Self-Assembled Sandwich-like Mixed Matrix Membrane of Defective Zr-MOF for Efficient Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UiO-66-NH2
2.3. Synthesis of Defective UiO-66-NH2
2.4. Synthesis of d-UiO-66-NH2 with Thin-Film Nanocomposite (TFN)
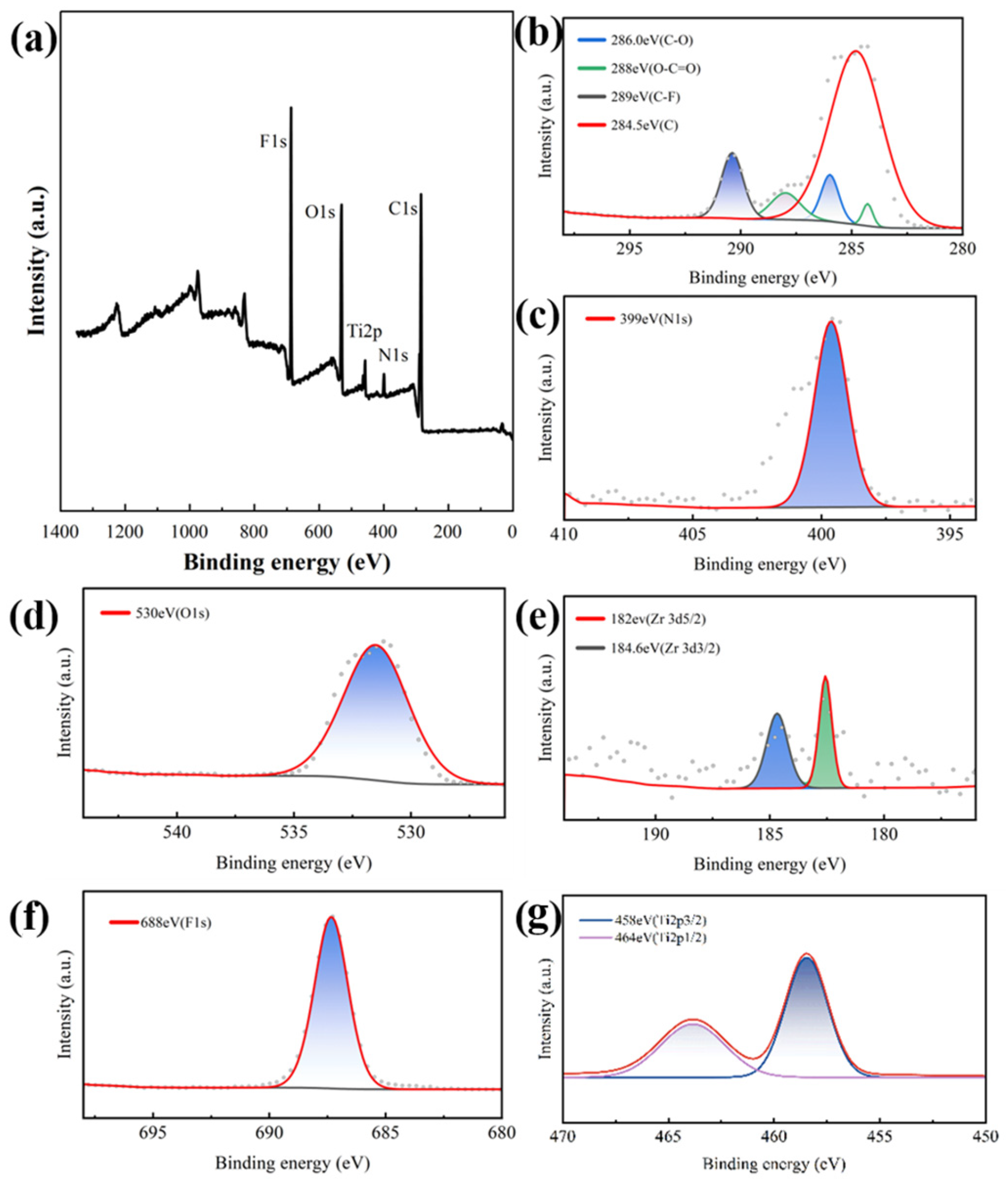
2.5. Characterization
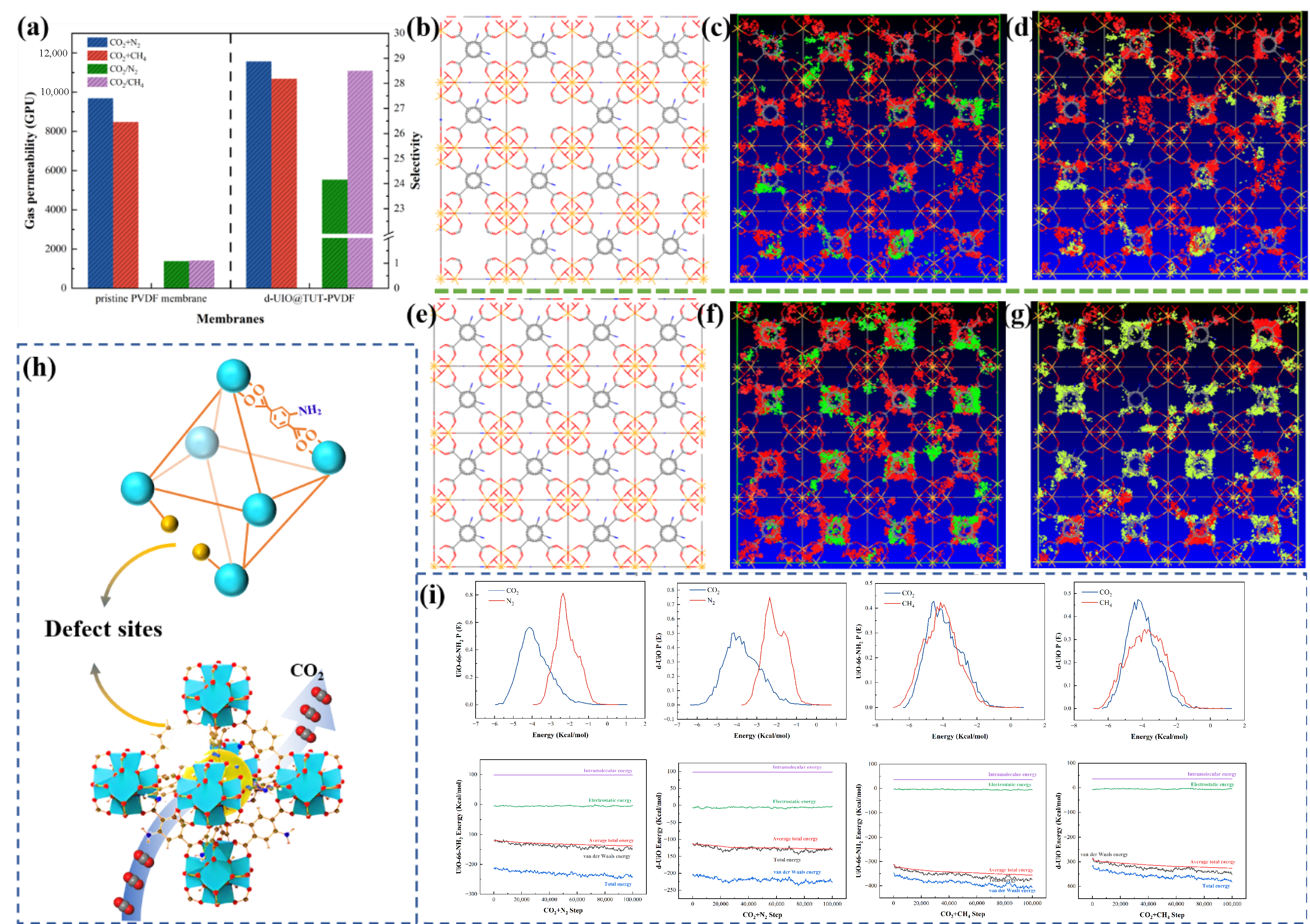
3. Results and Discussion
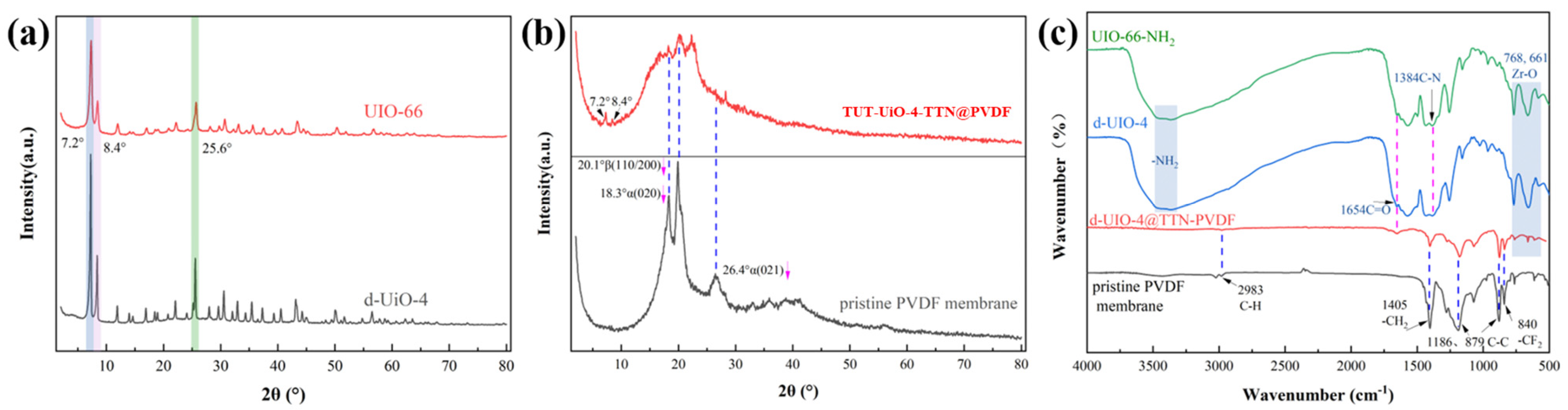
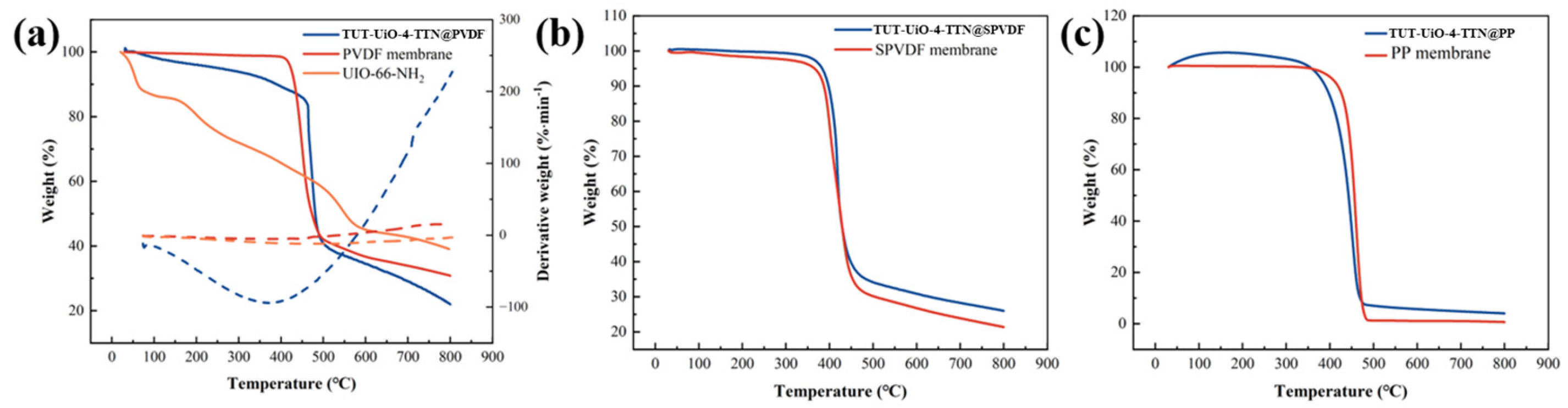
3.1. Characterization of TUT-UiO-TTN@PVDF Membranes
3.2. Crystal Structure of TUT-UiO-TTN@PVDF Membranes
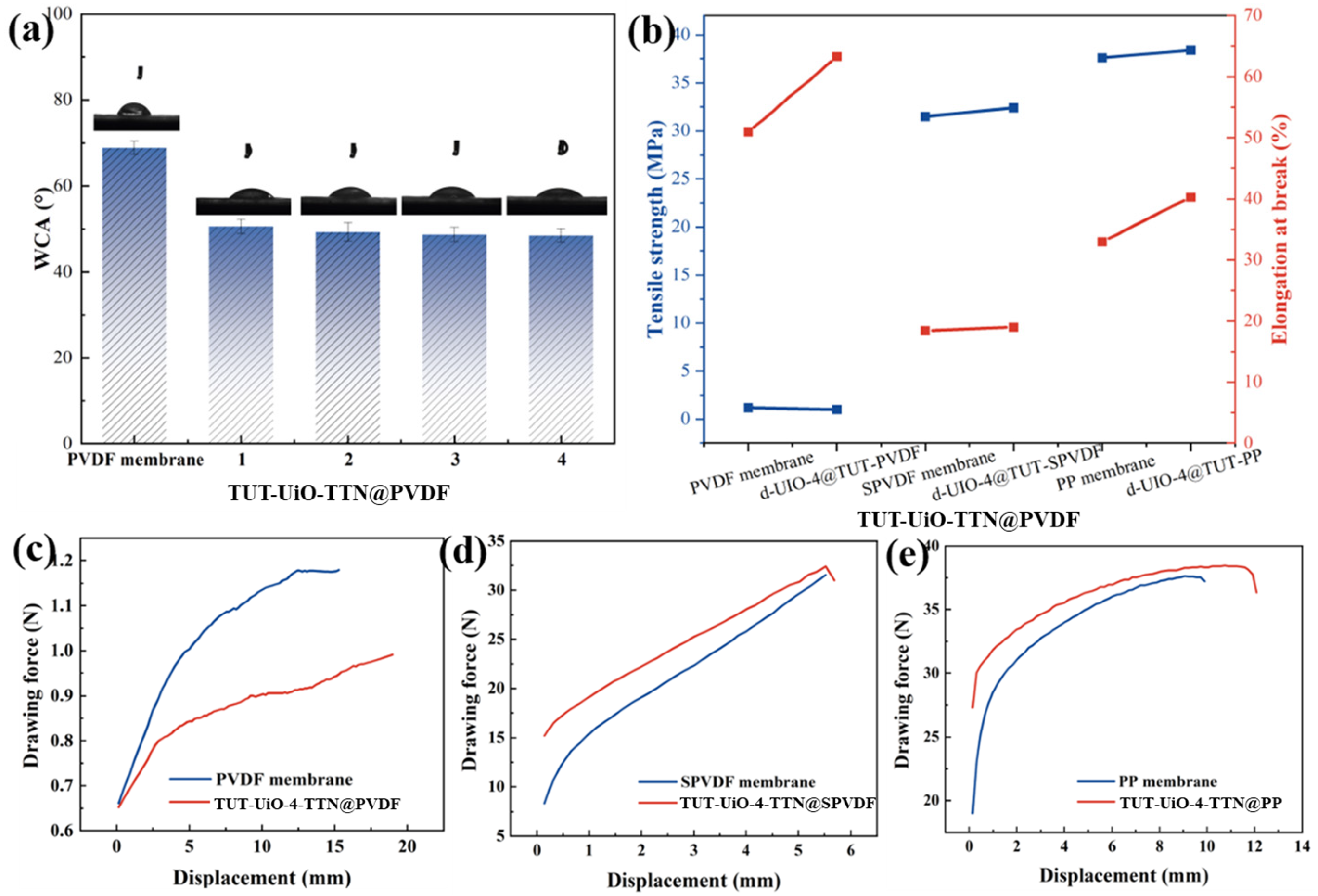
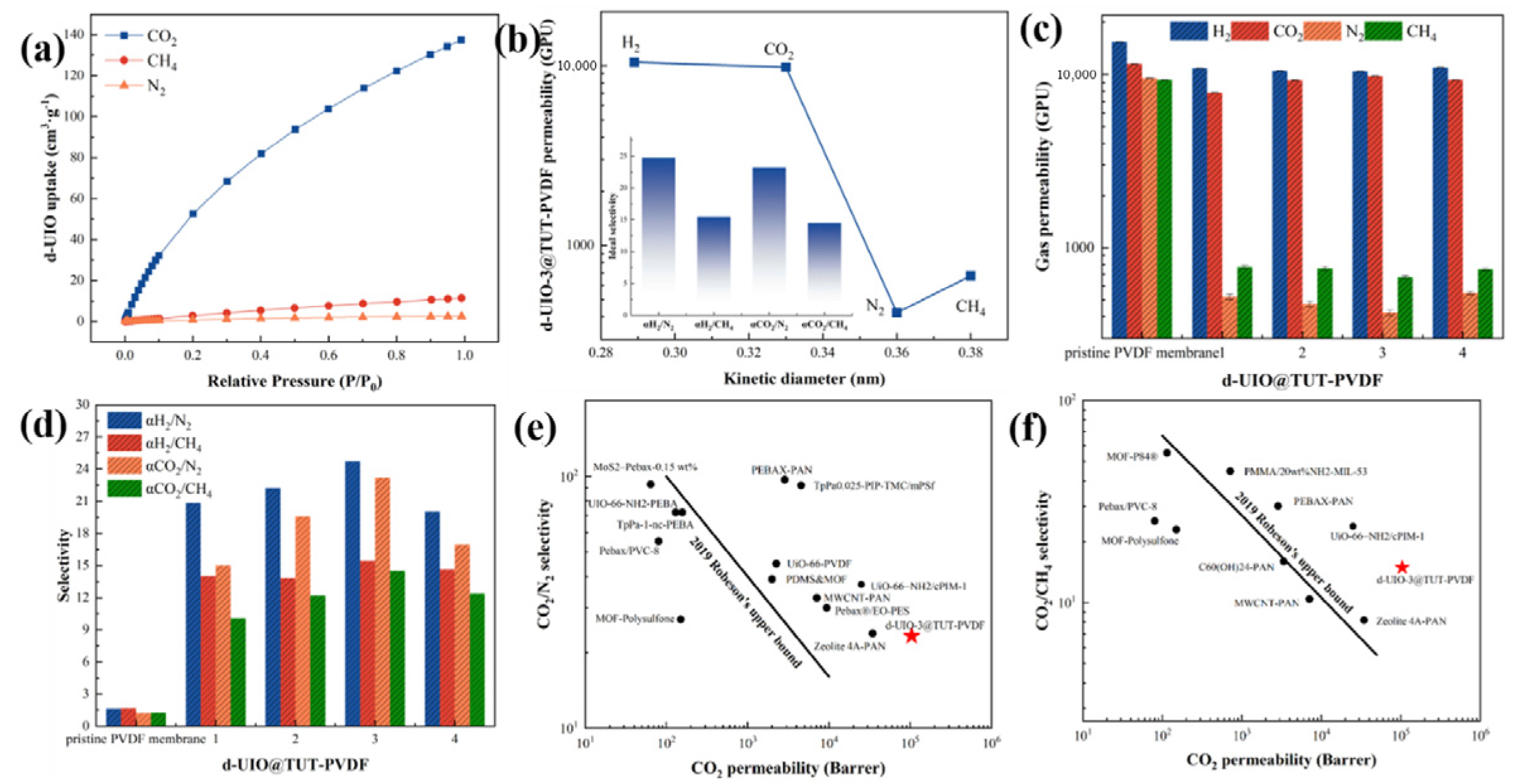
3.3. Performance of TUT-UiO-TTN@PVDF Membranes
3.4. Comprehensive Evaluation of Gas Separation Performances
3.4.1. Influence of Defect Strategy on Gas Separation Performance
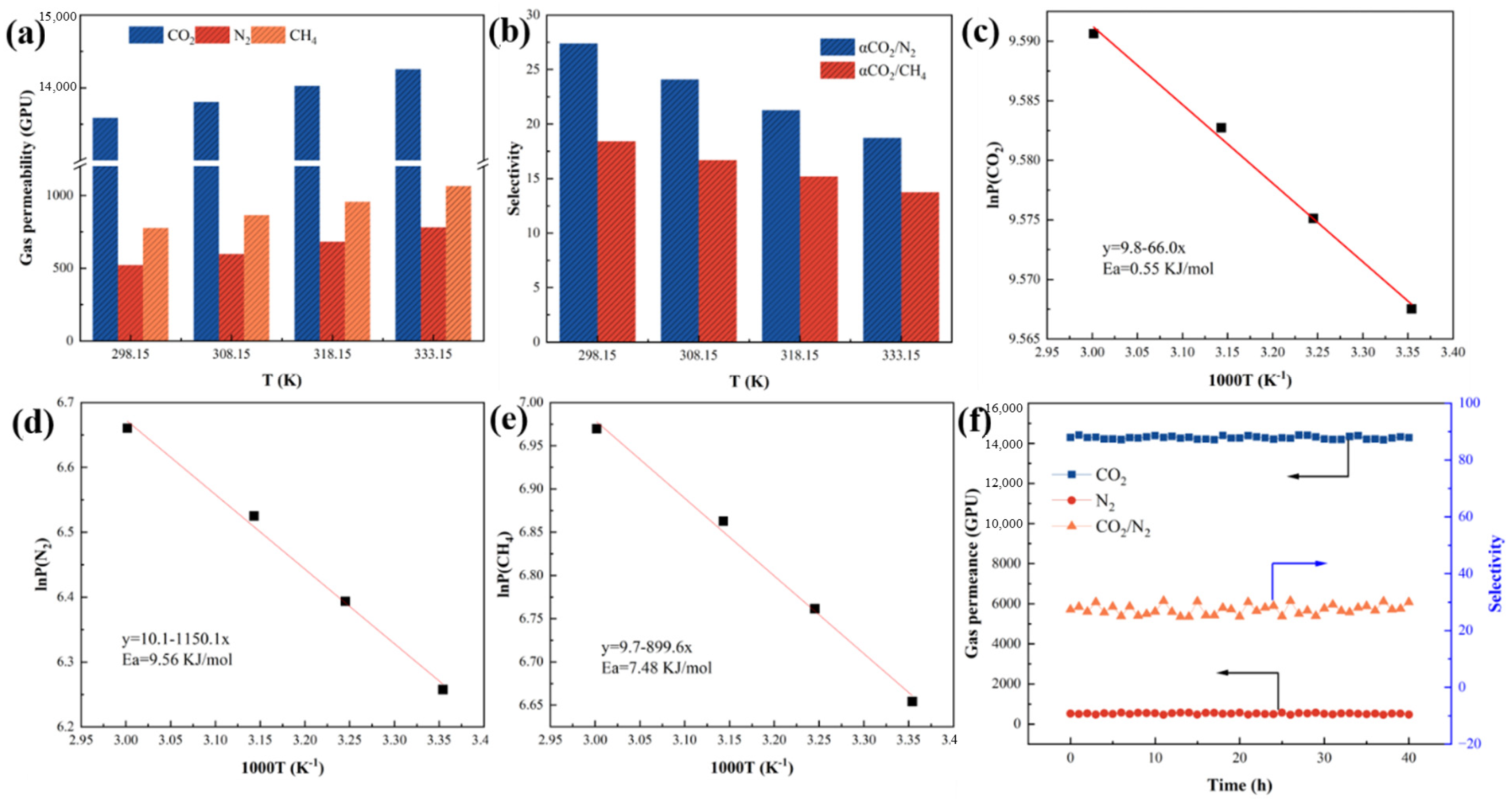
3.4.2. Influence of Gas Infiltration Activation Energy
3.4.3. Study on Separation Performance of Gas Mixture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMM | Mixed matrix membrane |
| TA | Tannic acid |
| Ti-BALDH | Polyvinylidene fluoride Dihydroxybis (ammonium lactato) Titanium (IV) |
| NH2-BDC | 2-Aminoterephthalic acid |
| TFN | Thin-film nanocomposite |
| TTN | Metal–phenolic coordination active coating |
| TTA | Titanium–tannic acid |
| SBET | Brunauer–Emmett–Teller (BET) specific surface area |
| SMicro | Surface area of micropores |
| VMicro | Microporous volume |
| VTotal | Total pore volume |
References
- Yoon, J.W.; Chang, H.; Lee, S.-J.; Hwang, Y.K.; Hong, D.-Y.; Lee, S.-K.; Lee, J.S.; Jang, S.; Yoon, T.-U.; Kwac, K.; et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 2017, 16, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, S.; Xue, R.; Liu, D.; Ren, H.; Zhang, R. Uncovering the characteristics of air pollutants emission in industrial parks and analyzing emission reduction potential: Case studies in Henan, China. Sci. Rep. 2021, 11, 23709. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Dziejarski, B.; Rodríguez-Estupiñán, P.; Fernández, V.B.; Giraldo, L.; Sreńscek-Nazzal, J.; Michalkiewicz, B.; Moreno-Piraján, J.C. Effective synthesis route of renewable activated biocarbons adsorbent for high CO2, CH4, H2, N2, C2H4 gas storage and CO2/N2, CO2/CH4, CO2/H2, C2H4/CH4 selectivity. Fuel 2024, 374, 132462. [Google Scholar] [CrossRef]
- Wei, R.; Alshahrani, T.; Chen, B.; Ibragimov, A.B.; Xu, H.; Gao, J. Advances in porous materials for efficient separation and purification of flue gas. Sep. Purif. Technol. 2025, 352, 128238. [Google Scholar] [CrossRef]
- dos Anjos Silva, G.R.; Moreira, V.R.; Amaral, M.C.S. Applications and advancements in membrane technologies for sustainable petroleum refinery wastewater treatment. J. Environ. Chem. Eng. 2025, 13, 115199. [Google Scholar] [CrossRef]
- Wu, T.; Shu, C.; Liu, S.; Xu, B.; Zhong, S.; Zhou, R. Separation Performance of Si-CHA Zeolite Membrane for a Binary H2/CH4 Mixture and Ternary and Quaternary Mixtures Containing Impurities. Energy Fuels 2020, 34, 11650–11659. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.-S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef]
- Hu, M.; Ni, J.; Zhang, B.; Neelakandan, S.; Wang, L. Crosslinked polybenzimidazoles containing branching structure as membrane materials with excellent cell performance and durability for fuel cell applications. J. Power Sources 2018, 389, 222–229. [Google Scholar] [CrossRef]
- Stepanova, E.A.; Atlaskin, A.A.; Kudryavtseva, M.S.; Shablykin, D.N.; Markin, Z.A.; Dokin, E.S.; Zarubin, D.M.; Prokhorov, I.O.; Vshivtsev, M.A.; Kazarina, O.V.; et al. Combining gas hydrate crystallization and membrane technology: A synergistic approach to natural gas separation. Chem. Eng. Process.—Process Intensif. 2025, 208, 110130. [Google Scholar] [CrossRef]
- Jin, K.; Pei, L.; Cai, Z.; Zhou, Y.; Zhang, X.; Zhao, Y.; Zhang, H.; Yang, Q.; Liang, S.; Wang, J. A smart gating microfiltration membrane to break aperture adaptability limit for industrial oily wastewater treatment. J. Membr. Sci. 2025, 719, 123762. [Google Scholar] [CrossRef]
- Conway, K.M.; Mi, B. Sacrificial membranes in water purification: Concepts, current status, and outlook. Sep. Purif. Technol. 2025, 362, 131748. [Google Scholar] [CrossRef]
- Park, H.W.; Baek, J.; Kim, W.-J. Forward osmosis and direct contact membrane distillation: Emerging membrane technologies in food and beverage processing. Innov. Food Sci. Emerg. Technol. 2024, 93, 103626. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Tonkonogovas, A.; Makarevicius, V. Thin-film composite membranes with tailored pore structures for enhanced gas separation performance. J. Environ. Chem. Eng. 2025, 13, 115527. [Google Scholar] [CrossRef]
- Usha, Z.R.; Liu, C.; Zhang, S.; Wang, Z. A comprehensive study of recent advances, challenges and factors of nanofiltration membrane in various industrial applications. Desalination 2025, 599, 118461. [Google Scholar] [CrossRef]
- Li, X.; Bian, H.; Huang, W.; Yan, B.; Wang, X.; Zhu, B. A review on anion-pillared metal–organic frameworks (APMOFs) and their composites with the balance of adsorption capacity and separation selectivity for efficient gas separation. Coord. Chem. Rev. 2022, 470, 214714. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y.; Li, X.; Wang, X.; Li, Y.; Feng, F.; Chai, X.; Zhong, J. Anion pillar inserted MOF-74-Cu with customized pore environment for efficient purification of natural gas. Fuel 2025, 382, 133678. [Google Scholar] [CrossRef]
- Ghaedi, S.; Rajabi, H.; Hadi Mosleh, M.; Sedighi, M. MOF biochar composites for environmental protection and pollution control. Bioresour. Technol. 2025, 418, 131982. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Hamarawf, R.F.; Omer, K.M. Adsorptive removal of toxic heavy metals from aquatic environment by metal organic framework (MOF): A review. J. Water Process Eng. 2025, 70, 106867. [Google Scholar] [CrossRef]
- Li, X.; Yan, B.; Huang, W.; Wang, X.; Peng, M.; Liu, L. A novel strategy of post defect modification (PDM) for synthesizing hydrophobic FA-UiO-66-CF3 with enhanced n-hexane vapor adsorption capacity under humidity. Microporous Mesoporous Mater. 2023, 356, 112595. [Google Scholar] [CrossRef]
- Xin, Q.; Dong, J.; Shao, W.; Ding, X.; Gao, N.; Zhang, L.; Jin, H.; Chen, H.; Zhang, Y. Reversible MOF-Based mixed matrix membranes for SO2/N2 separation: A photo-responsive approach. J. Membr. Sci. 2025, 714, 123431. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Guo, S.; Pan, Y.; Nezamzadeh-Ejhieh, A.; Lan, Q. Metal-organic frameworks (MOFs): A review of volatile organic compounds (VOCs) detection. Talanta 2025, 286, 127498. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, W.; Li, X.; Wang, X.; Yan, B.; Zhang, Z.; Zhong, J. Synthesis of dual-functionalized APTES-Bentonite/PVDF mixed-matrix membranes for the efficient separation of CO2/CH4 and CO2/N2. Mater. Today Commun. 2022, 31, 103431. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, J.; Ma, J.; Zheng, W.; Dong, G.; Li, X.; Tian, Z.; Zhang, Y.; Wang, J.; Wang, Y. Merging polymers of intrinsic microporosity and porous carbon-based zinc oxide composites in novel mixed matrix membranes for efficient gas separation. Green Energy Environ. 2025, 10, 203–213. [Google Scholar] [CrossRef]
- Wu, Q.; He, X.; Cui, C.; Qi, B.; Wei, J. Recent developments in Metal–organic framework-based mixed matrix membranes for hydrogen separation. Sep. Purif. Technol. 2025, 354, 128946. [Google Scholar] [CrossRef]
- Salehi, A.; Omidkhah, M.; Ebadi Amooghin, A.; Moftakhari Sharifzadeh, M.M. Improved gas separation performance of PMMA/Matrimid@5218/graphene oxide (GO) mixed matrix membranes. J. CO2 Util. 2025, 91, 103012. [Google Scholar] [CrossRef]
- Xin, Q.; Kong, S.; Cao, X.; Pan, Y.; Wan, K.; Chen, H.; Jin, H.; Gao, N.; Ding, X.; Zhang, Y. Heterostructure ZIF-8@MXene with sieving effect in mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2025, 355, 129779. [Google Scholar] [CrossRef]
- Neves, T.M.; Pollo, L.D.; Marcilio, N.R.; Tessaro, I.C. Insights into the development of carbon molecular sieve membranes from polymer blends for gas separation: A review. Gas Sci. Eng. 2024, 131, 205472. [Google Scholar] [CrossRef]
- Qiu, B.; Yu, M.; Luque-Alled, J.M.; Ding, S.; Foster, A.B.; Budd, P.M.; Fan, X.; Gorgojo, P. High Gas Permeability in Aged Superglassy Membranes with Nanosized UiO-66−NH2/cPIM-1 Network Fillers. Angew. Chem. Int. Ed. 2024, 63, e202316356. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Membr. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Imteyaz, S.; Maheshwari, A.; Rafiuddin. An outlook on membranes: Types, synthesis, and application in membrane technology. Hybrid Adv. 2025, 8, 100351. [Google Scholar] [CrossRef]
- Kanth, M.S.; Sandhya Rani, S.L.; Raja, V.K. Advancing ceramic membrane technology in chemical industries applications by evaluating cost-effective materials, fabrication and surface modification methods. Hybrid Adv. 2025, 8, 100380. [Google Scholar] [CrossRef]
- Peng, H.; Shah, V.; Li, K. Unprecedented water permeation in nanostructured PVDF membranes prepared by unidirectional freezing and surface melting method. J. Membr. Sci. 2023, 669, 121299. [Google Scholar] [CrossRef]
- Hao, B.; Jarman, W.; Peng, H.; Li, K. High performance crosslinked polyvinylidene fluoride (PVDF) membranes for solvent permeation. React. Funct. Polym. 2025, 209, 106174. [Google Scholar] [CrossRef]
- Tran, T.V.; Jalil, A.A.; Nguyen, D.T.C.; Hassan, N.S.; Alhassan, M.; Bahari, M.B. Highly enhanced chloramphenicol adsorption performance of MIL-53-NH2(Al)-derived porous carbons modified with tannic acid. Environ. Res. 2024, 259, 119447. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Zong, C.; Li, Y.; Jin, W. Study on membrane performance in vapor permeation of VOC/N2 mixtures via modified constant volume/variable pressure method. Sep. Purif. Technol. 2018, 200, 273–283. [Google Scholar] [CrossRef]
- Le, V.N.; Vo, T.K.; Yoo, K.S.; Kim, J. Enhanced CO2 adsorption performance on amino-defective UiO-66 with 4-amino benzoic acid as the defective linker. Sep. Purif. Technol. 2021, 274, 119079. [Google Scholar] [CrossRef]
- Xu, J.; Xie, A.; Sun, H.; Wu, Y.; Li, C.; Xue, C.; Cui, J.; Pan, J. Construction of tannic acid-Fe complex coated PVDF membrane via simple spraying method for oil/water emulsion separation. Colloids Surf. A 2023, 671, 131621. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; Chen, L. Selective adsorption and separation of dyes from aqueous solution by core-shell structured NH2-functionalized UiO-66 magnetic composites. J. Colloid Interface Sci. 2019, 539, 76–86. [Google Scholar] [CrossRef]
- Xu, H.; Feng, W.; Sheng, M.; Yuan, Y.; Wang, B.; Wang, J.; Wang, Z. Covalent organic frameworks-incorporated thin film composite membranes prepared by interfacial polymerization for efficient CO2 separation. Chin. J. Chem. Eng. 2022, 43, 152–160. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Zu, L.; Zhao, S.; Harvie, D.J.E.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrapermeable Composite Membranes Enhanced Via Doping with Amorphous MOF Nanosheets. ACS Cent. Sci. 2021, 7, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, Q.; Hua, Y.; Zhou, B.; Duan, J.; Jin, W. Mechanical Synthesis of COF Nanosheet Cluster and Its Mixed Matrix Membrane for Efficient CO2 Removal. ACS Appl. Mater. Interfaces 2017, 9, 29093–29100. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, H.; Zhang, X.; Zhang, Y. MoS2 Nanosheets Functionalized Composite Mixed Matrix Membrane for Enhanced CO2 Capture via Surface Drop-Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 23371–23378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Zhao, L.; Dutta, P.; Winston Ho, W.S. New Pebax®/zeolite Y composite membranes for CO2 capture from flue gas. J. Membr. Sci. 2015, 495, 415–423. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.-K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid®/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membr. Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Alavi, S.A.; Kargari, A.; Sanaeepur, H.; Karimi, M. Preparation and characterization of PDMS/zeolite 4A/PAN mixed matrix thin film composite membrane for CO2/N2 and CO2/CH4 separations. Res. Chem. Intermed. 2017, 43, 2959–2984. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Li, B.-Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 2012, 7, 504. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.-V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Membr. Sci. 2013, 425–426, 235–242. [Google Scholar] [CrossRef]


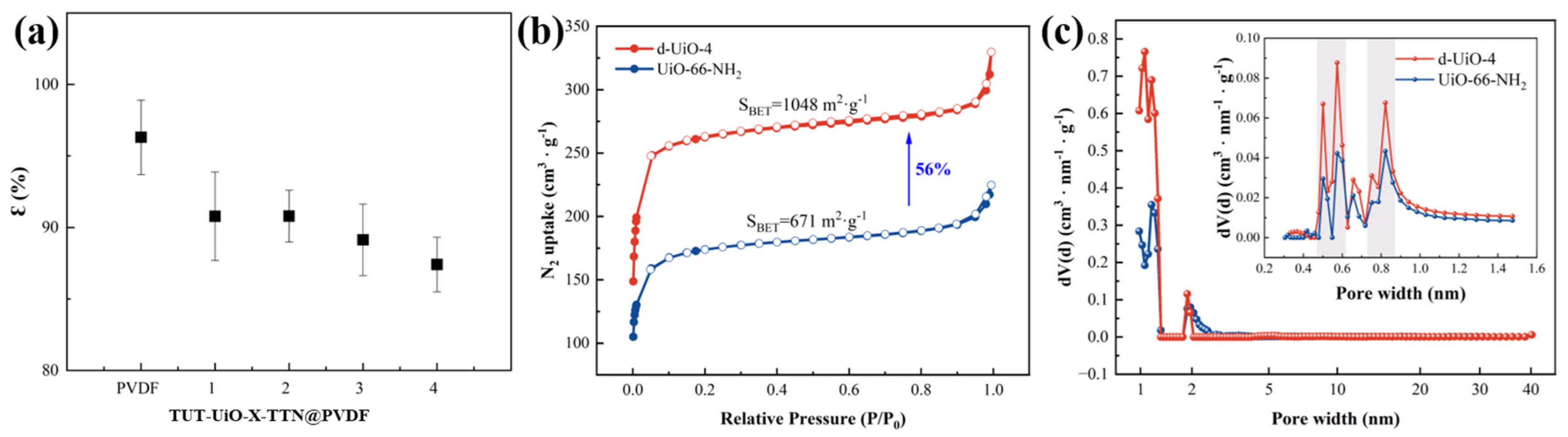
| Samples | SBET a (m2·g−1) | SMicro b (m2·g−1) | VMicro c (cm3·g−1) | VTotal d (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|---|---|
| d-UiO-4 | 1048 | 1013 | 0.40 | 0.51 | 1.95 |
| UiO-66-NH2 | 671 | 616 | 0.25 | 0.35 | 1.17 |
| Samples | Condition | Permeability | Selectivity | Ref. | |
|---|---|---|---|---|---|
| CO2/N2 | CO2/CH4 | ||||
| UiO-66−NH2/cPIM-1 | - | 2504 GPU | 37.20 | 23.80 | [28] |
| TpPa0.025-PIP-TMC/mPSf | 298 K, 0.15 MPa | 854 GPU 128 bar | 148.00 | - | [40] |
| TpPa0.025-PIP-TMC/mPSf | 0.5 MPa | 456 GPU | 92.00 | - | [40] |
| PDMS&MOF | 35 °C, 1.0 bar | 1990 GPU | 39.00 | - | [41] |
| TpPa-1-nc-PEBA | 298 K, 0.3 MPa | 156.9 bar | 72.00 | - | [42] |
| UiO-66-NH2-PEBA | 298 K, 0.2 MPa | 130 bar | 72.00 | - | [29] |
| MoS2–Pebax-0.15 wt% | 30 °C, 2 bar | 64 bar | 93.00 | - | [43] |
| Pebax®/EO-PES | 57 °C, 1.5 psig | 940 GPU | 30.00 | - | [44] |
| Pebax/PVC-8 | - | 8 GPU | 55.30 | 25.30 | [45] |
| MOF-Polysulfone | - | 15 GPU | 27.00 | 23.00 | [46] |
| Zeolite 4A-PAN | - | 3457 GPU | 23.80 | 8.18 | [47] |
| UiO-66-PVDF | - | 225 GPU | 45.00 | - | [30] |
| C60(OH)24-PAN | - | 338 GPU | 40–55 | 15–17 | [48] |
| MWCNT-PAN | 2 bar, 27 °C | 7090 bar | 32.90 | 10.40 | [49] |
| PEBAX-PAN | - | 287 GPU | 97.00 | 30.00 | [50] |
| TUT-UiO-3-TTN@PVDF | 30 °C, 1.5 bar | 10,439 GPU | 23.20 | 14.90 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Huang, W.; Li, X.; Xia, P.; Xu, X.; Feng, F. Self-Assembled Sandwich-like Mixed Matrix Membrane of Defective Zr-MOF for Efficient Gas Separation. Nanomaterials 2025, 15, 279. https://doi.org/10.3390/nano15040279
Li Y, Wang X, Huang W, Li X, Xia P, Xu X, Feng F. Self-Assembled Sandwich-like Mixed Matrix Membrane of Defective Zr-MOF for Efficient Gas Separation. Nanomaterials. 2025; 15(4):279. https://doi.org/10.3390/nano15040279
Chicago/Turabian StyleLi, Yuning, Xinya Wang, Weiqiu Huang, Xufei Li, Ping Xia, Xiaochi Xu, and Fangrui Feng. 2025. "Self-Assembled Sandwich-like Mixed Matrix Membrane of Defective Zr-MOF for Efficient Gas Separation" Nanomaterials 15, no. 4: 279. https://doi.org/10.3390/nano15040279
APA StyleLi, Y., Wang, X., Huang, W., Li, X., Xia, P., Xu, X., & Feng, F. (2025). Self-Assembled Sandwich-like Mixed Matrix Membrane of Defective Zr-MOF for Efficient Gas Separation. Nanomaterials, 15(4), 279. https://doi.org/10.3390/nano15040279







