Crystal Phase and Morphology Control for Enhanced Luminescence in K3GaF6:Er3+
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
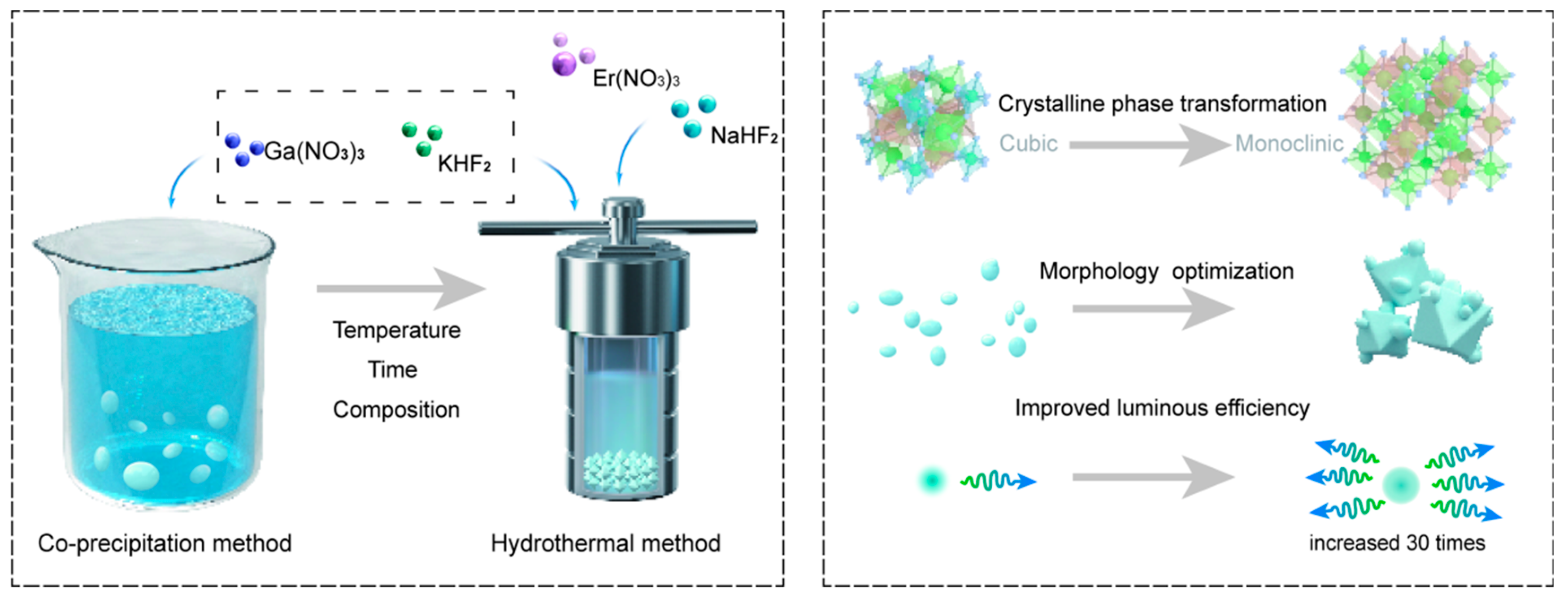
2.2. Synthesis Methods
2.2.1. Co-Precipitation Method for Preparing K3GaF6 Samples
2.2.2. Hydrothermal Method for Preparing K3GaF6 Samples
2.2.3. Hydrothermal Method for Preparing NaxK3-xGaF6 Samples
2.2.4. Hydrothermal Method for Preparing Na2.5K0.5GaF6:xEr3+ Samples
2.3. Testing Methods
3. Results and Discussion
3.1. Crystal Phase and Morphology
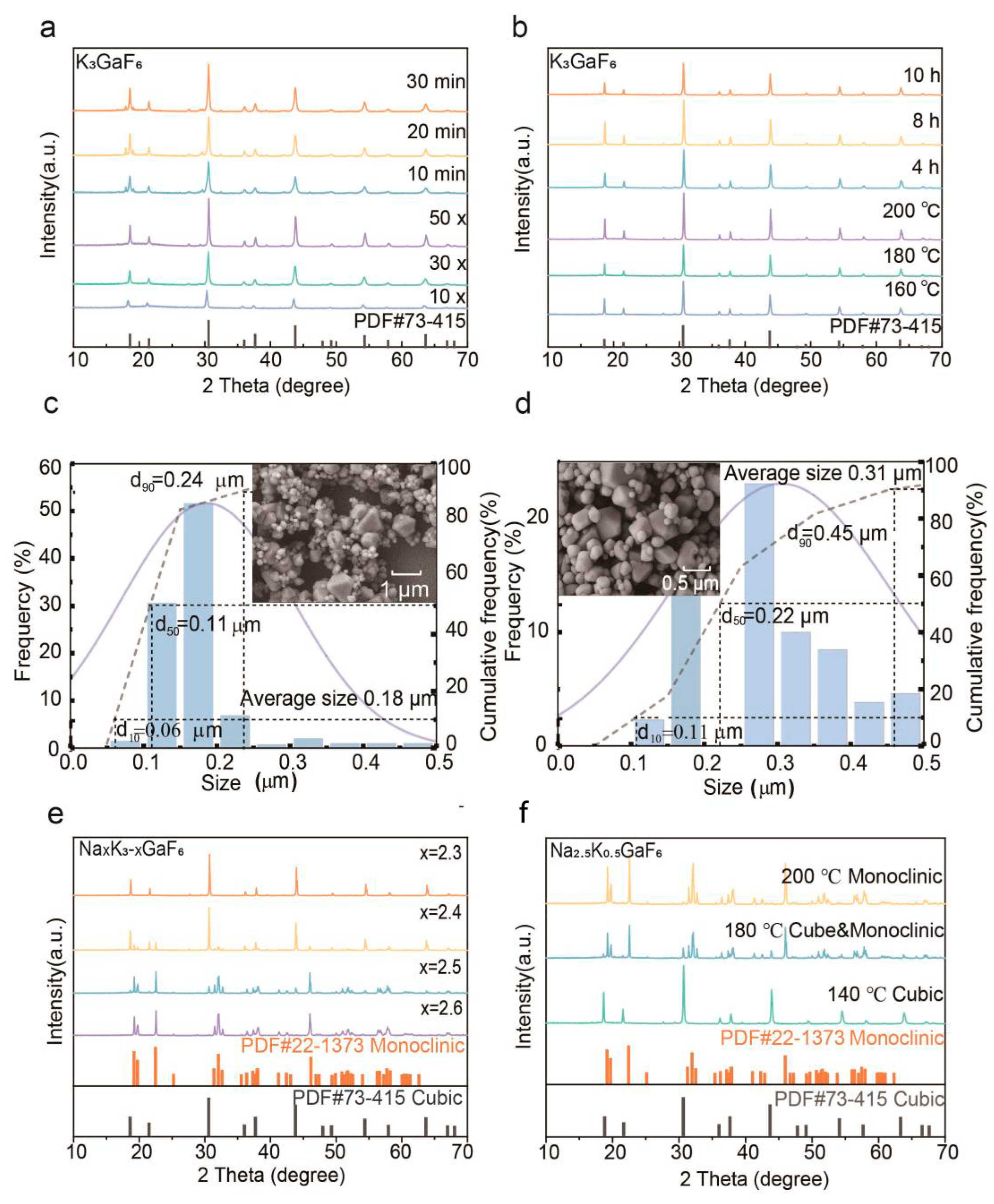
3.1.1. Phase Transition and Crystallinity
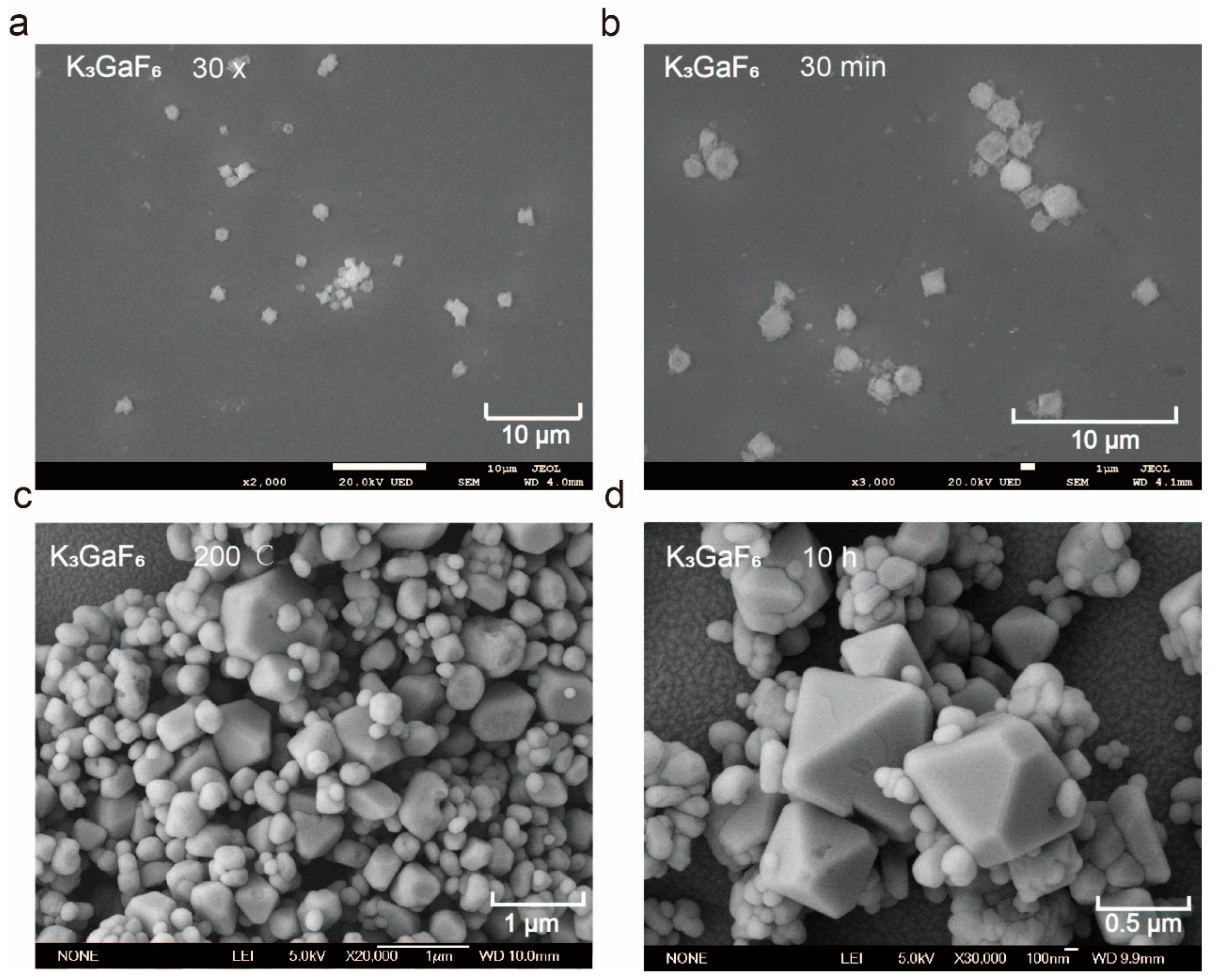
3.1.2. Morphology Evolution and Optimization
3.2. Luminescent Properties and Optimization
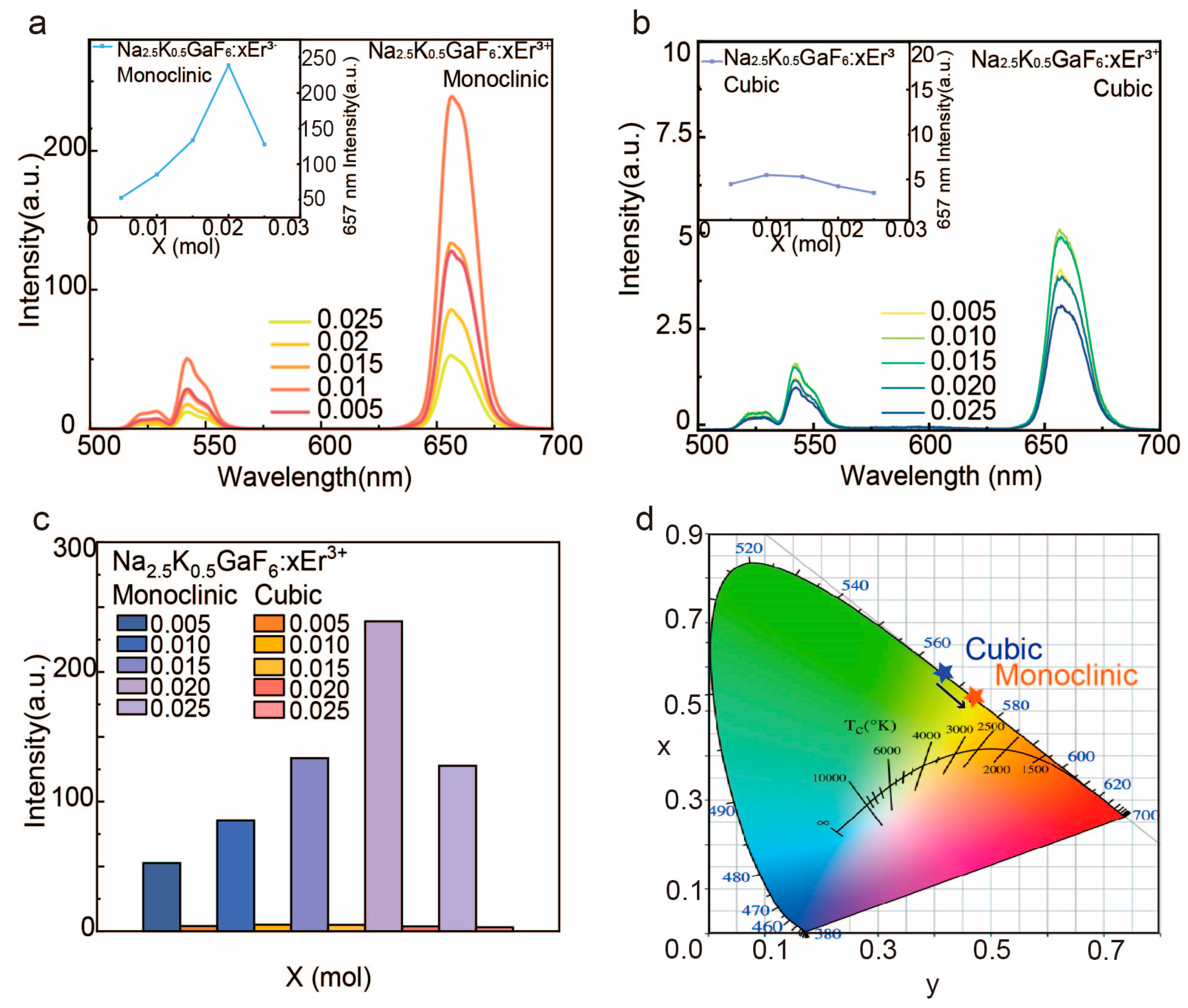
3.2.1. Spectral Characteristics of Cubic and Monoclinic Phases
3.2.2. Doping Concentration and Luminescent Efficiency
3.2.3. Synergistic Effects of Phase and Morphology Optimization
4. Practical Implications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auzel, F. Upconversion and Anti-Stokes processes with f and d ions in solids. ChemInform 2004, 104, 139–173. [Google Scholar]
- Wu, D.M.; Garcia-Etxarri, A.; Salleo, A.; Dionne, J.A. Plasmon-Enhanced Upconversion. J. Phys. Chem. Lett. 2014, 5, 4020–4031. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Qiu, H.L.; Prasad, P.N.; Chen, X.Y. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Pattelli, L.; Savo, R.; Burresi, M.; Wiersma, D.S. Spatio temporal Visualization of Light Transport in Complex Photonic Structures. Light Sci. Appl. 2016, 5, e16090. [Google Scholar] [CrossRef] [PubMed]
- Popov, N.; Ristic, M.; Kuncser, V.; Zadro, K.; Velinov, N.; Badica, P.; Alexandru-Dinu, A.; Iacob, N.; Krehula, L.K.; Musić, S.; et al. Influence of erbium doping on the structural, magnetic and optical properties of hematite (α-Fe2O3) nanorods. J. Phys. Chem. Solids 2022, 169, 110857. [Google Scholar] [CrossRef]
- Kruk, A.; Polnar, J. Investigation on the physicochemical properties of La-doped Er0.05Y1.95O3 nanopowders. J. Therm. Anal. Calorim. 2020, 139, 765–773. [Google Scholar] [CrossRef]
- Jiang, S.S.; Lin, J.; Huang, P. Nanomaterials for NIR-II Photoacoustic Imaging. Adv. Healthc. Mater. 2022, 12, 2202208. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, H.R.; Jin, Y.X.; Han, D.M. Recent Progress in Near-Infrared Organic Electroluminescent Materials. Top. Curr. Chem. 2022, 380, 6. [Google Scholar] [CrossRef]
- Zhou, Q.; Nozdriukhin, D.; Chen, Z.; Glandorf, L.; Hofmann, U.A.T.; Reiss, M.; Tang, L.; Deán-Ben, X.L.; Razansky, D. Depth-Resolved Localization Microangiography in the NIR-II Window. Adv. Sci. 2023, 10, 2204782. [Google Scholar] [CrossRef]
- Gao, C.; Zheng, P.; Liu, Q.; Han, S.; Li, D.; Luo, S.; Temple, H.; Xing, C.; Wang, J.; Wei, Y.; et al. Recent Advances of Upconversion Nanomaterials in the Biological Field. Nanomaterials 2021, 11, 2474. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2014, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Li, X.Y.; Chi, D.Z.; Zhang, H.J.; Liu, X.G. Lanthanide-Doped Upconversion Materials: Emerging Applications for Photovoltaics and Photocatalysis. Nanotechnology 2014, 25, 482001. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qi, D.; Zhao, H. Enhanced green upconversion luminescence in Ho3+ and Yb3+ codoped Y2O3 ceramics with Gd3+ ions. J. Lumin. 2013, 143, 388–392. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sun, X.; Si, R.; You, L.P.; Yan, C.H. Single-Crystalline and Monodisperse LaF3 Triangular Nanoplates from a Single Source Precursor. J. Am. Chem. Soc. 2005, 127, 3260–3261. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall Monodisperse NaYF4:Yb3+/Tm3+ Nanocrystals with Enhanced Near-Infrared to Near-Infrared Upconversion Photoluminescence. Acs Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Yin, Y.; McRae, C.; Piper, J.A.; Dawes, J.M.; Jin, D.; Goldys, E.M. Upconversion luminescence with tunable lifetime in NaYF4:Yb, Er nanocrystals: Role of nanocrystal size. Nanoscale 2013, 5, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, X.; Du, Y.; Qin, X.; Zhang, Y.; Ma, C.; Wen, S.; Ren, W.; Goldys, E.M.; Piper, J.A.; et al. Three-dimensional controlled growth of monodisperse sub-50nm heterogeneous nanocrystals. Nat. Commun. 2016, 7, 10254. [Google Scholar] [CrossRef]
- Ren, J.; Jia, G.; Guo, Y.; Wang, A.; Xu, S. Unraveling morphology and phase control of NaLnF4 upconverting nanocrystals. J. Phys. Chem. C 2016, 120, 1342–1351. [Google Scholar] [CrossRef]
- Huang, P.; Zheng, W.; Zhou, S.; Tu, D.; Chen, Z.; Zhu, H.; Li, R.; Ma, E.; Huang, M.; Chen, X. Lanthanide-Doped LiLuF4 Upconversion Nanoprobes for the Detection of Disease Biomarkers. Angew. Chem. 2014, 53, 1252–1257. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Synthesis of hexagonal-phase NaYF4: Yb, Er and NaYF4: Yb, Tm nanocrystals with efficient up-conversion fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, I.S.; Seo, H.; Park, Y.I.; Lee, J.H.; Kim, S.W.; Hyeon, T. Versatile PEG-derivatized phosphine oxide ligands for water-dispersible metal oxide nanocrystals. Chem. Commun. 2007, 48, 5167–5169. [Google Scholar] [CrossRef]
- Jang, H.S.; Woo, K.; Lim, K. Bright dual-mode green emission from selective set of dopant ions in β-Na(Y,Gd)F4:Yb,Er/β-NaGdF4:Ce,Tb core/shell nanocrystals. Opt. Express 2012, 20, 17107–17118. [Google Scholar] [CrossRef]
- Mendez-Ramos, J.; Tikhomirov, V.K.; Rodriguez, V.D.; Furniss, D. Infrared tuneable up-conversion phosphor based on Er3+-doped nano-glass–ceramics. J. Alloys Compd. 2007, 440, 328–332. [Google Scholar] [CrossRef]
- Goldner, P.; Pell, F.; Meichenin, D.; Auzel, F. Cooperative luminescence in ytterbium-doped CsCdBr3. J. Lumin. 1997, 71, 137–150. [Google Scholar] [CrossRef]
- Goldner, P.; Pell, F.; Auzel, F. Theoretical evaluation of cooperative luminescence rate in Yb3+: CsCdBr3 and comparison with experiment. J. Lumin. 1997, 72–74, 901–903. [Google Scholar] [CrossRef]
- Yue, X.Y.; Guo, Y.; Xua, J.; Hou, Y.; Zhu, H.K.; Wang, L.X.; Zhang, Q.T. Thermally stable fluoride phosphor Na3Sc2Li3F12:Cr3+ with broadband emission for NIR pc-LED applications. Opt. Mater. 2024, 149, 115040. [Google Scholar] [CrossRef]
- Jiao, M.X.; Portniagin, A.S.; Luo, X.L.; Jing, L.H.; Han, B.X.; Rogach, A.L. Semiconductor Nanocrystals Emitting in the Second Near-Infrared Window, Optical Properties and Application in Biomedical Imaging. Adv. Opt. Mater. 2022, 10, 2200226. [Google Scholar] [CrossRef]
- Yu, C.Y.; Yan, D.; Lou, S.Q.; Xia, C.; Cao, M.M.; Xuan, T.T.; Wang, J.; Li, H.L. Highly stabile ZnGa2O4: Eu nanocrystals as a fluorescence probe for bio-imaging. J. Lumin. 2018, 199, 492–498. [Google Scholar] [CrossRef]
- Dwivedi, A.; Srivastava, M.; Srivastava, A.; Kumar, A.; Chaurasia, R.N.; Srivastava, S.K. A Eu3+ doped functional core-shell nanophosphor as fluorescent biosensor for highly selective and sensitive detection of dsDNA. J. Photochem. Photobiol. B 2023, 249, 112802. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Rai, V.K. Ho3+-Yb3+, YMoO4 core@shell nanoparticles for enhanced visible upconversion and security applications. J. Alloys Compd. 2018, 750, 304–311. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Molokeev, M.S.; Liu, Q.L.; Xia, Z.G. Pure red upconversion luminescence and optical thermometry of Er3+ doped sensitizer-rich SrYbInO4 phosphors. J. Mater. Chem. C 2013, 6, 736–766. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.P.; Zhang, J.S.; Cao, C.Y.; Zhang, J.S.; Jin, Y.; Zhu, P.F.; Wei, G.D.; Wang, G.F.; Wang, L.L. Synthesis and green up-conversion fluorescence of colloidal La0.78Yb0.20Er0.02F3/SiO2 core/shell nanocrystals. Mater. Sci. 2007, 180, 2268–2272. [Google Scholar] [CrossRef]
- Rawat, P.; Saroj, S.K.; Gupta, M.; Prakash, G.V.; Nagarajan, R. Wet-chemical synthesis, structural characterization and optical properties of rare-earth doped halo perovskite K3GaF6. J. Fluor. Chem. 2017, 200, 1–7. [Google Scholar] [CrossRef]
- King, G. New examples of non-cooperative octahedral tilting in a double perovskite: Phase transitions in K3GaF6. Acta Cryst. 2020, B76, 789–794. [Google Scholar] [CrossRef]
- Shang, Y.F.; Hao, S.W.; Liu, J.; Tan, M.L.; Wang, N.; Yang, C.H.; Chen, G.Y. Synthesis of upconversion β-NaYF4:Nd3+/Yb3+/Er3+ particles with enhanced luminescent intensity through control of morphology and phase. Nanomaterials 2015, 5, 218–232. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Zhang, S.; Zhao, E.; Ren, J.; Liu, L.; Zhang, J. Mechanisms of Upconversion Luminescence of Er3+ Doped NaYF4 via 980 and 1530 nm Excitation. Nanomaterials 2021, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yang, Z.; Lai, S.; Shao, B.; Li, J.; Qiu, J.; Song, Z.; Yang, Y. Upconversion Emission Enhancement of NaYF4: Yb, Er Nanoparticles by Coupling Silver Nanoparticle Plasmons and Photonic Crystal Effects. J. Phys. Chem. C 2014, 118, 17992–17999. [Google Scholar] [CrossRef]
- Liao, J.; Yang, Z.; Shao, B.; Li, J.; Qiu, J.; Song, Z.; Yang, Y. Preparation and Photoluminescence Modification of NaGdF4: Eu3+ Nanorods in a Crystalline Colloidal Array. Sci. Adv. Mater. 2016, 8, 697–702. [Google Scholar] [CrossRef]
- Lee, C.; Bao, Z.; Fang, M.-H.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Leniec, G.; Kaczmarek, S.-M.; Brik, M.-G.; Tsai, Y.-T.; et al. Chromium(III)-Doped Fluoride Phosphors with Broadband Infrared Emission for Light-Emitting Diodes. Inorg. Chem. 2020, 59, 376–385. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Pan, X.; Zhang, Y.; Su, K.; Xie, R.-J.; Liao, J.; Mei, L.; Liao, L. Crystal Phase and Morphology Control for Enhanced Luminescence in K3GaF6:Er3+. Nanomaterials 2025, 15, 318. https://doi.org/10.3390/nano15040318
Guo Y, Pan X, Zhang Y, Su K, Xie R-J, Liao J, Mei L, Liao L. Crystal Phase and Morphology Control for Enhanced Luminescence in K3GaF6:Er3+. Nanomaterials. 2025; 15(4):318. https://doi.org/10.3390/nano15040318
Chicago/Turabian StyleGuo, Yilin, Xin Pan, Yidi Zhang, Ke Su, Rong-Jun Xie, Jiayan Liao, Lefu Mei, and Libing Liao. 2025. "Crystal Phase and Morphology Control for Enhanced Luminescence in K3GaF6:Er3+" Nanomaterials 15, no. 4: 318. https://doi.org/10.3390/nano15040318
APA StyleGuo, Y., Pan, X., Zhang, Y., Su, K., Xie, R.-J., Liao, J., Mei, L., & Liao, L. (2025). Crystal Phase and Morphology Control for Enhanced Luminescence in K3GaF6:Er3+. Nanomaterials, 15(4), 318. https://doi.org/10.3390/nano15040318






