Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of Nickel Wire, Copper Oxide and Cobalt Oxide (NW@CuO@Co3O4) Composite Wire
2.3. Characterization
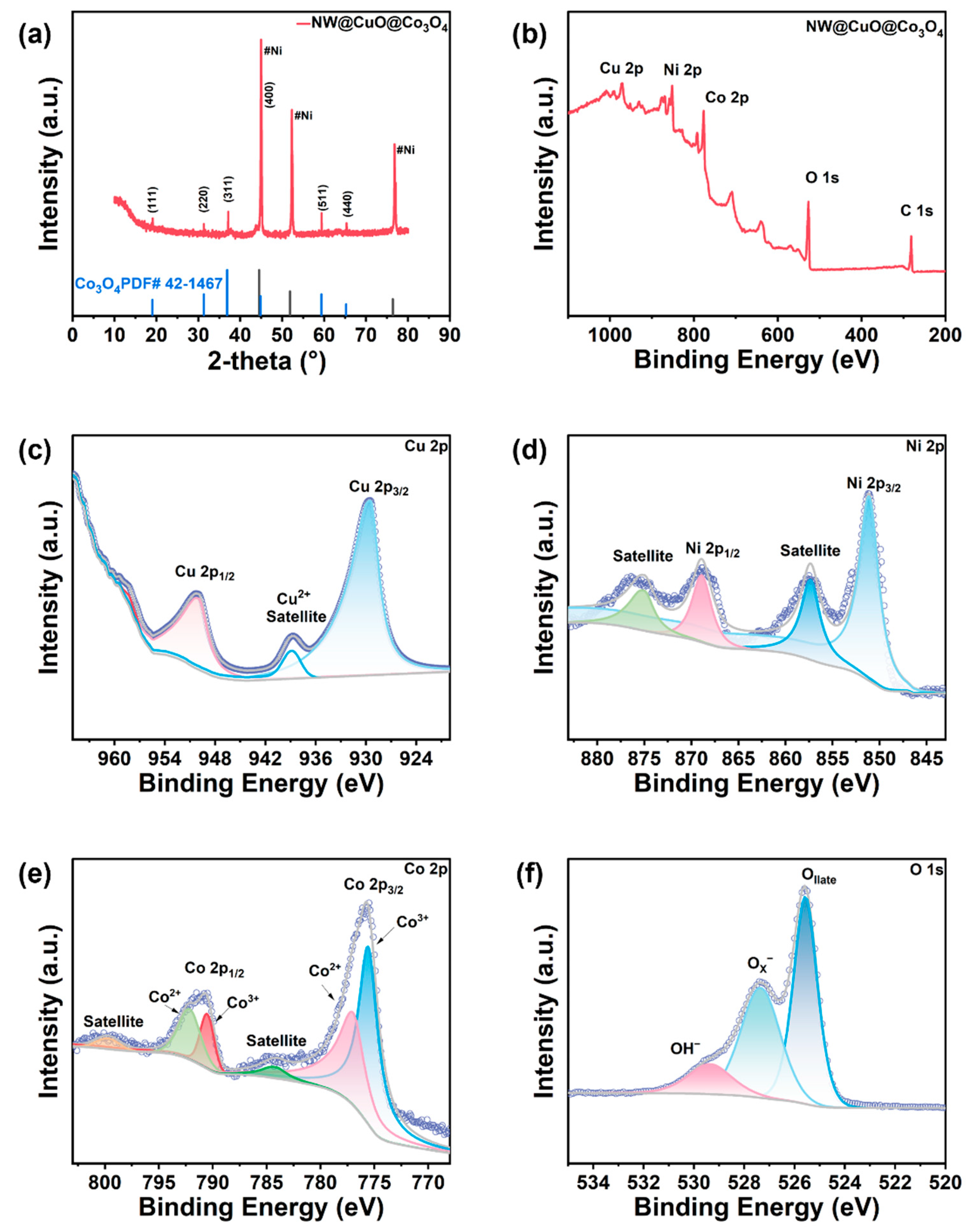
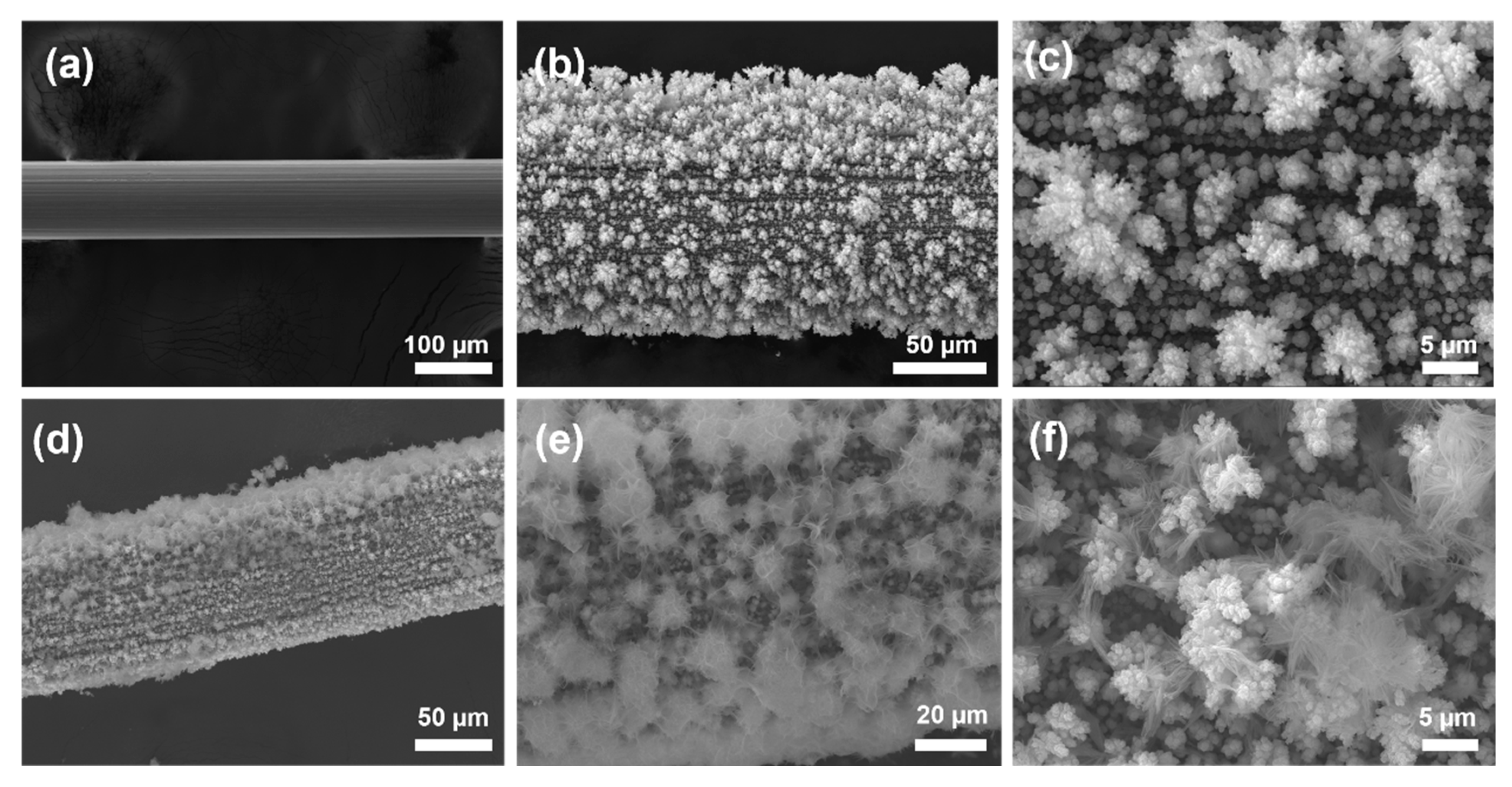
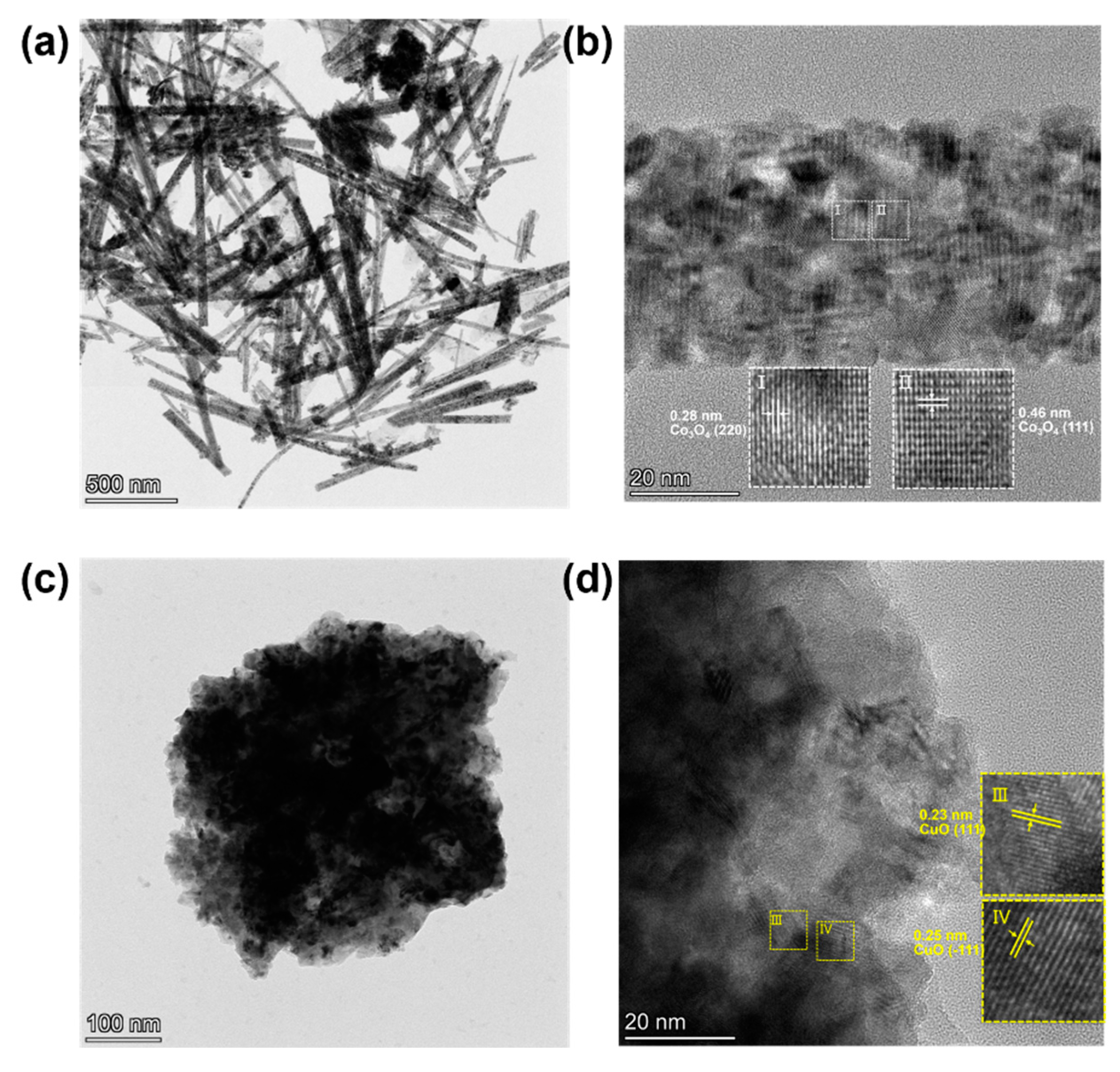
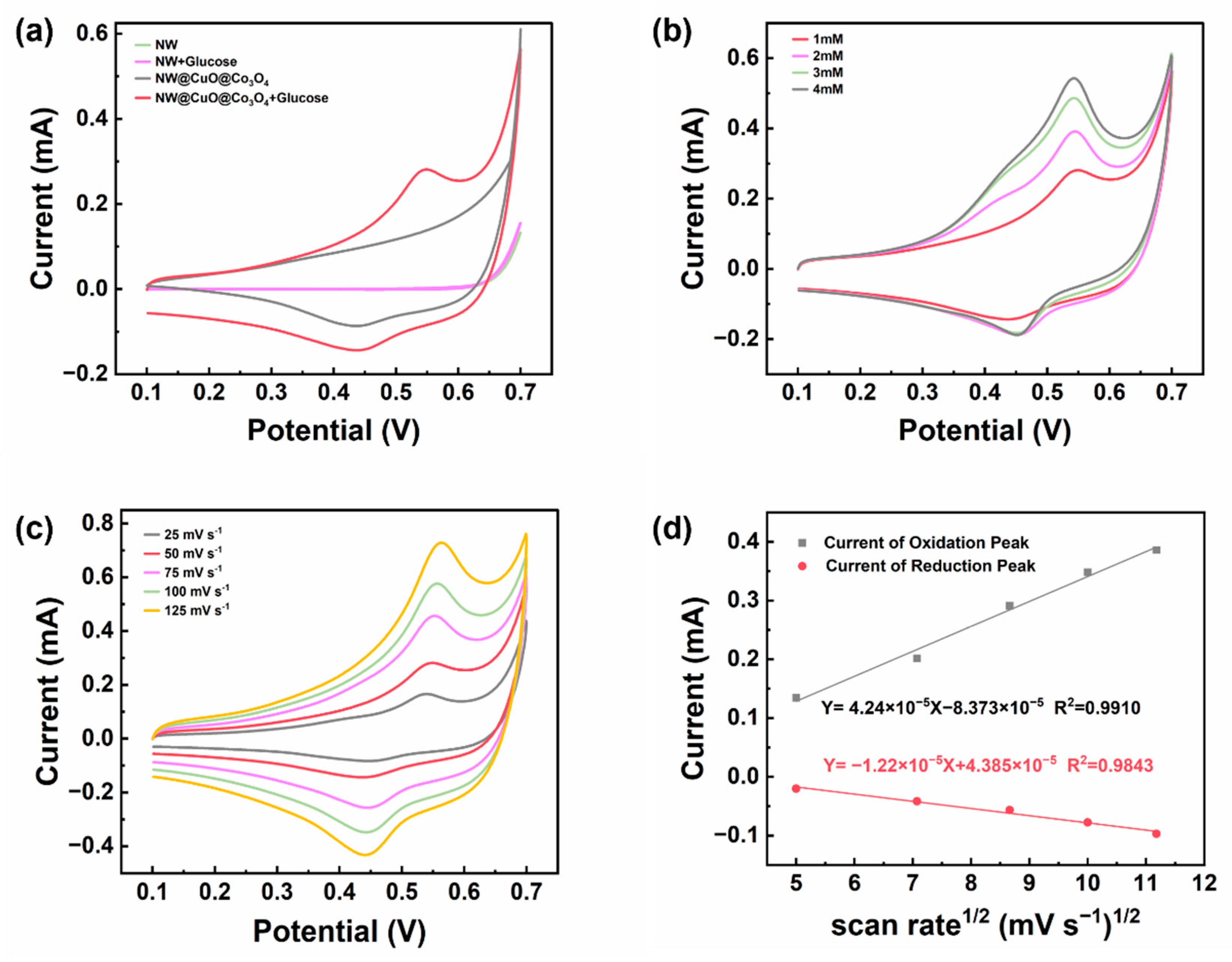
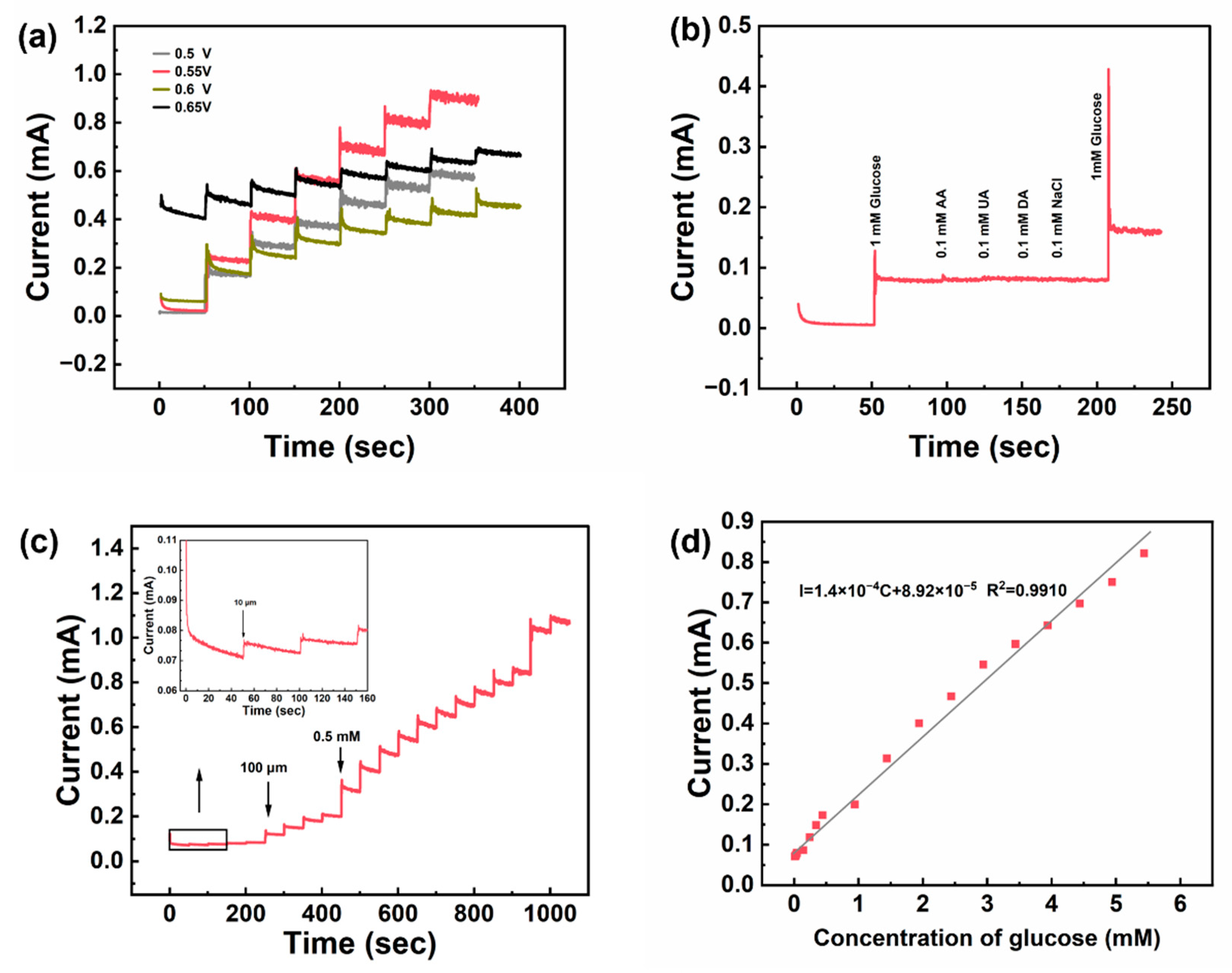
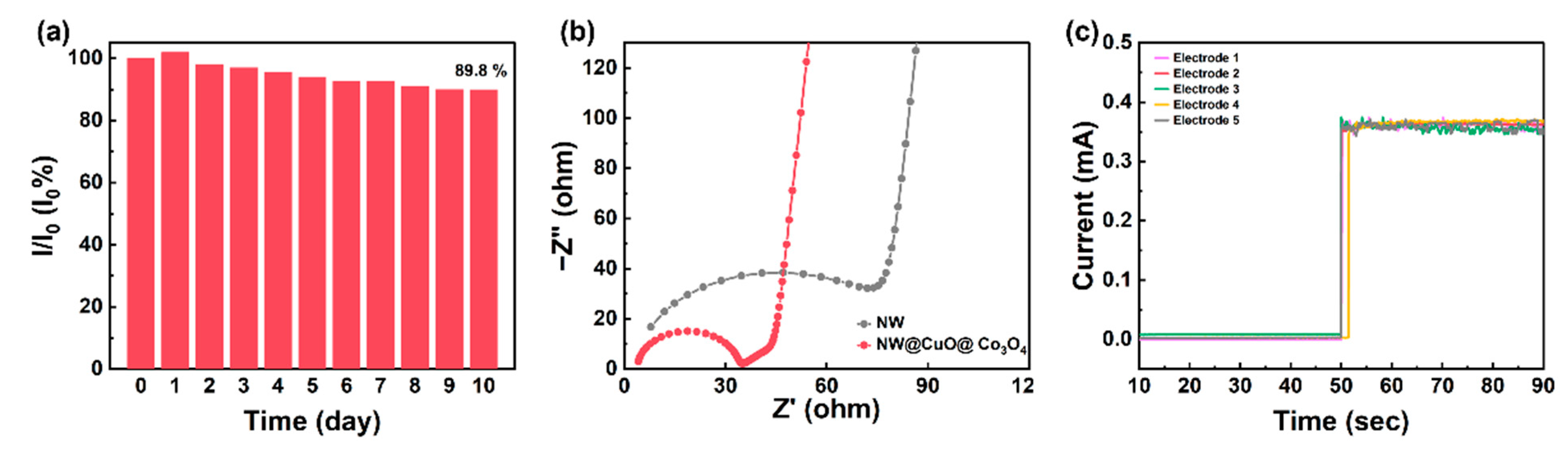
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadpour-Haratbar, A.; Mohammadpour-Haratbar, S.; Zare, Y.; Rhee, K.Y.; Park, S.J. A Review on Non-Enzymatic Electrochemical Biosensors of Glucose Using Carbon Nanofiber Nanocomposites. Biosensors 2022, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Glucose Electrochemical Biosensors: The Past and Current Trends. Int. J. Electrochem. Sc. 2021, 16, 210719. [Google Scholar] [CrossRef]
- Lipinska, W.; Grochowska, K.; Siuzdak, K. Enzyme Immobilization on Gold Nanoparticles for Electrochemical Glucose Biosensors. Nanomaterials 2021, 11, 1156. [Google Scholar] [CrossRef]
- Jarnda, K.V.; Wang, D.Q.; Qurrat-Ul-Ain; Anaman, R.; Johnson, V.E.; Roberts, G.P.; Johnson, P.S..; Jallawide, B.J.R.; Kai, T.H.; Ding, P. Recent Advances in Electrochemical Non-Enzymatic Glucose Sensor for the Detection of Glucose in Tears and Saliva: A Review. Sensor Actuat A-Phys. 2023, 363, 114778. [Google Scholar] [CrossRef]
- Zohaa; Arif, D.; Hassan, M.; Abdullah, M.; Miran, W.; Nasir, M.A.; Batool, S.; Baig, M.A.; Liaqat, U. An Electrochemical Sensor Based on Copper Oxide Nanoparticles Loaded on a Mesoporous Mcm-41 for Non-Enzymatic Detection of Glucose. Ceram. Int. 2024, 50, 12614–12620. [Google Scholar] [CrossRef]
- Osuna, V.; Martinez, E.P.A.; Dominguez, R.B.; Rios, A.V. A Review on the Advances in Nanomaterials for Electrochemical Non-Enzymatic Glucose Sensors Working in Physiological Conditions. Chemosensors 2024, 12, 159. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Yang, J.H.; Xue, Y.H.; Dai, L.M. Ultraviolet/Ozone Treatment for Boosting Oer Activity of Mof Nanoneedle Arrays. Chem. Eng. J. 2022, 427, 131498. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2020, 32, 1902670. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhai, Q.F.; Dong, D.S.; An, T.C.; Gong, S.; Shi, Q.Q.; Cheng, W.L. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Joshi, B.; Wang, J.J.; Shan, X.N.; Mo, Y.L.; Hsu, T.T.C. Effects of Heat, Moisture/Water, and Compressive Force on Frequency Response Spectrum of Super Sensitive Carbon Nanofiber Aggregates (Sscnfa). Cement Concrete Comp. 2024, 152, 105638. [Google Scholar] [CrossRef]
- Li, W.G.; Guo, Y.P.; Zhang, X.R.; Dong, W.K.; Li, X.H.; Yu, T.; Wang, K.J. Development of Self-Sensing Ultra-High-Performance Concrete Using Hybrid Carbon Black and Carbon Nanofibers. Cement Concrete Comp. 2024, 148, 105466. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Celesti, C.; Iannazzo, D.; Ampelli, C.; Giusi, D.; Costantino, V.; Neri, G. Functionalization of Carbon Nanofibers with an Aromatic Diamine: Toward a Simple Electrochemical-Based Sensing Platform for the Selective Sensing of Glucose. Acs Omega 2024, 9, 27085–27092. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Wu, H.Y.; Xu, Y.P.; Ma, S.W.; Sun, M.; Wang, Q. Msm Fiber Optic Surface Plasmon Resonance Glucose Sensor Based on Sno2 Nanofibers/Au Structure. Photonic Sens. 2024, 15, 250119. [Google Scholar] [CrossRef]
- Kasiviswanathan, U.; Kumar, C.; Sahi, A.K.; Kumar, A.; Jit, S.; Sharma, N.; Mahto, S.K. Electrospun Stannic Oxide Nanofiber Thin-Film Based Sensing Device for Monitoring Functional Behaviors of Adherent Mammalian Cells. IEEE T Nanobiosci 2025, 24, 120–126. [Google Scholar] [CrossRef]
- Gu, L.Y.; Hu, J.; Zhu, D.C.; Zhang, W.X.; Cheng, M.; Wei, T.; Liu, Q.Q.; Wang, R.R.; Li, W.F.; Ling, Y.; et al. A Flexible Gas Sensor Based on One-Dimensional Cu2O-SnO2/N-C Nanofibers toward for NO2 Detection at Low Temperature. Sensor Actuat A-Phys. 2025, 383, 116257. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, D.Q.; Gooding, J.J.; Xue, Y.H.; Dai, L.M. Flexible Fiber-Shaped Non-Enzymatic Sensors with a Graphene-Metal Heterostructure Based on Graphene Fibres Decorated with Gold Nanosheets. Carbon 2018, 136, 329–336. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Wu, Y.T.; Yang, J.H.; Xue, Y.H. Core-Sheath Fibers Composed of F-Doped Nickel Hydroxide Nanorods and Graphene Fibers for Effective Fiber-Shaped Nonenzymatic Glucose Sensors. J. Alloys Compd. 2021, 889, 161608. [Google Scholar] [CrossRef]
- Khayyat, S.A.; Ansari, S.G.; Umar, A. Glucose Sensor Based on Copper Oxide Nanostructures. J. Nanosci. Nanotechnol. 2014, 14, 3569–3574. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.L.; Liu, Z.Y.; Cheng, S.Y.; Huang, Y.Y.; Tao, X.X.; Liao, G.L.; Tang, Z.R. Ultrasensitive Non-Enzymatic Glucose Sensors Based on Different Copper Oxide Nanostructures by in-Situ Growth. Sensor Actuat B-Chem. 2016, 236, 326–333. [Google Scholar] [CrossRef]
- Li, J.M.; Jin, X.; Li, R.G.; Zhao, Y.; Wang, X.Q.; Liu, X.D.; Jiao, H. Copper Oxide Nanowires for Efficient Photoelectrochemical Water Splitting. Appl. Catal. B-Environ. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Vediyappan, V.; Sivakumar, M.; Chen, S.M.; Lai, Q.W.; Madhu, R. Nanolayers of Carbon Protected Copper Oxide Nanocomposite for High Performance Energy Storage and Non-Enzymatic Glucose Sensor. J. Alloys Compd. 2021, 875, 160063. [Google Scholar] [CrossRef]
- Cheng, C.E.; Tangsuwanjinda, S.; Cheng, H.M.; Lee, P.H. Copper Oxide Decorated Zinc Oxide Nanostructures for the Production of a Non-Enzymatic Glucose Sensor. Coatings 2021, 11, 936. [Google Scholar] [CrossRef]
- Naderi, L.; Shahrokhian, S. Metal-Organic Framework-Assisted Co3o4/Cuo@Comnp with Core-Shell Nanostructured Architecture on Cu Fibers for Fabrication of Flexible Wire-Typed Enzyme-Free Micro-Sensors. Chem. Eng. J. 2023, 456, 141088. [Google Scholar] [CrossRef]
- Xiong, L.Y.; Kim, Y.J.; Seo, W.C.; Lee, H.K.; Yang, W.C.; Xie, W.F. High-Performance Non-Enzymatic Glucose Sensor Based on Co3o4/Rgo Nanohybrid. Rare Metals 2023, 42, 3046–3053. [Google Scholar] [CrossRef]
- Jiang, F.; Su, Q.M.; Li, H.J.; Yao, L.B.; Deng, H.H.; Du, G.H. Growth of Ultrafine Cuco2o4 Nanoparticle on Graphene with Enhanced Lithium Storage Properties. Chem. Eng. J. 2017, 314, 301–310. [Google Scholar] [CrossRef]
- Xiao, X.C.; Zhang, Z.Y.; Nan, F.; Zhao, Y.; Wang, P.K.; He, F.; Wang, Y.D. Mesoporous Cuco2o4 Rods Modified Glassy Carbon Electrode as a Novel Non-Enzymatic Amperometric Electrochemical Sensors with High-Sensitive Ascorbic Acid Recognition. J. Alloys Compd. 2021, 852, 157045. [Google Scholar] [CrossRef]
- Xu, J.N.; Li, F.H.; Wang, D.D.; Nawaz, M.H.; An, Q.B.; Han, D.X.; Niu, L. Co3o4 Nanostructures on Flexible Carbon Cloth for Crystal Plane Effect of Nonenzymatic Electrocatalysis for Glucose. Biosens. Bioelectron. 2019, 123, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; DelaCruz, S.; Chen, C.; Tang, Z.R.; Shi, T.L.; Carraro, C.; Maboudian, R. Hierarchical Co3O4/CuO Nanorod Array Supported on Carbon Cloth for Highly Sensitive Non-Enzymatic Glucose Biosensing. Sensor Actuat B-Chem. 2019, 298, 126860. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.M. Synthesis and Characterizations of Biscuit-Like Copper Oxide for the Non-Enzymatic Glucose Sensor Applications. J. Colloid. Interf. Sci. 2017, 493, 349–355. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, T.; Zhang, G.X.; Wu, H.M.; Mei, H. A Novel Nonenzymatic Electrochemical Sensor Based on Double-Shelled Cuco2o4 Hollow Microspheres for Glucose and H2O2. J. Alloys Compd. 2020, 819, 153014. [Google Scholar] [CrossRef]
- Heidari, H.; Habibi, E. Amperometric Enzyme-Free Glucose Sensor Based on the Use of a Reduced Graphene Oxide Paste Electrode Modified with Electrodeposited Cobalt Oxide Nanoparticles. Microchim. Acta 2016, 183, 2259–2266. [Google Scholar] [CrossRef]
- Tran, T.H.; Thi, M.L.N.; Son, N.T.; Bui, Q.B.; Ai-Le, P.H.; Nhac-Vu, H.T. Novel Nanoneedle Structures of Zinc-Doped Cobalt Hydroxide as a Self-Supported Sensor for Sensitive Glucose Detection. Mater. Res. Bull. 2019, 120, 110580. [Google Scholar] [CrossRef]
| Sensitive Materials | Operating Potential | Linear Interval (Up to, mM) | Sensitivity (μA· mM−1·cm−2) | Detection Limit (μM) | Ref |
|---|---|---|---|---|---|
| CuO biscuits/SPCE | 0.5 V | 4.03 | 308.71 | 0.1 | [29] |
| Ni(OH)F@GF | 0.55 V | 7.66 | 595.3 | 1 | [17] |
| CuCo2O4 | 0.55 V | 7.9 | 400 | 0.75 | [30] |
| Co(NO3)2@rGO | 0.45 V | 4 | 1210 | 1.4 | [31] |
| Zn-Co-OH/3D NF | 0.6 V | 3.53 | 6100 | 8.33 | [32] |
| NW | 0.55 V | 0.5 | 270 | 45.2 | this work |
| NW@CuO@Co3O4 | 0.55 V | 5.44 | 4053 | 0.89 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhu, Z.; Liu, X.; Xue, Y. Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor. Nanomaterials 2025, 15, 411. https://doi.org/10.3390/nano15060411
Wu Y, Zhu Z, Liu X, Xue Y. Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor. Nanomaterials. 2025; 15(6):411. https://doi.org/10.3390/nano15060411
Chicago/Turabian StyleWu, Yuxin, Zhengwei Zhu, Xinjuan Liu, and Yuhua Xue. 2025. "Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor" Nanomaterials 15, no. 6: 411. https://doi.org/10.3390/nano15060411
APA StyleWu, Y., Zhu, Z., Liu, X., & Xue, Y. (2025). Multilayer Core-Sheath Structured Nickel Wire/Copper Oxide/Cobalt Oxide Composite for Highly Sensitive Non-Enzymatic Glucose Sensor. Nanomaterials, 15(6), 411. https://doi.org/10.3390/nano15060411







