Abstract
The introduction of nitrogen defects in graphitic carbon nitride (g-C3N4) has the important effect of improving its photocatalytic performance. This study employs a simple and environmentally friendly one-step pyrolysis method, successfully preparing g-C3N4 materials with adjustable N3C defect concentrations through the calcination of a urea and ammonium acetate mixture. By introducing N3C defects and adjusting the band structure, the conduction band of the g-C3N4 was shifted downward by 0.12 V, overcoming the traditional application limitations of N3C defects and enabling an innovative transition from enhanced oxidation to enhanced reduction capabilities. This transition significantly enhanced the adsorption and activation of O2. Characterization results showed that the introduction of N3C defects increased the specific surface area from 44.07 m2/g to 87.08 m2/g, enriching reactive sites, while narrowing the bandgap to 2.41 eV enhanced visible light absorption capacity. The g-C3N4 with N3C defects showed significantly enhanced photocatalytic activity, achieving peak performance of 54.8% for tetracycline (TC), approximately 1.5 times that of the original g-C3N4, with only a 5.4% (49.4%) decrease in photocatalytic efficiency after four cycles of testing. This study demonstrates that the introduction of N3C defects significantly enhances the photocatalytic performance of g-C3N4, expanding its potential applications in environmental remediation.
1. Introduction
With the acceleration of global industrialization and urbanization, the demand for fossil fuels has continuously increased, leading to severe environmental and energy issues [1]. Therefore, the development of green, clean, and sustainable energy sources has become an urgent global priority. Solar energy, being an abundant and clean resource, has garnered increasing attention for its potential applications in environmental remediation [2,3]. In recent years, photocatalytic technology, which can harness solar energy to degrade pollutants in water and air or convert solar energy into chemical energy, has been regarded as one of the most promising technologies for addressing energy crises and environmental pollution [4].
Graphitic carbon nitride (g-C3N4) is a non-metallic semiconductor material with a two-dimensional layered structure, unique electronic properties, and tunable optical characteristics. Due to its suitable band gap and band edge positions, it is considered one of the most promising photocatalysts [5,6,7]. This material has garnered significant attention in the field of photocatalysis due to its simple preparation method, excellent chemical and physical stability, and outstanding optical properties, enabling it to effectively absorb sunlight below 460 nm [8]. These advantages make g-C3N4 a promising semiconductor material for photocatalytic degradation of organic pollutants [9,10]. However, its photocatalytic activity is limited by a high rate of photogenerated charge carrier recombination, low light harvesting efficiency, and insufficient active sites [11,12]. To address these limitations, various modification techniques have been applied, including heterojunction construction, element doping, morphology and structural regulation, as well as defect engineering [13,14,15,16]. Among these methods, defect engineering is considered a successful approach to enhancing the photocatalytic efficiency of g-C3N4. By employing suitable strategies, defects can be introduced into the polymeric g-C3N4 which alter its electronic structure and optical properties, thereby extending its light absorption range, promoting the separation and transport of photogenerated electron–hole pairs, and enhancing its surface reactivity [17].
In recent years, defect engineering of g-C3N4 has achieved remarkable progress in enhancing its photocatalytic performance, while nitrogen [18] and carbon [19] vacancies play a critical role in improving the catalytic activity of the material. Studies on nitrogen vacancies are relatively extensive. For example, Niu et al. [20] successfully introduced nitrogen vacancies through high-temperature calcination (600 °C for 4 h), significantly altering the electronic structure of g-C3N4. Additionally, the addition of molten salts during reheating has also been used to introduce nitrogen vacancies at N2C sites. For instance, Dong et al. [21] mixed melamine with molten salts (NaCl, KCl, KOH) and processed it at high temperatures to produce abundant N2C vacancies. However, most studies focus on N2C vacancies, with limited attention to the role of tricoordinated nitrogen (N3C) vacancies in photocatalysis. Theoretically, N3C vacancies can provide more lone-pair electrons than N2C vacancies, helping accelerate photocatalytic oxidation [22]. Liang et al. [23] created N3C vacancies through self-assembly and thermal polymerization, optimizing the band structure and enhancing oxidation capacity, achieving a 97.1% degradation efficiency of ENR under visible light (NCN 0.48). Similarly, Gong et al. [24] used a hydrothermal method combined with glycerol as a reducing agent to etch the g-C3N4 surface, introducing N3C vacancies, which enhanced electron–hole pair separation efficiency and increased CO2 reduction activity to 12 times that of GCN (4.18 μmol g−1 h−1). Despite these methods improving the photocatalytic activity of g-C3N4, they commonly involve complex pretreatments, prolonged heating, and the use of harmful reagents, making it challenging to precisely control defect concentrations [25,26]. Furthermore, products generated during photocatalytic reactions tend to adsorb onto the catalyst surface, affecting nitrogen vacancy activity and stability. Therefore, a green and straightforward method is urgently needed to synthesize highly stable g-C3N4 with N3C vacancies under mild conditions without harmful reagents or reducing gases.
This study synthesizes N3C-defect-rich g-C3N4 photocatalytic materials (denoted as ACN-X) through a simple one-step calcination method, aided by additives. Urea is mixed with ammonium acetate, and the defect concentration is controlled by adjusting the amount of ammonium acetate. During the thermal decomposition process, urea and ammonium acetate react in situ to create a reducing atmosphere, facilitating the introduction of defects into g-C3N4. Through comprehensive characterization, we examined the morphology, microstructure, light absorption capacity, and charge transfer properties of defect-rich g-C3N4 to elucidate the role of N3C vacancies in photocatalysis. Additionally, the possible formation mechanism of N3C vacancies during synthesis was explored, and the photocatalytic performance of the prepared g-C3N4 samples was evaluated by degrading the pollutant TC under visible light. This study achieved multiple optimizations of the material through an effective defect engineering strategy, significantly enhancing the photocatalytic activity of g-C3N4 and providing a new perspective for future environmental purification technologies.
2. Materials and Methods
2.1. Chemicals and Reagents
Ammonium acetate (C2H7NO2, AR), urea (H2NCONH2, AR, 99.0%), disodium ethylenediaminetetraacetate (EDTA, C10H12N2Na4O8·XH2O, AR, 98.0%, 0.1 M), p-benzoquinone (C6H4O2, 99.0%), and tert-butanol (C4H10O, AR, 99.0%) were all purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals used in this study were of analytical grade and required no further purification.
2.2. Photocatalytic Degradation Test
The photocatalytic performance of the material was evaluated by measuring the degradation rate of tetracycline (TC) under visible light. A 20 mg photocatalyst was added to a reactor containing 100 mL of TC solution at a concentration of 80 mg/L, with the temperature maintained at 25 °C using a circulating water system. The solution was stirred in the dark for 30 min to achieve adsorption/desorption equilibrium and then irradiated with a 300 W xenon lamp (Beijing, China) equipped with a 420 nm cutoff filter. The reaction lasted for 1 h, with samples taken every 10 min. The filtrate was analyzed using a Persee General T9 Series Double Beam UV-Vis Spectrophotometer (Beijing, China) to assess the degree of TC degradation. To test the stability of the photocatalyst, the used catalyst was washed, dried, and recovered for subsequent cycling test experiments.
2.3. Sample Preparation
The preparation of N3C-vacancy g-C3N4 was processed as follows (Scheme 1): Different mass ratios of ammonium acetate (0.1–0.9 g) were mixed with urea (10 g) and synthesized using a one-step calcination method. Specifically, ammonium acetate and urea were thoroughly ground together in a mortar for 5 min, and the mixture was then placed in a crucible sealed with aluminum foil. The mixture was heated at a rate of 5 °C/min to 550 °C and maintained at that temperature for 2 h. Using this method, we successfully synthesized a series of samples labeled ACN-X, where X represents the amount of ammonium acetate added (0.1 g, 0.3 g, 0.5 g, 0.7 g, and 0.9 g). For comparative analysis, pure g-C3N4 was synthesized under the same experimental conditions using only 10 g urea.
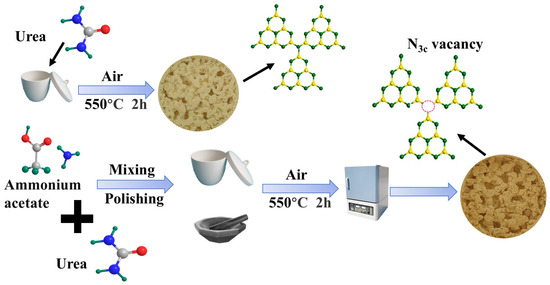
Scheme 1.
Process flow diagram for the preparation of g-C3N4 and ACN-X.
3. Results and Discussion
The phase structures of ACN-X and pure g-C3N4 were investigated using X-ray diffraction (XRD), as shown in Figure 1a. All samples exhibited two characteristic diffraction peaks around 13° and 27°, corresponding to the (100) and (002) crystal planes of g-C3N4, respectively. The peak at ~13° (100) is associated with the in-plane structural stacking of tri-s-triazine units, while the peak at ~27° (002) originates from the stacking of conjugated aromatic layers [27]. After the introduction of defects, no new characteristic peaks appeared or disappeared in the XRD patterns, indicating that the N3C vacancy defects did not significantly alter the bulk crystalline structure of CN. However, with increased amounts of ammonium acetate, the intensity of the (100) peak at 13° slightly decreased compared to that of the original g-C3N4, suggesting a partial disruption of long-range in-plane order [28]. This implies that the introduced vacancies may slightly affect the planar structure without significantly altering the crystalline phase. Similarly, the (002) peak at 27° showed reduced intensity and slightly broadened, which may be attributed to the presence of defects within the layers. These defects might alter interlayer interactions, leading to changes in stacking density and potential increases in interlayer spacing [29]. These observations indicate that, while the overall crystal structure of g-C3N4 is retained, minor structural modifications caused by the synthesis process lead to the formation of defects that affect in-plane and interlayer structural order. This is consistent with the morphological features observed in SEM and TEM analyses.
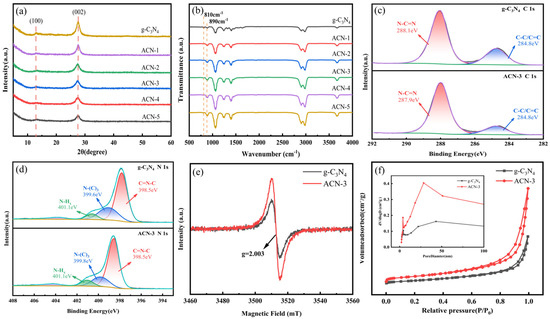
Figure 1.
(a) XRD spectra of g-C3N4 and ACN-X; (b) FTIR spectra of g-C3N4 and ACN-X; (c,d) C 1s and N 1s XPS spectra of g-C3N4 and ACN-3. (e) Room temperature EPR spectra of g-C3N4 and ACN-3; (f) nitrogen adsorption–desorption isotherms of g-C3N4 and ACN-3 (inset shows the corresponding pore size distribution curves).
To gain deeper insights into the chemical structure changes of the modified photocatalysts, detailed characterization was conducted using Fourier Transform Infrared Spectroscopy (FTIR). The FTIR analysis, as shown in Figure 1b, reveals that all samples exhibit the typical characteristics of graphitic carbon nitride and retain its basic structure. Specifically, the peaks at 810 cm−1 and 890 cm−1 correspond to the out-of-plane bending vibrations of the tri-s-triazine rings and the deformation vibrations of the N-H bond, respectively. The peaks in the range of 1200–1700 cm−1 are attributed to the stretching vibrations of N=C-N heterocycles, while the broad peaks between 3500 and 3800 cm−1 are primarily due to the stretching vibrations of N-H and O-H bonds, as well as adsorbed oxygen molecules [30]. With the addition of ammonium acetate, the peak intensity at 810 cm−1 was observed to gradually weaken and even disappear, suggesting that the structure of the heptazine rings may have been disrupted under the influence of ammonium acetate. This structural disruption could be attributed to the active radicals (NH2· and NH·) generated from the thermal decomposition of NH3 at high temperatures, which etched the N3C lattice sites, leading to an increase in N3C vacancies and partial degradation of the heptazine rings [31]. These changes indicate the successful introduction of N3C vacancies, and the greater the amount of ammonium acetate added, the more nitrogen vacancies were formed.
X-ray photoelectron spectroscopy (XPS) was performed to analyze the surface elemental composition, chemical states, and defect types after the addition of ammonium acetate. As shown in Figure S1, the presence of C and N elements is identified in both g-C3N4 and ACN-3, while the O element is likely due to surface adsorption occurring during the calcination process. As shown in Figure 1c, the high-resolution XPS spectra of C 1s display two main peaks at 284.8 eV and 288.1 eV, corresponding to graphitic carbon at the edges of triazine rings (C=C/C-C) and sp2 hybridized carbon (N–C=N), respectively. Notably, the peak at 288.1 eV shifts to a lower binding energy (287.9 eV), indicating that sp2 hybridized carbon has gained extra electrons from the missing nitrogen atoms, which partially confirms the presence of nitrogen vacancies [32]. In the high-resolution XPS spectra of N 1s (Figure 1d), there are four characteristic deconvolution peaks located at 398.5 eV (N2C), 399.6 eV (N3C), 401.1 eV (N-HX), and 404.1 eV (charge effects in heterocycles). For ACN-3, all peaks show no significant shift. Moreover, based on the XPS fitting analysis, the peak area of N3C decreases in ACN-3 after the addition of ammonium acetate, and the N3C/N2C peak area ratio decreases from 0.41 to 0.35. It is noteworthy that the N3C/N-HX ratio in both pure g-C3N4 and ACN-3 remains almost unchanged, suggesting that vacancies preferentially form at N3C sites [33].
To investigate in detail how the defect concentration of N3C vacancies changes with the increase in the amount of ammonium acetate, we conducted high-resolution XPS analysis of the C1s and N1s spectra of different samples (Figure S2) and examined the variations in the content of various chemical bonds in the ACN-X series samples (see Table 1 and Table 2). The results show that with the increased addition of ammonium acetate, the content of N3C in the samples gradually decreased, indicating that the nitrogen atoms at the N3C sites are more prone to being replaced or removed, leading to an increase in nitrogen vacancies. Meanwhile, the relative increase in the content of N2C suggests that after the nitrogen at N3C sites is removed, the surrounding structure may rearrange, causing some nitrogen atoms to transition to N2C coordination forms. This structural change particularly affects the bonding state of nitrogen atoms within the conjugated triazine framework, resulting in alterations in the electron distribution of the N=C=N structure. The enhancement of the infrared absorption peak corresponding to the N=C=N stretching vibrations (1700–1200 cm−1) in the FTIR spectra further confirms that significant changes in the surface and chemical structure of the material occurred due to the introduction of defects. Additionally, the gradual decrease in the N3C/N2C peak area ratio, from 0.41 in g-C3N4 to 0.32 in ACN-5, further confirms that the number of N3C vacancies increases with the increased addition of ammonium acetate [34]. Therefore, it can be concluded that the defect concentration increases as the amount of ammonium acetate added is increased.

Table 1.
Relative proportions of different chemical bonds in the N1s spectra of g-C3N4 and ACN-X samples.

Table 2.
Relative proportions of different chemical bonds in the C1s spectra of g-C3N4 and ACN-X samples.
The C1s spectral data also reveal that the content of graphitic carbon (C=C/C-C) decreases with the increased addition of ammonium acetate. This may be due to the disruption of the original arrangement of carbon atoms connected to N3C during the formation of nitrogen vacancies, leading to damage in the graphitized regions of the material. The reduction in the degree of graphitization implies a decline in the orderliness of the interlayer structure, resulting in a decrease in the intensity of the (002) peak and an increase in peak width in the XRD analysis. This indicates a more disordered crystalline structure and a higher defect rate, consistent with the crystalline changes observed in the XRD results.
During the reaction between urea and ammonium acetate to produce carbon nitride materials, although nitrogen vacancies were formed, the mechanism differs from the traditional nitrogen vacancy formation (i.e., reduction in nitrogen atoms). The C/N ratio shows a downward trend with increasing amounts of ammonium acetate (Table 3). This may be because, under high-temperature conditions, the reaction between ammonium acetate and urea not only promotes the formation of defects at N3C sites but also leads to the adsorption of some nitrogen-containing groups (such as NHX) on the material surface in a closed environment. Additionally, the presence of free amino groups may facilitate the formation of N-HX bonds, leading to an increase in nitrogen content in the final product. This also accounts for the slight increase in N-HX content. The NHx groups adsorbed on the material’s surface influence the surface charge distribution, enhance hydrophilicity, and facilitate the desorption of reaction products (such as intermediates or final products), thereby reducing their accumulation on the surface. This helps maintain the accessibility of active sites and enhances the stability of photocatalysis. In summary, the analysis indicates that by adjusting the reaction conditions (particularly the amount of ammonium acetate added), it is possible to effectively regulate the defect concentration in the material, which also triggers other surface chemical and structural changes, thereby affecting the overall photocatalytic performance of the material.

Table 3.
Elemental composition of g-C3N4 and ACN-X samples.
The presence of nitrogen vacancies was further confirmed by electron paramagnetic resonance (EPR) spectroscopy. As shown in Figure 1e, the EPR spectra of pure g-C3N4 and ACN-3 exhibit similar single Lorentzian lines, with a g-value of 2.003, indicating the presence of unpaired electrons in sp2 hybridized carbon atoms within the π-conjugated aromatic rings [35]. Compared to pure g-C3N4, the EPR signal of the material with introduced defects is significantly enhanced, which is attributed to the effective increase in unpaired electron concentration due to the introduction of N3C vacancies, thereby providing more free electrons to the sp2 carbon atoms [36]. This increase in unpaired electrons facilitates the generation and separation of charge carriers in the material, promoting the efficient transfer of photogenerated electrons. Based on the combined results of EPR and XPS spectra, the introduction of N3C vacancies has been confirmed to successfully modulate the electronic structure of carbon nitride, enhancing the concentration of unpaired electrons, which is expected to improve its activity and efficiency in photocatalytic reactions.
Based on the analysis results, a mechanism for the formation of nitrogen vacancies in ACN-3 is proposed. During the heating phase between 110 and 210 °C (Stage 1), ammonium acetate partially decomposes, releasing NH3 and CO2. Simultaneously, partial decomposition products of acetic acid and urea interact, leading to an acylation reaction that forms amide compounds. As the temperature continues to rise, the amide compounds gradually decompose, releasing a large volume of gaseous products (CO2 and NH3) and creating a self-generated reducing atmosphere. Under high-temperature conditions, the generated NH3 decomposes into reactive radicals (such as NH2· and NH·), which can effectively etch the N3C lattice sites in g-C3N4, participating in the reaction within the carbon nitride material and promoting the formation of nitrogen vacancies. Furthermore, the continuous release of gaseous products introduces a porous structure into the material, thereby increasing the specific surface area and further enhancing the material’s reactivity and photocatalytic performance.
The N3C defect-engineered g-C3N4 photocatalysts were synthesized via a one-step copolymerization method by uniformly mixing ammonium acetate and urea. During the thermal polycondensation process, ammonium acetate reacts with urea through an acylation reaction, accompanied by the release of gases (CO2 and NH3) and volume contraction, which induces the transformation of the morphology from irregular bulk structures to porous amorphous sheet-like structures [7,37,38,39]. To verify the effect of defect engineering on the morphological structure, the synthesized catalysts were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to examine their morphology and microstructure.
From the SEM images of g-C3N4 and ACN-3 (Figure 2a,b), it can be observed that under the influence of ammonium acetate, g-C3N4 transforms from a bulk structure into an amorphous, layered, porous structure. Compared to pure g-C3N4, ACN-3 exhibits a looser morphology and enhanced porosity. The TEM images of g-C3N4 and ACN-3 (Figure 2c,d) further demonstrate ACN-3’s distinctive porous structure, featuring stacked, sheet-like morphology with numerous in-plane pores. In addition, the HAADF-STEM and EDX elemental distribution maps in Figure 2a,b indicate that in ACN-3, the elements C and N are uniformly distributed within the selected area. To further investigate the surface characteristics of g-C3N4 and ACN-3, BET analysis was performed. The N2 adsorption–desorption isotherms and pore size distribution curves (Figure 1f) reveal that both materials exhibit typical type IV isotherms with H3 hysteresis loops, indicating the formation of mesoporous and macroporous structures, consistent with the SEM and TEM results. Compared to g-C3N4, the modified ACN-3 shows a higher specific surface area and pore volume (Table 4), along with a diverse pore structure ranging from micropores to macropores. The pore sizes of both catalysts are primarily distributed in the 2–40 nm range, suggesting the formation of numerous mesopores within the material. The introduction of defects did not lead to the collapse of the mesoporous structure but endowed ACN-3 with a more loosely packed morphology and rich porous characteristics. The increase in specific surface area provides more active sites on the catalyst surface [40], facilitating the adsorption of reactants and the desorption of products. These active sites can disperse the reaction centers, preventing the excessive accumulation of intermediates in localized regions and thereby reducing the shielding of active nitrogen vacancies. Simultaneously, this also promotes the diffusion and transport of reactants and products on the catalyst surface, accelerating the reaction process and enhancing the effective adsorption of pollutants and the photocatalytic performance [41].
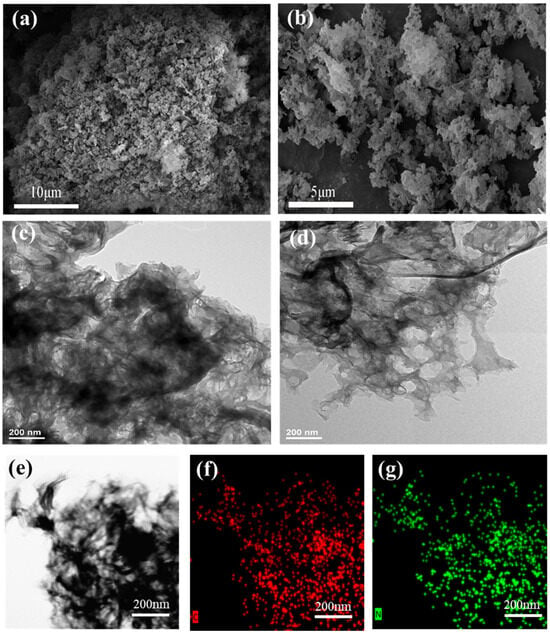
Figure 2.
(a,b) SEM images of g-C3N4 and ACN-3; (c,d) TEM images of g-C3N4 and ACN-3; (e) HAADF STEM image; and (f) EDX elemental distribution maps of carbon (C) and (g) nitrogen (N) in ACN-3.

Table 4.
Specific surface area and pore structure parameters of g-C3N4 and ACN-3.
The introduction of defects did not lead to the collapse of the mesoporous structure. Instead, it endowed ACN-3 with a looser morphology and abundant porosity, providing more active sites, which aids in the efficient adsorption of pollutants and enhances photocatalytic performance.
The influence of N3C vacancies on the electronic band structure and optical properties of g-C3N4 was analyzed through UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS). As shown in Figure 3a, pure g-C3N4 exhibits typical semiconductor absorption characteristics in the UV and part of the visible light region, with an absorption edge around 447 nm. In contrast, ACN-3 shows a significant redshift in the absorption edge (484 nm), and with the increasing amount of ammonium acetate, all samples exhibit further redshift (527 nm), indicating stronger visible light absorption. This phenomenon is likely due to the introduction of N3C vacancies, which creates intermediate band gaps in g-C3N4, enhancing both light absorption and charge transfer efficiency. In addition, the decomposition products of ammonium acetate disrupt the polymerization process of carbon nitride, introducing defects that lead to the distortion of its 2D planar structure, activating the n → π* electronic transition, and significantly broadening the light absorption range, particularly in the visible light region, thereby enhancing photocatalytic performance [42,43,44]. Figure 3b shows the relationship between the Kubelka–Munk function and photon energy for g-C3N4 and ACN-3. Based on the Kubelka–Munk theory, the band gap of ACN-3 is estimated to be 2.41 eV, lower than that of pure g-C3N4 2.66 eV, indicating that ACN-3 has a broader light absorption range. With the increased addition of ammonium acetate, the rising concentration of N3C vacancies further narrows the band gap, enabling wide-range tuning of the energy band (Figure S3). This provides advantageous conditions for improving the efficiency of the photocatalyst in the visible light region.
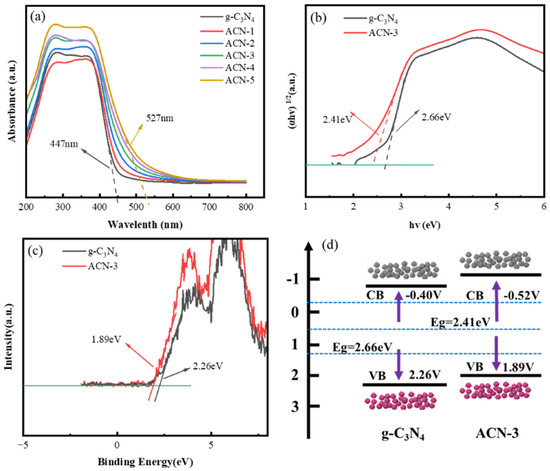
Figure 3.
(a) UV–visible absorption spectra of g-C3N4 and ACN-X; (b) transformation of the Kubelka–Munk function versus photon energy for g-C3N4 and ACN-3; (c) XPS valence band spectra of g-C3N4 and ACN-3; and (d) the corresponding band structure diagrams.
Furthermore, the valence band position of ACN-3 (Figure 3c) and its band structure (Figure 3d) were analyzed through XPS valence band spectroscopy. Compared with pure g-C3N4, the introduction of N3C vacancies results in a more negative conduction band potential in ACN-3, indicating that its photogenerated electrons possess a stronger reducing ability, allowing for more efficient generation of ·O2− radicals, which can effectively initiate photocatalytic reduction reactions [28]. Additionally, the narrower band gap of ACN-3 more effectively reduces the recombination of photogenerated electrons and holes, achieving enhanced spatial charge separation, which extends the lifetimes of these charge carriers. This improvement in photocatalytic activity provides a strong structural basis for increased photocatalytic efficiency [23].
To further investigate the effect of N3C vacancies on the efficiency of photogenerated charge separation and optical properties, we conducted electrochemical impedance spectroscopy (EIS) measurements on g-C3N4 and ACN-3. The EIS results (Figure 4a) show that, compared to pure g-C3N4, the arc radius in the impedance response of ACN-3 is significantly reduced, indicating lower surface resistance and faster interfacial charge transfer. These characteristics suggest that N3C vacancies greatly facilitate the transport of photogenerated electrons from the interior of the material to the surface, accelerating the migration of interfacial charges. Additionally, photoluminescence (PL) spectroscopy was used to further examine the behavior of photogenerated carriers [45]. At an excitation wavelength of 365 nm, the PL intensity of ACN-3 is much lower than that of pristine g-C3N4 (Figure 4b). This weaker PL signal indicates a significant reduction in the recombination rate of photogenerated charges, primarily due to the presence of nitrogen vacancies. The nitrogen vacancies help trap photogenerated holes, reducing the spatial overlap between photogenerated carriers and enhancing their separation efficiency [41,46]. Therefore, it can be inferred that the presence of N3C vacancies effectively suppresses the recombination of photogenerated carriers, thereby enhancing their transport and photocatalytic utilization efficiency. Through these measurements, we have confirmed the crucial role of N3C vacancies in improving the photoelectric performance of the material.
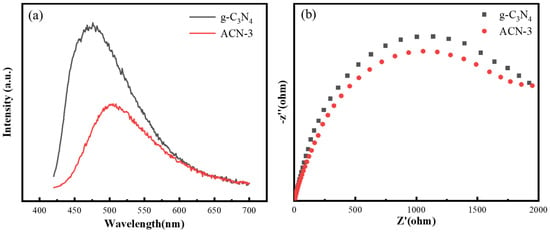
Figure 4.
(a) Nyquist plots from EIS measurements, and (b) PL spectra of g-C3N4 and ACN-3.
The photocatalytic performance of the prepared catalysts was evaluated using TC as the target pollutant. As shown in Figure 5a, the results indicated a significant improvement in the photocatalytic degradation efficiency of ACN-X samples with different defect concentrations compared to pure g-C3N4. The degradation rate of pure g-C3N4 was only 36.2%, while the ACN-X samples showed varying degrees of enhanced efficiency based on the defect concentration. A defect concentration that is too low cannot effectively inhibit the recombination of photogenerated electron–hole pairs, which is essential for improving the separation and transfer efficiency of charge carriers. Conversely, an excessively high defect concentration may lead to electron accumulation, forming recombination centers for charge carriers, which suppresses photocatalytic efficiency [17]. Therefore, precise control over the defect concentration is crucial. When the defect concentration was optimized, the degradation efficiency of ACN-3 reached 54.8%, which is 1.5 times that of pure g-C3N4. This significant improvement is primarily attributed to the introduction of N3C vacancies through defect engineering. These vacancies effectively reduced the recombination of photogenerated electron–hole pairs, enhancing the separation and transport efficiency of charge carriers. Moreover, the introduction of defects increased the specific surface area of the material and formed a rich porous structure, providing more active sites for the photocatalytic reaction, further promoting pollutant adsorption and improving the efficiency of photocatalytic degradation.
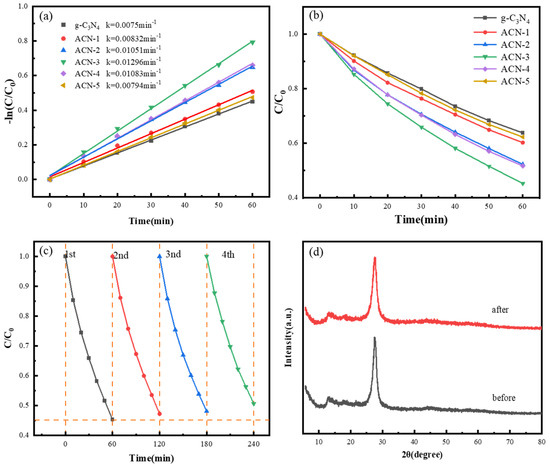
Figure 5.
(a) First-order kinetic curves for the degradation of TC; (b) photocatalytic degradation of TC by samples with varying ratios; (c) four-cycle photocatalytic degradation of TC using ACN-3; and (d) XRD patterns of ACN-3 before and after the photocatalytic experiment.
Figure 5b shows the first-order reaction rate constants of the samples with different defect concentrations. The reaction rate constant of pure g-C3N4 is 0.0075 min−1, while the rate constant of ACN-3, with the optimal defect concentration, increases to 0.0129 min−1, approximately 1.72 times that of unmodified g-C3N4. This indicates that the rational introduction of N3C vacancy defects effectively altered the electron distribution on the surface of g-C3N4, enhancing the transport efficiency of photogenerated electrons from the interior to the surface of the material, thereby significantly accelerating the photocatalytic reaction rate. Figure 5c presents the cyclic degradation performance of ACN-3 at the optimal defect concentration. According to the calculations, the photocatalytic degradation efficiency of ACN-3 in the first cycle was 54.8%. After four consecutive cycles, the efficiency remained at around 49.4%. This indicates that the material maintained high photocatalytic performance during repeated use, demonstrating excellent cyclic stability. Additionally, a comparison of the XRD patterns of ACN-3 before and after the reaction (Figure 5d) shows no significant changes in its crystal structure. The stability of the material can be attributed to its robust structure and the introduction of N3C vacancies through defect engineering, which effectively prevented the loss of active sites. This ensured that the photocatalytic efficiency remained high even after multiple uses, enhancing its feasibility for practical applications.
We conducted a radical scavenging experiment by adding quenching agents to investigate the photocatalytic mechanism of ACN-3. In the experiment, t-BuOH was used as a scavenger for ·OH, BQ was used to capture ·O2−, and EDTA-2Na was used to capture h+ [47]. As shown in Figure 6a, the photocatalytic degradation of TC was significantly inhibited when ·O2− scavenger BQ and h+ scavenger EDTA-2Na were added, reducing the degradation efficiency to 8.3% and 40.5%, respectively. In contrast, t-BuOH had a negligible effect on TC degradation. This indicates that hydroxyl radicals (·OH) are not the main active species in the photocatalytic reaction and play a relatively limited role. Instead, superoxide radicals (·O2−) act as the key active species, dominating the reaction, while the influence of h+ is secondary.
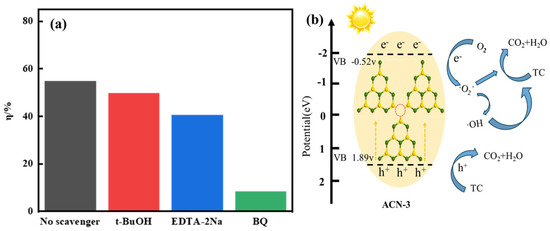
Figure 6.
(a) Radical scavenging experiments for TC degradation by ACN-3 under visible light; (b) proposed reaction mechanism for TC degradation by ACN-3 under visible light irradiation.
Based on the above findings, we propose a photocatalytic mechanism for g-C3N4 containing N3C vacancies, as illustrated in Figure 6b, which effectively explains its enhanced activity during the photocatalytic process. The introduction of N3C vacancies not only decreases the electron density of g-C3N4 but also enhances intersystem transition capability, facilitating the generation of photogenerated electrons and holes. Additionally, N3C vacancies serve as trap states for photogenerated electrons, effectively promoting the separation and transfer of electrons and holes, significantly reducing their recombination rate. Under visible light irradiation, photoexcitation of ACN-3 generates electron–hole pairs with a more negative conduction band potential, making its photogenerated electrons more prone to reacting with molecular oxygen to generate ·O2− radicals, which play a crucial role in the reaction. Concurrently, photogenerated holes (h+) also participate in the reaction by directly interacting with TC, catalyzing its oxidative decomposition into carbon dioxide (CO2) and water (H2O). Table 5 provides a summary comparison of recent studies with our work.

Table 5.
Photocatalytic activity for the TC degradation using carbon nitride in previous studies.
4. Conclusions
This study successfully synthesized g-C3N4 (ACN-X) with tricoordinated nitrogen vacancies (N3C) using a simple one-step calcination process assisted by additives. This method requires no harmful reducing agents, simplifying the preparation and defect generation process while enhancing the reproducibility and stability of the material. The defect engineering significantly optimized the electronic structure of the material, reducing the bandgap from 2.66 to 2.41 eV, thereby enhancing light absorption and energy capture efficiency, achieving effective separation of photogenerated electrons and holes, and reducing recombination rates. By precisely tuning the band structure, this study innovatively transformed the N3C-defect g-C3N4 material from enhanced oxidation capability to enhanced reduction capability, significantly improving its photocatalytic reduction performance. Under low dosage (20 mg), high concentration (80 mg/L, 100 mL), and short duration (1 h), ACN-3 achieved a TC degradation rate of 54.8%, with only a 5.4% decline in degradation efficiency after four cycles. This study provides new insights into the potential of carbon nitride materials in reductive photocatalytic applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15060466/s1, Figure S1: XPS spectra of g-C3N4 and ACN-3; Figure S2: XPS spectra of C 1s and N 1s for ACN-1 and ACN-5; Figure S3: The Kubelka–Munk function versus photon energy conversion plot for g-C3N4 and ACN-X.
Author Contributions
Conceptualization, Y.L., C.L., B.H. and L.Z.; methodology, Y.L.; software, F.C., J.Q. and X.M.; validation, Y.L., C.L. and L.Z.; formal analysis, Y.L.; investigation, Y.L.; resources, C.L. and Z.C.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L., C.L. and L.Z.; visualization, Y.L.; supervision, X.M. and S.Z.; project administration, C.L., L.Z. and Z.C.; funding acquisition, C.L. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Natural Science Foundation of Jiangsu Province (BK20180103, BK20180971), the Science and Technology Development Project of Suzhou (SS202036), and the Young Scientists Fund of the National Natural Science Foundation of China (No. 22408376) for their financial support.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary Information and from the corresponding author upon reasonable request.
Conflicts of Interest
We have no conflicts of interest to declare.
References
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; He, B.; Wei, S.; Wang, R.; Zhang, R.; Liu, R. Dynamic Fe-O-Cu induced electronic structure modulation on CuOx electrode for selective electrocatalytic oxidation of glycerol. Appl. Catal. B Environ. Energy 2025, 362, 124743. [Google Scholar] [CrossRef]
- He, B.; Zhong, S.; Li, K.; Wei, S.; Li, M.; Liu, R. Ionic liquids: The emerging “cardiotonic” for photocatalytic materials. Coord. Chem. Rev. 2025, 529, 216461. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Han, C.; Su, P.; Tan, B.; Ma, X.; Lv, H.; Huang, C.; Wang, P.; Tong, Z.; Li, G.; Huang, Y.; et al. Defective ultra-thin two-dimensional g-C3N4 photocatalyst for enhanced photocatalytic H2 evolution activity. J. Colloid Interface Sci. 2021, 581, 159–166. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.; Zhou, Y.; Qin, F.; Wang, H.; Wang, W.; Yang, Y.; Zeng, G. Dual optimization approach to Mo single atom dispersed g-C3N4 photocatalyst: Morphology and defect evolution. Appl. Catal. B Environ. 2022, 303, 120904. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhou, W.; Liu, Y.; Yin, L.; Hai, X.; Song, H.; Ye, J. Photoassisted Construction of Holey Defective g-C3N4 Photocatalysts for Efficient Visible-Light-Driven H2O2 Production. Small 2018, 14, 1703142. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, D.; Gu, L.; Xu, G.; Li, Z.; Yuan, Y. Awakening n → π* electronic transition by breaking hydrogen bonds in graphitic carbon nitride for increased photocatalytic hydrogen generation. Chem. Eng. J. 2020, 399, 125847. [Google Scholar] [CrossRef]
- Lu, S.; Shen, L.; Li, X.; Yu, B.; Ding, J.; Gao, P.; Zhang, H. Advances in the photocatalytic reduction functions of graphitic carbon nitride-based photocatalysts in environmental applications: A review. J. Clean. Prod. 2022, 378, 134589. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Akpotu, S.O.; Mombeshora, E.T.; Oladipo, A.O.; Ombaka, L.M.; Maria, B.B.; Idris, A.O.; Mamba, G.; Ndlwana, L.; Ayanda, O.S.; et al. Multi-dimensional applications of graphitic carbon nitride nanomaterials—A review. J. Mol. Liq. 2021, 344, 117820. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Du, X.; Dong, F.; Zhang, Y. Rational nanostructure design of graphitic carbon nitride for photocatalytic applications. J. Mater. Chem. A 2019, 7, 11584–11612. [Google Scholar] [CrossRef]
- Li, H.; Zhu, B.; Cheng, B.; Luo, G.; Xu, J.; Cao, S. Single-atom Cu anchored on N-doped graphene/carbon nitride heterojunction for enhanced photocatalytic H2O2 production. J. Mater. Sci. Technol. 2023, 161, 192–200. [Google Scholar] [CrossRef]
- Yang, P.; Shen, A.; Zhu, Z.; Wang, L.; Tang, R.; Yang, K.; Chen, M.; Dai, H.; Zhou, X. Construction of carbon nitride-based heterojunction as photocatalyst for peroxymonosulfate activation: Important role of carbon dots in enhancing photocatalytic activity. Chem. Eng. J. 2023, 464, 142724. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Yang, W.; Xiao, Y.; Zeng, L.; Wu, X.; Xu, Q.; Su, C.; He, Q. Homogeneous Carbon/Potassium-Incorporation Strategy for Synthesizing Red Polymeric Carbon Nitride Capable of Near-Infrared Photocatalytic H2 Production. Adv. Mater. 2021, 33, 2101455. [Google Scholar] [CrossRef]
- Dash, P.; Thirumurugan, S.; Nataraj, N.; Lin, Y.C.; Liu, X.; Dhawan, U.; Chung, R.J. Near-Infrared Driven Gold Nanoparticles-Decorated g-C3N4/SnS2 Heterostructure through Photodynamic and Photothermal Therapy for Cancer Treatment. Int. J. Nanomed. 2024, 19, 10537–10550. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Zhang, W.; Kheradmand, A.; Gu, S.; Kobielusz, M.; Macyk, W.; Li, H.; Huang, J.; Jiang, Y. Near-Infrared-Triggered Nitrogen Fixation over Upconversion Nanoparticles Assembled Carbon Nitride Nanotubes with Nitrogen Vacancies. ACS Appl. Mater. Interfaces 2021, 13, 32937–32947. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective polymeric carbon nitride: Fabrications, photocatalytic applications and perspectives. Chem. Eng. J. 2022, 427, 130991. [Google Scholar] [CrossRef]
- Wang, J.; Gao, B.; Dou, M.; Huang, X.; Ma, Z. A porous g-C3N4 nanosheets containing nitrogen defects for enhanced photocatalytic removal meropenem: Mechanism, degradation pathway and DFT calculation. Environ. Res. 2020, 184, 109339. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-vacancy modified graphitic carbon nitride: Enhanced CO2 photocatalytic reduction performance and mechanism probing. J. Mater. Chem. A 2019, 7, 1556–1563. [Google Scholar] [CrossRef]
- Niu, P.; Liu, G.; Cheng, H.-M. Nitrogen Vacancy-Promoted Photocatalytic Activity of Graphitic Carbon Nitride. J. Phys. Chem. C 2012, 116, 11013–11018. [Google Scholar] [CrossRef]
- Dong, G.; Qiu, P.; Chen, Q.; Huang, C.; Chen, F.; Liu, X.; Li, Z.; Wang, Y.; Zhao, Y. K+-Intercalated carbon nitride with electron storage property for high-efficiency visible light driven nitrogen fixation. Chem. Eng. J. 2022, 433, 133573. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Yuan, X.; Guo, J.; Liang, J.; Tang, W.; Chen, Y.; Li, X.; Wang, H.; Chu, W. Defect engineering in polymeric carbon nitride photocatalyst: Synthesis, properties and characterizations. Adv. Colloid Interface Sci. 2021, 296, 102523. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shan, R.; Gu, J.; Wang, S.; Cheng, L.; Yuan, H.; Chen, Y. Unraveling the impact of three coordinate nitrogen (N3C) vacancies in porous carbon nitride nanobelt for boosted photocatalytic degradation of microplastics and antibiotics. Appl. Catal. B Environ. Energy 2024, 358, 124402. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, P.; Ma, D.; Zhong, J. Construction of three-coordinated (N3C) nitrogen vacancies in g-C3N4 for efficient photocatalytic CO2 reduction. Ceram. Int. 2024, 50 Pt B, 33131–33142. [Google Scholar] [CrossRef]
- Liao, J.; Cui, W.; Li, J.; Sheng, J.; Wang, H.; Dong, X.; Chen, P.; Jiang, G.; Wang, Z.; Dong, F. Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4. J. Chem. Eng. J. 2020, 379, 122282. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.-L.; Wang, B.; Chen, C.; Huang, Y.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of Dopants and Defects in Graphitic Carbon Nitride with Exceptionally Modulated Band Structures for Efficient Photocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Wang, H.; Bu, Y.; Wu, G.; Zou, X. The promotion of the photocatalytic nitrogen fixation ability of nitrogen vacancy-embedded graphitic carbon nitride by replacing the corner-site carbon atom with phosphorus. Dalton Trans. 2019, 48, 11724–11731. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Zhang, X.; Fan, J.; Lv, K.; Carabineiro, S.A.; Dong, F. 2D g-C3N4 for advancement of photo-generated carrier dynamics: Status and challenges. Mater. Today 2020, 41, 270–303. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhang, X.; Tian, J. Structure engineering of 1T/2H multiphase MoS2 via oxygen incorporation over 2D layered porous g-C3N4 for remarkably enhanced photocatalytic hydrogen evolution. Mater. Today Nano 2022, 18, 100204. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Liu, S.; Tong, J.; Kong, F.; Sun, N.; Han, X.; Zhang, Y. Enhanced photocatalytic activity and optical response mechanism of porous graphitic carbon nitride (g-C3N4) nanosheets. Mater. Res. Bull. 2021, 140, 111263. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Gan, L.; Meng, J.; Feng, Y.; Wang, K.; Zhou, K.; Wang, C.; Han, X.; Zhou, X. Amorphous Carbon Nitride with Three Coordinate Nitrogen (N3C) Vacancies for Exceptional NO Abatement in Visible Light. Adv. Energy Mater. 2021, 11, 2004001. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Cheng, C.; Dai, X.; Chen, F.; Ning, J.; Wang, W.; Hu, Y. Photochemical Tuning of Tricoordinated Nitrogen Deficiency in Carbon Nitride to Create Delocalized π Electron Clouds for Efficient CO2 Photoreduction. ACS Catal. 2024, 14, 10204–10213. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Zhou, Q.; Zhang, H.; Zhang, H.; An, B.; Ning, H.; Xing, T.; Wang, M.; Wu, M.; et al. Boosting C3H6 Epoxidation via Tandem Photocatalytic H2O2 Production over Nitrogen-Vacancy Carbon Nitride. ACS Catal. 2023, 13, 13101–13110. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Tang, Y.; Zhang, H.; Li, K. Preparation of phosphorus-doped mesoporous g-C3N4 and its photocatalytic degradation of tetracycline hydrochloride. Microporous Mesoporous Mater. 2023, 360, 112733. [Google Scholar] [CrossRef]
- Dong, G.; Ho, W.; Wang, C. Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies. J. Mater. Chem. A 2015, 3, 23435–23441. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Lv, K.; Wu, X.; Li, Q.; Li, Y.; Li, X.; Sun, J. Drastic promoting the visible photoreactivity of layered carbon nitride by polymerization of dicyandiamide at high pressure. Appl. Catal. B Environ. 2018, 232, 330–339. [Google Scholar] [CrossRef]
- Kang, Y.; YANG, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Qiao, W.; Li, L.; Zhu, Z. Ammonia-induced robust photocatalytic hydrogen evolution of graphitic carbon nitride. Nanoscale 2015, 7, 18887–18890. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Che, G.; Liao, G.; Dong, H.; Li, C.; Song, N.; Li, C. Facile construction of porous intramolecular g-C3N4-based donor-acceptor conjugated copolymers as highly efficient photocatalysts for superior H2 evolution. Nano Energy 2020, 67, 104273. [Google Scholar] [CrossRef]
- Gou, N.; Yang, W.; Gao, S.; Li, Q. Incorporation of ultrathin porous metal-free graphite carbon nitride nanosheets in polyvinyl chloride for efficient photodegradation. J. Hazard. Mater. 2023, 447, 130795. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Zhang, M.; Zhang, X.; Lv, K.; Liu, Y.; Ho, W.; Dong, F. C3N4 with engineered three coordinated (N3C) nitrogen vacancy boosts the production of 1O2 for Efficient and stable NO photo-oxidation. Chem. Eng. J. 2020, 389, 124421. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Chen, Z. A solid-state chemical reduction approach to synthesize graphitic carbon nitride with tunable nitrogen defects for efficient visible-light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 535, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Tong, H.; Odutola, J.; Song, J.; Peng, L.; Tkachenko, N.; Antonietti, M.; Pelicano, C.M. Boosting the Quantum Efficiency of Ionic Carbon Nitrides in Photocatalytic H2O2 Evolution via Controllable n → π* Electronic Transition Activation. Adv. Mater. 2024, 36, 2412753. [Google Scholar] [CrossRef]
- Zhan, X.; Zeng, Y.; Zhang, H.; Wang, X.; Jin, D.; Jin, H.; Luo, S.; Yang, L.; Hong, B. The coral-like carbon nitride array: Rational design for efficient photodegradation of tetracycline under visible light. J. Environ. Chem. Eng. 2023, 11, 109201. [Google Scholar] [CrossRef]
- Fu, J.; Mo, Z.; Chen, H.; Chen, Z.; Zhu, X.; Yan, J.; Liu, J.; Wei, Y.; Li, H.; Xu, H. Three coordinate nitrogen (N3C) vacancies from in-situ hydrogen bond breaking over polymeric carbon nitride for efficient photocatalysis. J. Environ. Chem. Eng. 2023, 11, 109495. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Li, T.; Zhang, L.; Hu, C. Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant. Appl. Catal. B Environ. 2020, 268, 118397. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, L.; Bibi, R.; Wang, K.; Hao, D.; Zhou, J.; Li, N. Boosting photocatalytic degradation of tetracycline under visible light over hierarchical carbon nitride microrods with carbon vacancies. J. Hazard. Mater. 2021, 413, 125376. [Google Scholar] [CrossRef]
- Sun, H.; Guo, F.; Pan, J.; Huang, W.; Wang, K.; Shi, W. One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem. Eng. J. 2021, 406, 126844. [Google Scholar] [CrossRef]
- Chang, L.-L.; Hu, C.; Wang, C.-Y.; Chen, W.-L.; Yamada, K.; Wu, A.-Y.; Yoshida, M.; Chien, S.-C.; Tung, K.-L. Synergistic effects of carbon and nitrogen vacancies in carbon nitride for photocatalytic H2 production and tetracycline oxidation. Sep. Purif. Technol. 2025, 354, 129346. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, Z.; Fu, N.; Li, X.; Hu, L.; Li, X.; Zhang, C. Novel feathery P/S Co-doped graphitic carbon nitride for highly efficient synergistic photocatalytic H2O2 generation and tetracycline degradation. RSC Adv. 2024, 14, 38391–38402. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Kunnel, E.S.; Trivedi, S.; Sasidharan, R.; Kumar, A.; Chinthala, M. Mechanistic Insights into Simultaneous Oxygen-Doped and Defect-Engineered Carbon Nitride as a Multifunctional Photocatalyst for Tetracycline Degradation, N2 Fixation, and H2O2 Production. Ind. Eng. Chem. Res. 2024, 63, 18975–18988. [Google Scholar] [CrossRef]
- Cai, W.; Wu, X.; Ren, G.; Tan, J.; He, X.; Tu, C.; Liu, Y.; He, Y. Photocatalytic degradation of tetracycline by g-C3N4 homostructure modified by cyano group and nitrogen defect. Diam. Relat. Mater. 2024, 143, 110898. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Y.; Xu, J.; Xing, J.; Lin, H.; Wang, L. Introducing Br, K, and cyano group into carbon nitride for efficient photocatalytic hydrogen peroxide production then in situ tetracycline mineralization. J. Colloid Interface Sci. 2024, 667, 433–440. [Google Scholar] [CrossRef]
- Xing, J.; Huang, X.; Yong, X.; Li, X.; Li, J.; Wang, J.; Wang, N.; Hao, H. N-doped synergistic porous thin-walled g-C3N4 nanotubes for efficient tetracycline photodegradation. Chem. Eng. J. 2023, 455, 140570. [Google Scholar] [CrossRef]
- Lian, X.; Pelicano, C.M.; Huang, Z.; Yi, X.; Savateev, A.; Antonietti, M. Rational Design of TaON/Potassium Poly(Heptazine Imide) Heterostructure for Multifunctional Environmental Remediation. Adv. Funct. Mater. 2024, 34, 2403653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).