H.; Guo, Z.-Y.; Liu, Z.-L.; Shi, H.-J.; Cui, J.-L.; Li, H.; Li, H.-H.; Li, C.-Z. Steering CO2 electroreduction to C2+ products via enhancing localized *CO coverage and local pressure in conical cavity. Adv. Mater. 2024, 36, 2312204. [Google Scholar]
- Su, J.-W.; Pan, D.-H.; Dong, Y.; Zhang, Y.-Y.; Tang, Y.-L.; Sun, J.; Zhang, L.-J.; Tian, Z.-Q.; Chen, L. Ultrafine Fe2C iron carbide nanoclusters trapped in topological carbon defects for efficient electroreduction of carbon dioxide. Adv. Energy Mater. 2023, 13, 2204391. [Google Scholar]
- Zheng, T.-T.; Jiang, K.; Ta, N.; Hu, Y.-F.; Zeng, J.; Liu, J.-Y.; Wang, H.-T. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 2019, 3, 265–278. [Google Scholar] [CrossRef]
- Liu, M.-H.; Liu, S.-J.; Xu, Q.; Miao, Q.-Y.; Yang, S.; Hanson, S.; Chen, G.-Z.; He, J.; Jiang, Z.; Zeng, G.-F. Dual atomic catalysts from COF-derived carbon for CO2RR by suppressing HER through synergistic effects. Carbon. Energy 2023, 5, e300. [Google Scholar] [CrossRef]
- Jing, X.; Dong, X.; Liu, C.-X.; Li, J.-W.; Dai, Y.-Z.; Xue, W.-Q.; Luo, L.-H.; Ji, Y.; Zhang, X.; Li, X.; et al. Turning copper into an efficient and stable CO evolution catalyst beyond noble metals. Nat. Commun. 2024, 15, 5998. [Google Scholar]
- Li, J.-W.; Zeng, H.-L.; Dong, X.; Ding, Y.-M.; Hu, S.-P.; Zhang, R.-H.; Dai, Y.-Z.; Cui, P.-X.; Xiao, Z.; Zhao, D.-H.; et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat. Commun. 2023, 14, 340. [Google Scholar] [CrossRef]
- Azaiza-Dabbah, D.; Wang, F.; Haddad, E.; Solé-Daura, A.; Carmieli, R.; Poblet, J.M.; Vogt, C.; Neumann, R. Heterometallic transition metal oxides containing lewis acids as molecular catalysts for the reduction of carbon dioxide to carbon monoxide with bimodal activity. J. Am. Chem. Soc. 2024, 146, 27871–27885. [Google Scholar] [CrossRef]
- Yun, H.; Yoo, S.; Son, J.; Kim, J.-H.; Wu, J.-W.; Jiang, K.; Shin, H.; Hwang, Y.-J. Strong cation concentration effect of Ni–N–C electrocatalysts in accelerating acidic CO2 reduction reaction. Chem 2025, 102461. [Google Scholar] [CrossRef]
- Cho, J.-H.; Ma, J.-H.; Lee, C.-H.; Lim, J.-W.; Kim, Y.; Jang, H.-Y.; Kim, J.; Seo, M.-g.; Choi, Y.-H.; Jang, Y.-J.; et al. Crystallographically vacancy-induced MOF nanosheet as rational single-atom support for accelerating CO2 electroreduction to CO. Carbon Energy 2024, 6, e510. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.-R.; Ren, H.-A.; Wang, X.-Y.; Li, W.-J.; Hu, H.-C.; Chen, X.-Y.; Liu, Y.-Q.; Guan, Q.-X.; Li, W. Stabilizing Ni single-atom sites through introducing low-valence Ni species for durably efficient electrochemical CO2 reduction. Appl. Catal. B-Environ. 2025, 368, 125151. [Google Scholar] [CrossRef]
- Zhu, W.-L.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.-J.; Wright, C.-J.; Sun, X.-L.; Peterson, A.-A.; Sun, S.-H. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Liu, S.-B.; Tao, H.-B.; Zeng, L.; Liu, Q.; Xu, Z.-H.; Liu, Q.-X.; Luo, J.-L. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef]
- Chang, Q.-W.; Kim, J.; Lee, J.-H.; Kattel, S.; Chen, J.-G.; Choi, S.-I.; Chen, Z. Boosting activity and selectivity of CO2 electroreduction by prehydridizing Pd nanocubes. Small 2020, 16, 2005305. [Google Scholar] [CrossRef]
- Yang, H.-B.; Hung, S.-F.; Liu, S.; Yuan, K.-D.; Miao, S.; Zhang, L.-P.; Huang, X.; Wang, H.-Y.; Cai, W.-Z.; Chen, R.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar]
- Gu, J.; Hsu, C.-S.; Bai, L.-C.; Chen, H.-M.; Hu, X.-L. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [PubMed]
- Wang, J.-J.; Wang, G.-J.; Zhang, J.-F.; Wang, Y.-D.; Wu, H.; Zheng, X.-R.; Ding, J.; Han, X.-P.; Deng, Y.-D.; Hu, W.-B. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angew. Chem. Int. Ed. 2021, 60, 1–6. [Google Scholar]
- Ma, M.-B.; Tang, Q. Axial coordination modification of M–N4 single-atom catalysts to regulate the electrocatalytic CO2 reduction reaction. J. Mater. Chem. C 2022, 10, 15948–15956. [Google Scholar]
- Jiang, X.-X.; Zhao, Y.-X.; Kong, Y.; Sun, J.-J.; Feng, S.-Z.; Lu, X.; Hu, Q.; Yang, H.-P.; He, C.-X. Support effect and confinement effect of porous carbon loaded tin dioxide nanoparticles in high-performance CO2 electroreduction towards formate. Chin. Chem. Lett. 2025, 36, 109555. [Google Scholar]
- Yang, X.-F.; Wang, A.-Q.; Qiao, B.-T.; Li, J.; Liu, J.-Y.; Zhang, T. A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Zhang, Y.; Jiao, L.; Yang, W.-J.; Xie, C.-F. Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction. Angew. Chem. Int. Ed. 2021, 60, 7607–7611. [Google Scholar]
- Chen, Y.-Q.; Zhang, J.-R.; Yang, L.-J.; Wang, X.-Z.; Wu, Q.; Hu, Z. Recent advances in non-precious metal–nitrogen–carbon single-site catalysts for CO2 electroreduction reaction to CO. Electrochem. Energy Rev. 2022, 5, 11. [Google Scholar] [CrossRef]
- Li, X.-G.; Bi, W.-T.; Chen, M.-L.; Sun, Y.-X.; Ju, H.-X.; Yan, W.-S.; Zhu, J.-F.; Wu, X.-J.; Chu, W.-S.; Wu, C.-Z.; et al. Exclusive Ni–N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction. J. Am. Chem. Soc. 2017, 139, 14889–14892. [Google Scholar]
- Huang, J.-R.; Qiu, X.-F.; Zhao, Z.-H.; Zhu, H.-L.; Liu, Y.-C.; Shi, W.; Liao, P.-Q.; Chen, X.-M. Single-product faradaic efficiency for electrocatalytic of CO2 to CO at current density larger than 1.2 A cm−2 in neutral aqueous solution by a single-atom. Nanozyme Angew. Chem. Int. Ed. 2022, 61, e202210985. [Google Scholar]
- Xu, F.; Wang, X.-H.; Liu, X.; Li, C.-Y.; Fan, G.-H.; Xu, H. Computational screening of TMN4 based graphene-like BC6N for CO2 electroreduction to C1 hydrocarbon products. Mol. Catal. 2022, 530, 112571. [Google Scholar]
- Xu, S.-X.; Ding, Y.-X.; Du, J.; Zhu, Y.; Liu, G.-Q.; Wen, Z.-B.; Liu, X.; Shi, Y.-B.; Gao, H.; Sun, L.-C.; et al. Immobilization of iron phthalocyanine on pyridine-functionalized carbon nanotubes for efficient nitrogen reduction reaction. ACS Catal. 2022, 12, 5502–5509. [Google Scholar]
- Zheng, W.-Q.; Zhu, R.; Wu, H.-J.; Ma, T.; Zhou, H.-J.; Zhou, M.; He, C.; Liu, X.-K.; Li, S.; Cheng, C. Tailoring bond microenvironments and reaction pathways of single-atom catalysts for efficient water electrolysis. Angew. Chem. Int. Ed. 2022, 61, e202208667. [Google Scholar]
- Ping, D.; Huang, S.-G.; Wu, S.-D.; Zhang, Y.-F.; Wang, S.-W.; Yang, X.-Z.; Han, L.-F.; Tian, J.-F.; Guo, D.-J.; Qiu, H.-J.; et al. Confinement effect and 3D design endow unsaturated single Ni atoms with ultrahigh stability and selectivity toward CO2 Electroreduction. Small 2023, 20, 2309014. [Google Scholar]
- Ping, D.; Feng, Y.-C.; Wu, S.-D.; Yi, F.; Cheng, S.-Y.; Wang, S.-W.; Tian, J.-F.; Wang, H.; Yang, X.-Z.; Guo, D.-J.; et al. Maximizing surface single-Ni sites on hollow carbon sphere for efficient CO2 electroreduction. ACS Sustain. Chem. Eng. 2024, 12, 3034–3043. [Google Scholar]
- Cui, Y.; Ren, C.-J.; Li, Q.; Ling, C.-Y.; Wang, J.-L. Hybridization state transition under working conditions: Activity origin of single-atom catalysts. J. Am. Chem. Soc. 2024, 146, 15640–15647. [Google Scholar] [CrossRef]
- Mao, Z.-C.; Wei, G.-P.; Liu, L.-L.; Hao, T.-T.; Wang, X.-J.; Tang, S.-B. Synergistic effect of multi-metal site provided by Ni-N4, adjacent single metal atom, and Fe6 nanoparticle to boost CO2 activation and reduction. J. Colloid. Interface Sci. 2025, 679, 860–867. [Google Scholar] [PubMed]
- Zhang, W.-Y.; Mehmood, A.; Ali, G.; Liu, H.; Chai, L.-Y.; Wu, J.; Liu, M. Nickel nanocluster-stabilized unsaturated Ni–N3 atomic sites for efficient CO2-to-CO electrolysis at industrial-level current. Angew. Chem. Int. Ed. 2025, e202424552. [Google Scholar]
- Zhao, C.-G.; Dai, X.-Y.; Yao, T.; Chen, W.-X.; Wang, X.-Q.; Wang, J.; Yang, J.; Wei, S.-Q.; Wu, Y.; Li, Y.-D. Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar]
- Ren, X.-Y.; Liu, H.; Wang, J.-G.; Yu, J.-Y. Electrospinning-derived functional carbon-based materials for energy conversion and storage. Chin. Chem. Lett. 2024, 35, 109282. [Google Scholar]
- Hajikhani, M.; Lin, M.-S. A review on designing nanofibers with high porous and rough surface via electrospinning technology for rapid detection of food quality and safety attributes. Trends Food Sci. Technol. 2022, 128, 118–128. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Ren, W.-H.; Ni, W.-Y.; Lee, S.; Hu, X.-L. Ionomers modify the selectivity of Cu-catalyzed electrochemical CO2 reduction. ChemSusChem 2023, 16, e202201687. [Google Scholar] [CrossRef]
- Wang, X.-J.; Wang, Y.-J.; Cui, L.-Y.; Gao, W.-Q.; Li, X.; Liu, H.; Zhou, W.-J.; Wang, J.-G. Coordination-based synthesis of Fe single-atom anchored nitrogen-doped carbon nanofibrous membrane for CO2 electroreduction with nearly 100% CO selectivity. Chin. Chem. Lett. 2024, 35, 110031. [Google Scholar] [CrossRef]
- Koh, D.; McCool, B.A.; Deckman, H.; Lively, R. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes. Science 2016, 353, 804–807. [Google Scholar] [CrossRef]
- Yao, Y.-J.; Gao, M.-X.; Zhang, Y.-Y.; Zheng, H.-D.; Hu, H.-H.; Yin, H.-Y.; Wang, S.-B. Nonprecious bimetallic (Mo, Fe)-N/C nanostructures loaded on PVDF membrane for toxic Cr VI reduction from water. J. Hazard. Mater. 2020, 389, 121844. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Wang, Z.; Zong, X.; Jin, Y.-M.; Li, D.; Xiong, Y.-P.; Wu, G. N- & S-co-doped carbon nanofiber network embedded with ultrafine Ni-Co nanoalloy for efficient oxygen electrocatalysis and Zn–air batteries. Nanoscale 2020, 12, 9581–9589. [Google Scholar]
- Chen, Y.-B.; Zou, L.-L.; Liu, H.; Chen, C.; Wang, Q.; Gu, M.; Yang, B.; Zou, Z.-Q.; Fang, J.-H.; Yang, H. Fe and N Co-doped porous carbon nanospheres with high density of active sites for efficient CO2 electroreduction. J. Phys. Chem. C. 2019, 123, 16651–16659. [Google Scholar] [CrossRef]
- Wu, S.-D.; Lv, X.-N.; Ping, D.; Zhang, G.-W.; Wang, S.-W.; Wang, H.; Yang, X.-Z.; Guo, D.-J.; Fang, S.-M. Highly exposed atomic Fe–N active sites within carbon nanorods towards electrocatalytic reduction of CO2 to CO. Electrochim. Acta 2020, 340, 135930. [Google Scholar] [CrossRef]
- Ping, D.; Dong, C.-J.; Zhao, H.; Dong, X.-F. A novel hierarchical RuNi/Al2O3–carbon nanotubes/Ni foam catalyst for selective removal of CO in H2-rich fuels. Ind. Eng. Chem. Res. 2018, 57, 5558–5567. [Google Scholar] [CrossRef]
- Liu, J.; Song, P.; Ruan, M.-B.; Xu, W.-L. Catalytic properties of graphitic and pyridinic nitrogen doped on carbon black for oxygen reduction reaction. Chin. J. Catal. 2016, 37, 1119–1126. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Zhang, T.; Ma, J.; Liu, S.-H.; Zhuang, X.-D.; Yuan, C.; Feng, X.-L. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv. Mater. 2016, 29, 1604480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Wang, C.-M.; Wu, Z.-Y.; Xiong, Y.-J.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.-S.; Chen, Y.; Chen, Y.-G.; Li, Y.-L.; Li, R.-Y.; Sun, X.-L.; Ye, S.-Y.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760. [Google Scholar] [CrossRef]
- Qu, L.-T.; Liu, Y.; Baek, J.; Dai, L.-M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Kattel, S.; Wang, G.-F. A density functional theory study of oxygen reduction reaction on Me–N4 (Me = Fe, Co, or Ni) clusters between graphitic pores. J. Mater. Chem. A 2013, 1, 10790. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhai, P.-L.; Zhang, Y.-X.; Wu, Y.-Z.; Wang, C.; Ran, L.; Gao, J.-F.; Li, Z.-W.; Zhang, B.; Fan, Z.-Z.; et al. Engineering single-atomic Ni-N4-O sites on semiconductor photoanodes for high-performance photoelectrochemical water splitting. J. Am. Chem. Soc. 2021, 143, 20657–20669. [Google Scholar]
- Gong, L.; Chen, B.-T.; Gao, Y.; Yu, B.-Q.; Wang, Y.-H.; Han, B.; Lin, C.-X.; Bian, Y.-Z.; Qi, D.-D.; Jiang, J.-Z. Covalent organic frameworks based on tetraphenyl-p-phenylenediamine and metalloporphyrin for electrochemical conversion of CO2 to CO. Inorg. Chem. 2022, 9, 3217–3223. [Google Scholar] [CrossRef]
- Lyu, X.; Anastasiadou, D.; Raj, J.; Wu, J.-J.; Bai, Y.-C.; Li, J.-L.; Cullen, D.-A.; Yang, J.; Gonçalves, L.-P.-L.; Lebedev, O.-I.; et al. Large-scale synthesis of metal/nitrogen Co-doped carbon catalysts for CO2 electroreduction. Electrochim. Acta 2023, 455, 142427. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wei, X.-F.; Cai, R.-M.; Ren, J.-Z.; Ju, M.; Lu, X.-Q.; Long, X.; Yang, S.-H. Composition-Tuned surface binding on CuZn-Ni catalysts boosts CO2RR selectivity toward CO generation. ACS Materials Lett. 2022, 4, 497–504. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Liu, Y.; Song, Z.-L.; Miao, Z.-C.; Zhao, J.-P. Ni-N-Doped carbon-modified reduced graphene oxide catalysts for electrochemical CO2 reduction reaction. Catalysts 2021, 11, 561. [Google Scholar] [CrossRef]
- Sheng, J.-Y.; Gao, M.-S.; Zhao, N.; Zhao, K.; Shi, Y.-A.; Wang, W. Bimetallic Ni/Fe functionalized, 3D printed, self-supporting catalytic-electrodes for CO2 reduction reaction. Fuel 2025, 382, 133703. [Google Scholar]
- Liu, E.; Liu, T.-X.; Ma, X.-J.; Zhang, Y.-P. The electrocatalytic performance of Ni–AlO(OH)3@RGO for the reduction of CO2 to CO. New J. Chem. 2022, 46, 12023. [Google Scholar]
- Cheng, Y.; Zhao, S.-Y.; Li, H.-B.; He, S.; Veder, J.-P.; Johannessen, B.; Xiao, J.-P.; Lu, S.-F.; Pan, J.; Chisholm, M.-F.; et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2. Appl. Catal. B Environ. 2019, 243, 294–303. [Google Scholar]
- Varela, A.-S.; Ju, W.; Bagger, A.; Franco, P.; Rossmeisl, J.; Strasser, P. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts. ACS Catal. 2019, 9, 7270–7284. [Google Scholar]
- Lin, L.; Li, H.-B.; Yan, C.-C.; Li, H.-F.; Si, R.; Li, M.-R.; Xiao, J.-P.; Wang, G.-X.; Bao, X.-H. Synergistic catalysis over iron-nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv. Mater. 2019, 31, 1903470. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Meng, C.; Yu, W.; Wang, Y.; Cui, L.; Li, T.; Wang, J. Constructing of Ni-Nx Active Sites in Self-Supported Ni Single-Atom Catalysts for Efficient Reduction of CO2 to CO. Nanomaterials 2025, 15, 473. https://doi.org/10.3390/nano15060473
Zhou X, Meng C, Yu W, Wang Y, Cui L, Li T, Wang J. Constructing of Ni-Nx Active Sites in Self-Supported Ni Single-Atom Catalysts for Efficient Reduction of CO2 to CO. Nanomaterials. 2025; 15(6):473. https://doi.org/10.3390/nano15060473
Chicago/Turabian StyleZhou, Xuemei, Chunxia Meng, Wanqiang Yu, Yijie Wang, Luyun Cui, Tong Li, and Jingang Wang. 2025. "Constructing of Ni-Nx Active Sites in Self-Supported Ni Single-Atom Catalysts for Efficient Reduction of CO2 to CO" Nanomaterials 15, no. 6: 473. https://doi.org/10.3390/nano15060473
APA StyleZhou, X., Meng, C., Yu, W., Wang, Y., Cui, L., Li, T., & Wang, J. (2025). Constructing of Ni-Nx Active Sites in Self-Supported Ni Single-Atom Catalysts for Efficient Reduction of CO2 to CO. Nanomaterials, 15(6), 473. https://doi.org/10.3390/nano15060473





