Abstract
The optical properties of Mn5+ ions, which are responsible for the intense green–turquoise–blue coloration of Mn5+-based pigments and the near-infrared emission of phosphors, are the focus of this article. Mn5+ ions enter crystalline matrices in four-fold coordinated positions and can maintain their 5+ valence state when crystalline hosts meet the conditions described in this work. Mn5+ ions have [Ar]3d2 electronic configuration and always experience a strong crystal field due to a high electric charge; therefore, their lower electronic states have the 3A2 < 1E < 1A1 < 3T2 < 3T1 progression in energy. We present the properties of several Mn5+-based pigments and discuss the electronic transitions responsible for their coloration. Specifically, we show that the color is determined by the spin-allowed 3A2 → 3T1(3F) absorption, which extends across the orange–red–deep red spectral region and is strongly influenced by crystal field strength. The narrow-band emission Mn5+-activated near-infrared phosphors arise from the spin-forbidden 1E → 3A2 transition, whose energy is independent of the crystal field strength and determined by the nephelauxetic effect. We demonstrate the linear relationship between 1E state energy and the nephelauxetic parameter β1 using Racah parameter literature data for Mn5+ phosphors. Lastly, we address the recent applications of these Mn5+ phosphors in luminescence thermometry.
1. Introduction
The vivid colors of materials have captivated individuals since antiquity. The principal factor influencing a material’s color is its interaction with light in the visible spectrum (380–750 nm) perceptible to the human eye. Unlike organic dyes, inorganic pigments have superior resistance to heat, light, weathering, solvents, and chemicals, rendering them preferable for artist pigments, exterior coatings, and heat-reflective paints. Inorganic pigments come in a wide variety of colors, from subtle earth tones to vivid blues, greens, and reds. Their colors are highly saturated and fade-resistant, ensuring long-term use in a wide range of applications [1], such as automotive and industrial coatings, paints, plastics, printing inks, cosmetics, and construction materials. Furthermore, pigments play a vital part in the creation of paper, rubber, glass, porcelain, and glazes. Inorganic pigments have high chemical stability, making them resistant to degradation from light, heat, and chemical interactions. This stability makes them ideal for outdoor applications where weather resistance is critical. They also provide a variety of opacity levels, from wholly opaque to transparent, allowing for fine control of visual effects in paints, varnishes, and other materials. Although the fundamental principles of color science for gemstones and minerals are comprehended, precisely predicting the color of inorganic solids continues to be difficult until they are experimentally produced [2].
Pigments are classified based on their different properties. Color is one approach to categorize; white pigments, colorful pigments, and black pigments are the three primary divisions, each with its own set of components. Among white pigments, titanium dioxide, in both rutile and anatase forms [3,4]; zinc sulfide, including lithopones [5]; and zinc oxide take precedence. Colored pigments come in a variety of colors, ranging from blues like complex metal oxides, ultramarine, and Prussian blue [6] to greens like chromium(III) oxide. Yellows include iron(III) oxide hydroxide [7], lead chromate, bismuth vanadate [8], and cadmium sulfide, and reds vary from iron(III) oxide to lanthanum tantalum oxynitride. Carbon black represents the realm of black pigments [9]. Classification based on chemical composition highlights a pigment’s underlying characteristics. Oxides, such as iron oxide and titanium dioxide, are known for their opacity and durability. Sulfides, such as cadmium and zinc sulfide, provide bright colors and resistance to heat. Carbonates, silicates, and hydrated oxides, along with pigments such as zinc carbonate and ultramarine blue, create a flexible palette suitable for a variety of applications. Furthermore, various pigments, such as carbon black and metallic powders, have specialized applications, particularly in the ink and decorative finishes industries.
Transition metal ions are crucial as pigments, offering a broad spectrum of vibrant colors and exceptional stability [10,11]. These ions possess unique properties that make them indispensable in applications such as coatings, plastics, ceramics, and cosmetics. In terms of properties, transition metal pigments exhibit a remarkable variability in color, ranging from deep blues and greens to vivid reds and yellows. Their high opacity and coverage ensure uniform coloration with minimal application, while their chemical stability ensures longevity and resistance to environmental factors like light, heat, and chemicals [12]. Additionally, their compatibility with various binders and substrates facilitates their incorporation into different materials and formulations. Transition metals having a coordination environment with less symmetry and more mixing between the p and d orbitals are more likely to produce bright colors due to the relaxation of selection rules for d–d transitions. Charge transfer transitions typically have high transition probabilities (Laporte permitted), resulting in colorful hues, and they tend to dominate crystal field transition colors when both are present.
Cobalt, copper, iron, nickel, and chrome compounds are commonly used transition metal pigments, each with a unique color and application. Cobalt is known for its brilliant blue and purple hues; copper is prized for its vibrant green and blue shades; iron produces reds, browns, and yellows; nickel provides yellow, green, and gray tones; and chrome, bright yellows and greens. Despite their broad application, these pigments have several drawbacks. Cobalt pigments have been linked to health risks, as cobalt compounds can cause skin sensitivity and respiratory problems in vulnerable persons. Moreover, cobalt pigments’ color may fade or change, especially when exposed to extreme climatic conditions or reactive substances. Copper-based pigments may corrode over time. They can oxidize when exposed to environmental elements such as moisture and oxygen, resulting in color changes and damage to the substance to which they are applied. Iron pigments, while durable and stable, can exhibit a relatively limited color range compared to other pigments. Additionally, they may lack the vibrancy and intensity found in pigments derived from other metals. Moreover, iron-based pigments can be prone to color fading over time, particularly when exposed to harsh environmental conditions such as prolonged sunlight or moisture. Nickel-based pigments pose health risks due to the carcinogenic properties of nickel compounds. Prolonged exposure to nickel pigments, whether through inhalation or skin contact, can lead to allergic reactions and respiratory issues. Additionally, nickel pigments may exhibit limited chemical stability, making them prone to degradation when exposed to certain environmental conditions or chemical agents. Chromium-based pigments raise environmental and health concerns due to the toxicity of chromium compounds. Exposure to chromium pigments can adversely affect human health and the environment, prompting regulatory restrictions and environmental management protocols. Despite these drawbacks, ongoing research and development efforts focus on mitigating the adverse effects of chromium, copper, nickel, and cobalt pigments. Strategies include exploring alternative ions, enhancing production processes, and developing safer substitutes to address environmental and health concerns while maintaining the desired color properties.
One of the solutions is the use of manganese, since it is an essential trace element in the human body and has good biocompatibility. Manganese is known for its ability to stabilize in different oxidation states in crystalline solids, ranging from 1+ to 7+. Each manganese oxidation state has unique electronic and optical properties, which provide opportunities to develop pigments having a wide range of coloration. Well-known examples include manganese brown pigments based on Mn3O4, manganese violet (NH4MnP2O7), manganese pink (Al2O3:Mn), orange/red Mn4+-activated oxide phosphors [13], black pigments with high reflectivity in the near-infrared spectral range [14], and Mn5+-based pigments which are discussed in detail in this paper. Important progress has been achieved recently with the development of blue pigment based on Mn3+ optical centers in the hexagonal YInO3 and, similarly, in the trigonal bipyramidal sites of hexagonal ScGaZnO4, LuGaZnO4, and LuGaMgO4 [15]. The Mn3+ optical centers provide red coloration when introduced into indium sites of the monoclinic Li3InB2O6 [16], purple in YGaO3, brown in YAlO3 [17] and CaAl12O19 [18], and violet in LaAlGe2O7 [19]. Mn2+ can facilitate brown coloration when it is introduced to the Zn site in Zn2SiO4 [20].
Mn5+-based pigments are distinguished by their ability to generate vibrant blue and turquoise/green colors [21,22]. Moreover, Mn5+-based pigments often exhibit excellent opacity, leading to substantial coverage and consistent coloration in paints, coatings, and other substances. This characteristic is effective for attaining desired aesthetic outcomes with minimal application. Mn5+ pigments provide significant stability against deterioration from conditions including light exposure, heat, and chemical interactions. This guarantees the durability and color retention of items containing these pigments, enhancing their lasting attractiveness. Mn5+ pigments exhibit compatibility with various binders and substrates, enabling their integration into multiple formulations and materials. This adaptability increases their usefulness across multiple industries and applications. Mn5+ pigments play an important part in the creation of eye-catching glazes, tiles, and decorative pottery. Beyond traditional applications, Mn5+ pigments may exhibit unique optical and magnetic properties in specialized matrices, offering opportunities for innovation in fields such as electronics, optics, and magnetic materials. These matrices enable the development of novel materials and technologies that leverage the distinctive properties of Mn5+ pigments for diverse applications.
Upon activation with low concentrations of Mn5+ ions, certain phosphors exhibit light emission in the near-infrared (NIR) spectral range, specifically at wavelengths exceeding 1100 nm and characterized by narrow spectral bands. The narrowband near-infrared luminescence of Mn5+ is beneficial for near-infrared lasers [23,24,25] and for narrow-band near-infrared light sources designed for the targeted detection of chemical substances [26]. Recent studies demonstrate that nanoparticles activated by Mn5+ function as effective probes for luminescence imaging in deep tissues and luminescence thermometry within the second biological transparency window (1000–1350 nm). Moreover, these nanoparticles demonstrate significant resistance to photochemical degradation [27,28].
This article discusses the electronic processes that regulate the color and emission properties of Mn5+ pigments and phosphors, demonstrates the use of crystal field engineering for the control of their color and emission, and reviews the current landscape of Mn5+ pigments and phosphors.
2. Electronic and Optical Properties of Mn5+ Ions
The Mn5+ ion has two electrons in the outer 3d electron shell, so its electron configuration is [Ar]3d2, where [Ar] denotes the electron configuration of argon with completely filled electron shells 1s22s22p63s23p6. Coulomb interaction between two 3d electrons produces 45 degenerated microstates, which are grouped in five LS terms: two spin-triplets—3F and 3P—and three spin-singlets—1D, 1G, and 1S. Here, the 2S+1L notation is used, where S is the total spin, and L is the orbital momentum. According to Hund’s rule, the 3F term is the ground state. The degree of degeneracy of these terms is as follows: 21 for 3F, 9 for 3P, 5 for 1D, 9 for 1G, and 1 for 1S.
The quantitative description of the energy level scheme of a free Mn5+ ion requires knowledge of the so-called Racah parameters B and C, which have dimensions of energy and are linear combinations of the Slater integrals. In terms of the Racah parameters, the energies of the above-given terms are as follows (the energy of the ground term 3F is taken as zero): 15B for the 3P term, 5B + 2C for the 1D term, 12B + 2C for the 1G term, and 22B + 7C for the 1S term. The values of B and C fitted to the experimentally observed energy levels of free Mn5+ ions are B0 = 1223 cm−1 and C0 = 4613 cm−1 [29]. The Hartree–Fock calculated values (which are typically 15–20% overestimated if compared to the experimental values) of the same parameters are B0 = 1436 cm−1 and C0 = 5450 cm−1 [11].
The degenerated energy levels will undergo splitting in the crystal field, with the splitting pattern governed by the point group symmetry of the impurity ion’s site and the magnitude of the splitting reliant on the interionic distances and charges of the adjacent ions. Mn5+ ions often occupy four-fold coordinated sites in solids. If such a position has the ideal tetrahedral symmetry described by the Td point group, then the splitting patterns of all above-mentioned LS terms are as follows (the notation of the irreducible representations of the Td group is used): 3F → 3A2 + 3T1 + 3T2, 3P → 3T1, 1D → 1E + 1T2, 1G → 1A1 + 1E + 1T1 + 1T2, and 1S → 1A1. If the true symmetry of the Mn5+ site is reduced, namely resembling a deformed tetrahedron, the orbitally degenerate states will experience additional splitting, consistent with the symmetry characteristics of the corresponding point group.
The conventional method for analyzing the spectra of transition metal ions in crystals relies on Tanabe–Sugano diagrams [30], which illustrate the splitting of the terms of free ions within cubic crystal fields (in fact, the term “cubic” is a very general one and covers both tetrahedral and octahedral symmetries). These diagrams are usually plotted for a fixed ratio of C/B; the horizontal axis corresponds to the non-dimensional ratio Dq/B (Dq is the crystal field strength), and the vertical axis is the energy E of the crystal field states in terms of the Racah parameters B and E/B.
Figure 1 shows the Tanabe–Sugano diagram for the 3d2 electron configuration in the tetrahedral crystal field (since the Mn5+ ions occupy the four-fold coordinated sites). In a majority of crystal field books, the Tanabe–Sugano diagrams are plotted for the octahedral crystal field, but the tetrahedral Tanabe–Sugano diagrams can easily be obtained from the octahedral ones if the conjugate electron configurations dn and d10-n are considered. Thus, the Tanabe–Sugano diagram for Ni2+ (3d8 configuration) in the octahedral crystal field corresponds to the Tanabe–Sugano diagram for Mn5+ or Cr4+ (3d2 configuration) in the tetrahedral crystal field. The vertical dashed line separates the diagram into two sections designated as the “weak” and “strong” crystal fields. In the former case, the first excited state is the orbital and the spin-triplet 3T2, and the emission will correspond to the broad spin-allowed 3T2 → 3A2 transition. This can be the case with the ions with a smaller electric charge, V2+ for example. In the latter case, the first excited state is the orbital doublet and spin-singlet 1E, and the emission will be due to the sharp spin-forbidden 1E → 3A2 transition. The Mn5+ ions, because of their high electric charge, are always in a strong crystal field situation. Figure 1 also shows a typical way of excitation of the Mn5+ emission: at first, the absorption takes place to the 3T2 state, then the non-radiative relaxation to the 1E state occurs, and, finally, the 1E → 3A2 emission transition is realized in the near-infrared spectral range.

Figure 1.
Tanabe–Sugano diagram for the Mn5+ ions in the tetrahedral coordination.
A particular feature of the 3d2 electron configuration is that the energy difference between the 3A2 and 3T2 states coming from the 3F term is equal to the 10Dq, which gives an easy way of estimating the crystal field strength from the position of the lowest in energy excitation (absorption) peak.
3. Spectroscopic Properties, Color, and Photoluminescence of Mn5+ in Crystalline Solids
The Mn5+ ions tend to enter crystalline matrices in four-fold coordinated positions. From the point of view of charge compensation, the easiest way would be if they substituted for the pentavalent ions, such as P5+, V5+, or As5+. Therefore, the phosphates, vanadates, and arsenates are the most promising materials for doping with the Mn5+ ions. With certain charge compensation, it is possible to substitute Mn5+ ions for Si4+ ions in various silicate hosts [31].
The typical absorption (or excitation) spectrum of Mn5+ ions in solids is dominated by two broad structured bands, Figure 2, which are due to the spin-allowed transitions 3A2 → 3T2 (3F) at about 10,000–12,000 cm−1 and 3A2 → 3T1 (3F) at about 15,000–17,000 cm−1, depending on the host. The intensity of the former is usually somewhat lower because, in the Td symmetry, the 3A2 → 3T2 transition is forbidden by the group selection rules. The electric dipole-allowed 3A2 → 3T1 (3P) transition that corresponds to a two-electron jump occurs at higher energies of about 24,000–27,000 cm−1. The absorption from the spin-forbidden transitions to the singlet state 3A2 → 1A1 (1G) occurring around 13,500 cm−1 is weak, sharp, and does not depend on the crystal field strength. It is only weakly dependent on the host material’s properties because of the covalent effects (the nephelauxetic effect). The 3A2 → 1E (1D) spin-forbidden transition is barely seen in the absorption spectra. Charge transfer bands (CTBs) occur at high energies and are strictly host-dependent.
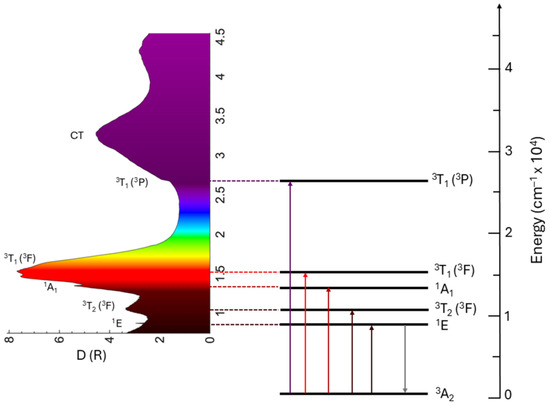
Figure 2.
Kubelka–Munk transformation of the diffuse reflectance spectrum of the Ca6Ba(PO4)4O:Mn5+ (left) and Mn5+ energy levels and electronic transitions responsible for color and photoluminescence emission of Ca6Ba(PO4)4O:Mn5+ (right).
The color of Mn5+ pigments is primarily determined by the 3A2 → 3T1 (3F) absorption, which extends across the orange–red–deep red spectral region. The energy of the barycenter of this absorption band is significantly influenced by the crystal field strength surrounding Mn5+ ions; refer to Figure 1. Therefore, it is possible to tune the pigment color through crystal field engineering, which involves modifying the host crystal structure to alter the distances between Mn5+ ions and ligand ions, as well as changing the type of ligand ions. The absorption strength of this transition is influenced by the Mn5+ concentration, and any change in this concentration also affects the intensity of pigment color. The CT and 3A2 → 3T1 (3P) transitions allow absorption in the violet spectral region, with the extent of absorption influenced by the crystal field strength. As a result, the color of the Mn5+ pigments is a blend of green and blue, with a slight addition of violet in certain cases.
The 1E (1D) → 3A2 transition determines the emission spectra of the Mn5+ ions. It occurs at about 8200–8900 cm−1. In certain cases, the weak and broad emission from the 3T2 (1D) → 3A2 transition can be observed at above-room temperature, usually in systems with low crystal field splitting. The 1E emission band is narrow due to weak electron–phonon interaction (the lateral displacement between 3A2 and 1E states is quite small). The 1E lifetime is relatively long (half a millisecond) due to the spin-forbidden character of the 1E → 3A2 transition. The Tanabe–Sugano diagram for the 3d2 configuration in a tetrahedral crystal field (Figure 1) indicates that the energy difference between the ground state 3A2 and the first excited state 1E is unaffected by the crystal field strength, as both states are parallel to one another. This energy difference closely approximates the energy interval between the 3F and 1D states of the free ion, defined using the Racah parameters as 8B + 2C, where B and C differ among various hosts due to covalent effects. Figure 3a shows a perfect correlation between the energy of the 1E state and (8B + 2C) for Mn5+ in different hosts listed in Table 1. Thus, the nephelauxetic effect predominantly influences the energy of the 1E state.

Figure 3.
(a) The correlation between the energy of the 1E state and (8B + 2C); the full line shows the linear correlation with the slope of 0.9817 ≈ 1, and (b) the dependence of the 1E state energy on the nephelauxetic parameter β1 for Mn5+ in different hosts listed in Table 1.
For the quantitative analysis of the nephelauxetic effect, a new nephelauxetic parameter β1 was introduced earlier for the ions with the 3d3 electron configuration [32,33] and derived in Ref. [34]:
It has been demonstrated in those references that the dependence of energy of the 2E→4A2 spin-forbidden transition for the 3d3 electron configuration is a linear function of the β1 parameter. Later on, the energy of the 1E→3A2 spin-forbidden transition of the Ni2+ ions in the octahedral coordination (whose energy level sequence is identical to the case of the Mn5+ ions in the tetrahedral coordination, considered in the present work) was also shown to be a linear function of the β1 parameter. Following that approach, the dependence of the Mn5+ ions’ 1E state energy on the β1 parameter is shown in Figure 3b for data taken from Table 1; it appears to be linear (R2 of 0.94),

Table 1.
Crystal field strength Dq, Racah parameters B and C, nephelauxetic parameter β1, and the energy of the 1E state for the tetrahedrally coordinated Mn5+ ions in different crystals.
Table 1.
Crystal field strength Dq, Racah parameters B and C, nephelauxetic parameter β1, and the energy of the 1E state for the tetrahedrally coordinated Mn5+ ions in different crystals.
| Host Material | Dq [cm−1] | B [cm−1] | C [cm−1] | β1 | 1E [cm−1] | Reference |
|---|---|---|---|---|---|---|
| Y2SiO5 | 1133 | 550 | 2255 | 0.6642 | 8754.0 | [35] |
| Sr10(VO4)6F2 | 1088 | 518 | 2321 | 0.6577 | 8642.4 | [36] |
| Sr5(PO4)3Cl | 1053 | 510 | 2407 | 0.6679 | 8710 | [37] |
| Ca2PO4Cl | 1162 | 455 | 2657 | 0.6857 | 8849.6 | [37] |
| Li3PO4 | 1208 | 475 | 2556 | 0.6767 | 8802.0 | [37] |
| Ca2AsO4Cl | 1030 | 530 | 2245 | 0.6516 | N.A. * | [38] |
| Ca2VO4Cl | 1000 | 535 | 2253 | 0.6557 | N.A. | [38] |
| Li3VO4 | 1049 | 646 | 2006 | 0.6842 | 8950.9 | [39] |
| YAlO3 | 1100 | 485 | 2256 | 0.6296 | 8267.2 | [40] |
| Ba3(VO4)2 | 1000 | 530 | 2250 | 0.6525 | 8499.1 | [41] |
| Ca6Ba(PO4)4O | 1060 | 544 | 2292 | 0.669 | 8773.9 | [42] |
| Ba2SiO4 | 1131 | 419 | N.A. | N.A. | 8403.4 | [43] |
* N.A.—not applicable.
It can be noted from the data collected in Table 1 that both Racah parameters B and C are strongly reduced from their free ion values. This is a clear indication of a high degree of covalency of the Mn5+–O2− chemical bonds, which is caused by a high electric charge of the manganese ions pulling the charge density from the oxygen ions towards manganese and thus enhancing the overlap between their wave functions. Higher values of the β1 parameter are indications of the more ionic nature of the “impurity ion–ligand” chemical bonds, whereas lower β1 values correspond to enhanced covalent interactions between the impurity ions and ligands.
Oxide complexes including two or more metals represent an important category of thermally stable pigments. The color spectrum of these pigments is generally determined by the presence of various 3d transition metals (V, Cr, Mn, Fe, Co, Ni, or Cu) and a number of coordination polyhedra that can accommodate 3d transition metal ions (tetrahedral, pentahedral, octahedral, or prismatic sites). Phosphate compounds of alkali or alkaline earth metals doped with transition metal ions have been thoroughly examined across many fields, including catalysis [44,45,46], energy research (notably with lithium phosphate cathodes currently under intense study [47,48,49]), and healthcare (where calcium phosphate materials are pivotal biomaterials and continue to be the preferred option for biomedical applications, as substantiated by recent reviews [50,51,52,53]). Moreover, these compounds play a significant role in the coloration of inorganic substances, functioning as dyes or pigments based on their resistance to corrosive environments [54,55,56,57].
Oxide matrices activated in tetrahedral sites by 3d2 transition-metal ions, such as Cr4+, Mn5+, and Fe6+, showcase vibrant colors thanks to their significant absorption cross sections in the visible spectral region. Furthermore, these ions exhibit exceptional luminescent characteristics. While Mn5+ and Fe6+ activated materials display sharp line luminescence in the NIR range [58], compounds activated with Cr4+ display a broad emission due to a much weaker nephelauxetic effect. This feature renders them appropriate for use as tunable lasers in the NIR region [59].
Manganese blue, an industrial pigment, is formed by the introduction of Mn5+ ions into BaSO4. Nonetheless, due to environmental issues, production of this pigment has almost come to a halt. The high price of indium-containing pigments activated by Mn5+ has limited their commercial use. To reintroduce Mn5+ in commercial pigments, new host material ought to be found [60]. There is a clear need to find cheaper blue pigments with comparable optical characteristics to those currently in use. The main limitation of Mn5+ is the need for a host material that can stabilize manganese ions in their 5+ oxidation state. According to Shannon [61], Mn5+ exists only in a tetrahedral coordination, with a small effective ionic radius of 33 pm. The (VO4)3− group in Ba3(VO4)2 is a good example of a structure for the successful Mn5+ ion incorporation at V5+ sites (V5+ ion radius in tetrahedral coordination is approximately 35.5 pm). Isovalent tetrahedral molecular ions such as (PO4)3− or (VO4)3− can be easily substituted with (MnO4)3− ions, with no charge compensation required because of the same electric charge and similar effective ionic radius (see Table 2). These replacements produce phosphate and vanadate pigments in bright, vivid colors ranging from blue to green [62,63]. These newly developed pigments are easy to synthesize, demonstrate resistance to heat and acids, and are environmentally friendly, offering significant potential for diverse applications where thermal resistance is critical, such as roofing materials. Similar substitutions can be made to (AsO4)3− without charge compensation and to (SiO4)2− with charge compensation, for example, with Al.

Table 2.
Effective ionic radii in tetrahedral coordination of selected metals, according to Shannon [57].
Several compounds with Mn5+ have been documented for their bright coloration. For instance, Mn takes 5+ valence and occupies tetrahedral sites in the brownmillerite-type Ba2In2-xMnxO5+x, providing a light yellow (x = 0), intense turquoise (x = 0.1), green (x = 0.2, 0.3), or dark green (x ≥ 0.4) color [60]. The distinct color of these compounds arises from the substantial absorption of visible light at 500 nm. Under reducing conditions, Mn5+ is converted to Mn3+, resulting in Ba2In2−xMnxO5+x turning black. The colors and diffuse reflectance spectra of Ba2In2−xMnxO5+x phases are shown in Figure 4. The light-yellow appearance of pure Ba2In2O5 results from the band edge tailing into the visible spectrum. Replacing indium with manganese produces a turquoise to green hue due to increased optical absorption in both high- and low-energy parts of the visible spectrum. The high-energy peak predominantly originates from the In–O charge-transfer transitions, whereas a low-energy shoulder results from the Mn–O charge transfer, affecting the visible hue.
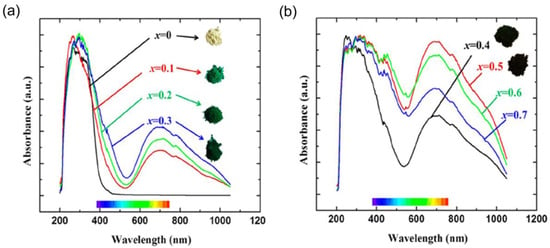
Figure 4.
Diffuse reflectance spectra of Ba2In2−xMnxO5+x samples and their corresponding powder color variations: (a) Ba2In2−xMnxO5+x (x = 0, 0.1, 0.2, 0.3), where the spectrum for x = 0 (Ba2In2O5, white color) is shown for comparison; and (b) Ba2In2−xMnxO5+x (x = 0.4, 0.5, 0.6, 0.7). Reprinted with permission from Ref. [60]. 2013, American Chemical Society.
The apatite-type structure A5(MO4)3X with A as Ca2+, Sr2+, Ba2+, or Pb2+; M as P5+, Mn5+, Cr5+, or V5+; and X as F−, Cl−, OH−, provides substantial possibilities for the combining of various A-, M-, and X-site elements, facilitating the creation of compounds with a wide range of advantageous features [64,65]. Notably, apatite-type compounds featuring Mn5+ tetrahedrally coordinated by oxygen exhibit promising characteristics as materials for pigments that produce vibrant blue and green colors [66,67].
The 5+ oxidation state of manganese is infrequent in inorganic oxides because of its instability, as Mn5+ typically takes more stable oxidation states like 4+ and 7+. However, apatite-type compounds that include Mn5+ in tetrahedral coordination at the M-site with alkaline earth elements, especially Ba2+, at the A-site exhibit stability because of the high first ionization potential of alkaline earth elements (approximately 5 eV for Ba2+), which promotes the preservation of this uncommon oxidation state of Mn [68]. A variety of compounds with an apatite structure have been synthesized, and their structural, optical, and magnetic characteristics and coloration have been studied [69]. Table 3 presents the compounds doped with Mn5+ and the synthesis techniques, structural characteristics, and powder color variations resulting from Mn5+ ion doping.

Table 3.
Synthesized materials doped with Mn5+ used as pigments.
The optical properties of Mn5+-doped Ba5Mn3-xMxO12Cl (M = V, P) apatite structures have been presented by Medina et al. [69]. The doped samples show bright colors from light to dark turquoise and dark green, while undoped Ba5(PO4)3Cl and Ba5(VO4)3Cl are white, Figure 5a. L*, a*, and b* denote the coordinates of the CIELAB color space, with L* indicating lightness, the a*-axis representing the green–red range, and the b*-axis spanning from blue (−b*) to yellow (+b*) [74]. The color coordinates (L*, a*, b*) exhibit an increase in L* and a* values alongside a decrease in b* values with higher Mn content (larger x values) in Ba5Mn3-xMxO12Cl (M = V, P) samples (Figure 5). The diffuse reflectance spectra of Ba5Mn3-xVxO12Cl pigments, presented in Figure 5b, reveal substantial absorption of red/orange light (around 630 nm) and purple light (around 400 nm), with low absorption in the green/blue spectral region (around 500–520 nm). These optical properties result in the manifestation of green or turquoise colors. Ba5V3O12Cl and Ba5P3O12Cl are white because they do not absorb visible light. The high-energy absorption peak in the UV spectral region is caused by the Mn5+–O2− charge transfer. Reduction of manganese concentration decreases absorbance and lightens samples. The authors measured near-infrared reflectance spectra of pigments to assess their cool pigments (Figure 5c). We found out that the reflection intensity decreases with an increase in Mn content, i.e., for pigments of a darker color. Reflectance in the 750–2500 nm range is about 70–85%, making all compounds promising cool pigments.
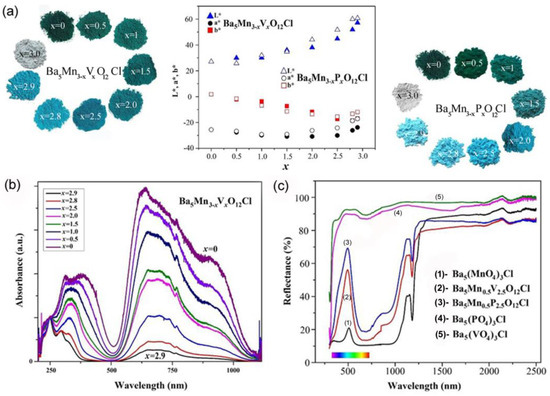
Figure 5.
(a) L*, a*, b* color parameters of Ba5Mn3-xVxO12Cl and Ba5Mn3−xPxO12Cl (x = 0; 0.5; 1; 1.5; 2.0; 2.5; 2.8; 2.9; 3.0) samples as a function of the Mn content (x) and color changes with Mn5+ doping. (b) Diffuse-reflectance spectra of the Ba5Mn3−xVxO12Cl series; (c) UV-vis and NIR reflectance (%) of Ba5Mn3−xVxO12Cl (x = 0, 2.5, 3) and Ba5Mn3−xPxO12Cl (x = 0, 2.5, 3) samples as a function of wavelength (nm). Adapted with permission from Ref. [69]. 2016, Elsevier.
Sun Woog Kim et al. [71] analyzed novel inorganic sky-blue pigments, Ca6Ba(P1-xMnx)4O17 (0 ≤ x ≤ 0.13), produced using a solid-state reaction technique, emphasizing its chromatic characteristics alongside thermal and chemical stability. The most vivid sky-blue hue, Figure 6, was obtained for Ca6Ba(P0.99Mn0.01)4O17, which has CIE color parameters of L* = 75.47, a* = 11.67, b* = 10.29, C = 15.6, and H = 221.4 (see the upper table on Figure 6.)
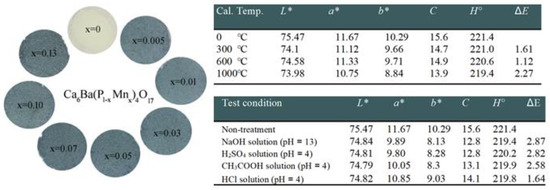
Figure 6.
Photographs of the Ca6Ba(P1−xMnx)4O17 (0 ≤ x ≤ 0.13) pigments. Tables present color coordination values in the L*, a*, b*, C, H⁰, and ΔE system of Ca6Ba(P0.99Mn0.01)4O17 pigments after the thermal stability test and acid and alkali resistance test. Adapted with permission from Ref. [71]. 2017, Elsevier.
To evaluate the color thermal stability of this pigment, the authors utilized the Ca6Ba(P0.99Mn0.01)4O17 sample. Firstly, the authors heated the sample at 300 °C, 600 °C, and 1000 °C for 6 h to evaluate the thermal stability of its color performance. Using the equation, the color difference (ΔE) of the samples was determined [74] thus:
The samples heated at 300 °C and 600 °C showed color difference (ΔE) values of 1.61 and 1.12, respectively, below the minimum difference in color perceptible by humans (ΔEmin = 2) [75]. The sample heated at 1000 °C had a ΔE value of 2.27. The result suggests that the pigment possesses excellent thermal stability within the 0–600 °C region; however, its stability reduces as temperature rises into the 600–1000 °C temperature range. These pigments displayed some chemical color instability in acidic and alkaline solutions; see the upper table in Figure 6. Despite the necessity for enhancements in chemical color stability, this pigment may serve as a promising option for a novel ecologically friendly inorganic sky-blue pigment.
In comparison to Mn2+ and Mn4+ optical centers, emissions from Mn5+ centers have been observed in a much smaller number of host materials. These are, for example, Li3PO4 [76], Sr5(VO4)3F4,[25,77], Ba5(PO4)3Cl [78], Sr5(PO4)3Cl [78,79,80], Ca2PO4Cl [78,79], Ca2VO4Cl [78,79], Sr2VO4Cl [78,79], Ca6Ba(PO4)4O [42], Y2SiO5 [31], and M2SiO4 (M = Ba, Sr, Ca) [43].
The early application of Mn5+ emission has been concentrated on the development of NIR-emitting solid-state lasers [23,25]. The laser action is characterized using a three-level laser scheme, and it has been demonstrated that the long lifetime of the 1E state, together with its substantial visible absorption, makes the Mn5+ system suitable for flashlamp pumping. However, the Mn5+ laser’s tunability is severely constrained due to its narrow emission. The typical internal quantum efficiencies (IQE) of Mn5+-activated phosphors vary from 20% to 40%, with new findings indicating a potential enhancement in IQE with Bi3+ co-doping [81].
4. Luminescence Thermometry with Mn5+ Ions
Mn5+ has the benefit of activating nanoparticles, making them ideal probes for luminescent imaging in deep tissues and luminescent thermometry in the second window of biological transparency (1000–1350 nm). Recently, Piotrowski et al. [82] demonstrated Mn5+ lifetime-based thermal imaging in the optical transparency windows through skin-mimicking tissue phantoms. Dramicanin et al. [42] presented and explained the near-infrared luminescence of Ca6Ba(PO4)4O:Mn5+ and demonstrated its use for temperature sensing in the near-infrared spectral region. When excited within a broad and strong absorption band spanning from 500 to 1000 nm, this phosphor provides an ultra-narrow emission (FWHM = 5 nm) centered at 1140 nm, originating from the spin-forbidden 1E→3A2 transition. They discovered that the 1E emission is quenched due to thermally assisted crossover with the 3T2 state and that the relatively high Debye temperature of 783 K for Ca6Ba(PO4)4O facilitates efficient emission. A high Debye temperature indicates the material’s rigid structure. The increased structural rigidity restricts the non-radiative transition of photons, resulting in enhanced quantum efficiency. This phosphor has been effective in luminescence intensity ratio thermometry, with a relative sensitivity of 1.92% K−1 and temperature resolution of 0.2 K within the physiological temperature range. The relative sensitivity of a thermometer is defined as the rate of the temperature-induced change in a measured luminescence feature divided by the magnitude of this feature, and the temperature resolution is the smallest change in a temperature that causes a perceptible change in the measured luminescence feature [83]. While there are no established thresholds at which the materials are considered promising for thermometers since different applications require different measurement performances and temperature operating ranges, it is generally assumed that thermometers with relative sensitivities exceeding 1% K−1 and temperature resolution better than 0.5 K around room temperature may be considered promising [84,85].
As illustrated in Figure 7a, an increase in temperature results in the enhancement of the broad emission peak from the 3A2 level within the 950 nm to 1030 nm range, while the narrow emission peak from the 1E level around 1140 nm decreases. This phenomenon occurs due to thermalization between the 1E and 3T2 levels, with the energy difference (ΔET = 1216 cm−1) being overcome by thermally excited electrons. To experimentally determine the uncertainty in the luminescence intensity ratio (LIR), 50 emission spectra were recorded at each temperature. The mean of these measurements was used as the LIR value, and the standard deviation (σLIR) was considered the uncertainty in LIR, as depicted in the inset of Figure 7b (distribution measured at 30 °C). Figure 5c shows that the relative sensitivity value, represented by blue dots for measurements taken at 30 °C, ranges from 2.35%K−1 to 1.26%K−1 over the temperature range, with a value of 1.92% K−1 at 30 °C. This sensitivity is among the highest recorded for luminescent thermometers operating within the second biological transparency window.
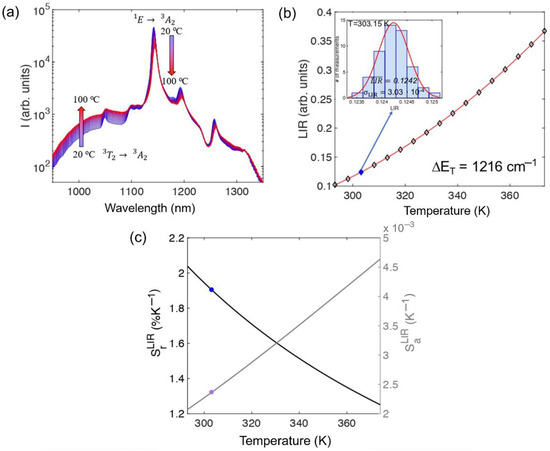
Figure 7.
(a) Photoluminescence emission spectra of Ca6Ba(PO4)4O:Mn5+ powder measured at different temperatures; (b) luminescence intensity ratio (LIR) as a function of temperature (experimental data—diamond markers). The insert shows the LIR distribution histogram measured at 303.15 K (30 °C)—filled diamond marker; (c) calculated absolute and relative sensitivities (marked values at 303.15 K (30 °C)). Reprinted from Ref. [42].
5. Conclusions
The color of Mn5+ pigments is influenced by the spectral positions and intensity of absorptions from the spin-allowed 3A2 → 3T1 (3F) (the orange–deep red spectral region) and 3A2 → 3T1 (3P) (the violet spectral region) electronic transitions. The former can be controlled by engineering the crystal field surrounding the Mn5+ ions (by varying manganese-ligand bond lengths, bond angles, and ligand type), whereas the latter can be altered by changing Mn5+ concentration. The charge transfer between Mn5+ and ligands also has an effect on violet absorption. The turquoise-blue coloring of the Mn5+ pigments is a result of these absorptions. The narrow-band near-infrared emission with a long lifetime, typically around a half millisecond, occurs in low-doped materials. It is composed of emission from the spin-forbidden 1E → 3A2 transition and its vibrational sidebands. The energy of the 1E emission is strongly influenced by the nephelauxetic effect (covalency) and is not dependent on the crystal field strength. We showed here that the energy of the 1E state is linearly dependent on the value of the nephelauxetic parameter β1. The weak and broad emission from the spin-allowed 3T2 (1D) → 3A2 transition can be observed at temperatures above room temperature. This phenomenon is typically observed in systems with small crystal field splitting when the energy difference between 1E and 3T2 is sufficiently small to allow thermalization. Despite the difficulty of maintaining manganese’s 5+ valence state when added into solids, the number of reported Mn5+ pigments and phosphors is growing over time. Suitable hosts for Mn5+ doping typically consist of (PO4)3−, (VO4)3−, or (SiO4)2− groups, where Mn5+ replaces P5+, V5+, or Si4+ in tetrahedral coordination, and contain electropositive alkaline earth metals such as Ba, Sr, and Ca. Mn5+ pigments are generally non-toxic and possess favorable stability, which presents a significant opportunity for their future application. Their narrow-band near-infrared emission was initially evaluated for solid-state laser development but has recently been applied in bioimaging and biothermal imaging within the second biological transparency window. Luminescence thermometry sensors utilizing Mn5+ emission typically exhibit a large relative sensitivity of approximately 2% K⁻¹ at room temperature. This, combined with small uncertainties in the measurements of intense 1E emissions, results in high precision and accuracy, achieving around 0.2 K in measurements.
Author Contributions
Conceptualization, S.K. and M.D.D.; methodology, S.K., T.D., A.I.P., M.G.B. and M.D.D.; formal analysis, S.K., T.D., A.I.P., M.G.B. and M.D.D.; investigation, S.K., T.D., A.I.P. and M.D.D.; data curation, M.G.B. and M.D.D.; Visualization, S.K. and T.D.; writing—original draft preparation, S.K., M.G.B. and M.D.D.; writing—review and editing, S.K. and M.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia under contract 451-03-66/2024-03/200017.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pfaff, G. Inorganic Pigments; Walter de Gruyter: Berlin, Germany, 2017; pp. 54–56. [Google Scholar]
- Li, J.; Kumari, L.S.; Subramanian, M.A. Solid state inorganic color pigments: Ancient to modern. In Comprehensive Inorganic Chemistry III, 3rd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 16, pp. 154–196. [Google Scholar]
- Auer, G. Titanium dioxide. In Industrial Inorganic Pigments, 3rd ed.; Buxbaum, G., Pfaff, G., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 51–81. [Google Scholar]
- Pfaff, G. Titanium dioxide pigments. In Encyclopedia of Color, Dyes, Pigments, 1st ed.; Pfaff, G., Ed.; Walter de Gruyter: Berlin, Germany, 2022; Volume 3, pp. 1177–1194. [Google Scholar]
- Gao, H.; Yang, S.; Mao, D.; Long, M.; Qu, X. Significant zinc release from widely-used commercial lithopone pigments under solar irradiation. Environ. Pollut. 2022, 292, 118352. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, S.; Basha, R.; Sreeram, K.J.; Sangilimuthu, S.N.; Nair, B.U. Functional pigments from chromium(III) oxide nanoparticles. Dye. Pigment. 2012, 94, 548–552. [Google Scholar] [CrossRef]
- Wiese, J. Iron oxide pigments. In Industrial Inorganic Pigments, 3rd ed.; Buxbaum, G., Pfaff, G., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 99–111. [Google Scholar]
- Tücks, A.; Beck, H.P. The photochromic effect of bismuth vanadate pigments. Part I: Synthesis, characterization and lightfastness of pigment coatings. J. Solid State Chem. 2005, 178, 1145–1156. [Google Scholar] [CrossRef]
- Bhakare, M.A.; Wadekar, P.H.; Khose, R.V.; Bondarde, M.P.; Some, S. Eco-friendly biowaste-derived graphitic carbon as black pigment for conductive paint. Prog. Org. Coat. 2020, 147, 105872. [Google Scholar] [CrossRef]
- Marciniak, L.; Kniec, K.; Elżbieciak-Piecka, K.; Trejgis, K.; Stefanska, J.; Dramićanin, M.D. Luminescence thermometry with transition metal ions. A review. Coord. Chem. Rev. 2022, 469, 214671. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.-G. Theoretical Spectroscopy of Transition Metal and Rare Earth Ions: From Free State to Crystal Field, 1st ed.; Jenny Stanford Publishing: Singapore, 2019; pp. 35–37. [Google Scholar]
- Kung, H.H. Spectroscopy of Transition Metal Ions on Surfaces. Appl. Catal. A Gen. 2001, 213, 141–142. [Google Scholar] [CrossRef]
- Zhou, Q.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.; Shi, J.; Dramićanin, M.D.; Brik, M.G.; Wu, M. Mn2+ and Mn4+ Red Phosphors: Synthesis, Luminescence and Applications in WLEDs. A Review. J. Mater. Chem. C 2018, 6, 2652–2671. [Google Scholar] [CrossRef]
- Oka, R.; Masui, T. Synthesis and characterization of black pigments based on calcium manganese oxides for high near-infrared (NIR) reflectance. RSC Adv. 2016, 6, 90952–90957. [Google Scholar] [CrossRef]
- Smith, A.E.; Mizoguchi, H.; Delaney, K.; Spaldin, N.A.; Sleight, A.W.; Subramanian, M.A. Mn3+ in trigonal bipyramidal coordination: A new blue chromophore. J. Am. Chem. Soc. 2009, 131, 17084–17086. [Google Scholar] [CrossRef]
- Divya, S.; Das, S. Eco-friendly Li3InB2O6 based red pigments for various IR blocking cool coating applications. Opt. Mater. 2020, 109, 110410. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Sarma, D.; Reddy, M.L.P.; Natarajan, S.; Gopalakrishnan, J. YGa1−xMnxO3: A novel purple inorganic pigment. RSC Adv. 2013, 3, 3199–3202. [Google Scholar] [CrossRef]
- Medina, E.A.; Li, J.; Subramanian, M.A. Colored oxides with hibonite structure II: Structural and optical properties of CaAl12O19-type pigments with chromophores based on Fe, Mn, Cr and Cu. Prog. Solid State Chem. 2017, 45–46, 9–29. [Google Scholar] [CrossRef]
- Kim, S.W.; Saito, Y.; Hasegawa, T.; Toda, K.; Uematsu, K.; Sato, M. Development of a novel nontoxic vivid violet inorganic pigment—Mn3+-doped LaAlGe2O7. Dye. Pigment. 2017, 136, 243–247. [Google Scholar] [CrossRef]
- Hwang, D.-H.; Han, K.-S.; Lee, B.-H. Synthesis and formation mechanism of Mn-doped Zn2SiO4 brown pigment. Mater. Sci. Forum 2011, 695, 295–298. [Google Scholar] [CrossRef]
- Aso, S.; Onoda, H. Synthesis of cobalt-substituted manganese phosphate purple pigments. Materials 2023, 16, 4132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, P.; Lei, H.; Li, Y.; Cao, W.; Kuang, J. Synthesis and properties of novel turquoise-green pigments based on BaAl2-xMnxO4+y. Dye. Pigment. 2018, 155, 212–217. [Google Scholar] [CrossRef]
- Merkle, L.D.; Pinto, A.; Verdún, H.R.; McIntosh, B. Laser action from Mn5+ in Ba3(VO4)2. Appl. Phys. Lett. 1992, 61, 2386–2388. [Google Scholar] [CrossRef]
- Moncorgé, R.; Manaa, H.; Boulon, G. Cr4+ and Mn5+ active centers for new solid state laser materials. Opt. Mater. 1994, 4, 139–151. [Google Scholar] [CrossRef]
- Merkle, L.D.; Guyot, Y.; Chai, B.H.T. Spectroscopic and laser investigations of Mn5+: Sr5(VO4)3F. J. Appl. Phys. 1995, 77, 474–480. [Google Scholar] [CrossRef]
- Ma, L.; Peng, Y.; Pei, Y.; Zeng, J.; Shen, H.; Cao, J.; Qiao, Y.; Wu, Z. Systematic discovery about NIR spectral assignment from chemical structural property to natural chemical compounds. Sci. Rep. 2019, 9, 9503. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, P.M.; Keevend, K.; Aellen, M.; Gogos, A.; Krumeich, F.; Herrmann, I.K.; Pratsinis, S.E. Bi2O3 boosts brightness, biocompatibility and stability of Mn-doped Ba3(VO4)2 as NIR-II contrast agent. J. Mater. Chem. B 2021, 9, 3038–3046. [Google Scholar] [CrossRef]
- Cao, R.; Yu, X.; Cao, C.; Qiu, J. Near-infrared emission Ba3(PO4)2: Mn5+ phosphor and potential application in vivo fluorescence imaging. Spectrochim. Acta Part A 2014, 128, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Andreici, E.L.; Gruia, A.S.; Avram, N.M. The parameters of the free ions Mn5+ and Fe6+. Phys. Scr. 2012, 2012, 014060. [Google Scholar] [CrossRef]
- Sugano, S.; Tanabe, Y.; Kamimura, H. Multiplets of Transition-Metal Ions in Crystals. In Pure and Applied Physics; Massey, H.S.W., Brueckner, K.A., Eds.; Academic Press: New York, NY, USA, 1970; pp. 107–111. [Google Scholar]
- Hömmerich, U.; Eilers, H.; Yen, W.M.; Verdun, H.R. The optical center MnO43− in Y2SiO5:Mn, X (X = Al, Ca). Chem. Phys. Lett. 1993, 213, 163–167. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Brik, M.G. Crystal field studies of the Mn4+ energy levels in the perovskite, LaAlO3. Opt. Mater. 2013, 35, 1544–1548. [Google Scholar] [CrossRef]
- Brik, M.G.; Camardello, S.J.; Srivastava, A.M. Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2015, 4, 39–43. [Google Scholar] [CrossRef]
- Ma, C.-G.; Wang, Y.; Liu, D.-X.; Li, Z.; Hu, X.-K.; Tian, Y.; Brik, M.G.; Srivastava, A.M. Origin of the β1 parameter describing the nephelauxetic effect in transition metal ions with spin-forbidden emissions. J. Lumin. 2018, 197, 142–146. [Google Scholar] [CrossRef]
- Shen, Y.; Riedener, T.; Bray, K.L. Effect of pressure on site-symmetry distortions of Mn5+ and Cr4+ in Y2SiO5. Phys. Rev. B 2000, 61, 9277–9286. [Google Scholar] [CrossRef]
- Scott, M.A.; Henderson, B.; Gallagher, H.G.; Han, T.P.J. Optical spectroscopy of (MnO4)3− and (VO4)5− in Sr10(VO4)6F2. J. Phys. Condens. Matter 1997, 9, 9893–9908. [Google Scholar] [CrossRef]
- Brik, M.G.; Cavalli, E.; Borromei, R.; Bettinelli, M. Crystal field parameters and energy level structure of the MnO43− tetroxo anion in Li3PO4, Ca2PO4Cl and Sr5(PO4)3Cl crystals. J. Lumin. 2009, 129, 801–806. [Google Scholar] [CrossRef]
- Wu, X.-X.; Yu, X.-P.; Zheng, W.-C. Studies of EPR parameters for Mn5+-doped Ca2(MO4)Cl (M = P, As, V) crystals from a two-mechanism model. Eur. Phys. J. Appl. Phys. 2014, 68, 30601. [Google Scholar] [CrossRef]
- Andreici, E.-L. Modeling of crystal field and spin-hamiltonian parameters for tetrahedral coordinated Mn5+ doped in Li3VO4. AIP Conf. Proc. 2012, 1472, 101–107. [Google Scholar]
- Brik, M.G.; Sildos, I.; Berkowski, M.; Suchocki, A. Spectroscopic and crystal field studies of YAlO3 single crystals doped with Mn ions. J. Phys. Condens. Matter 2009, 21, 025404. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Schmidt, J.; Chan, I.Y.; Singel, D.J. Electron spin-echo-detected excitation spectroscopy of manganese-doped Ba3(VO4)2: Identification of tetrahedral Mn5+ as the active laser center. Phys. Rev. B 1995, 51, 6215. [Google Scholar] [CrossRef] [PubMed]
- Dramićanin, M.D.; Marciniak, Ł.; Kuzman, S.; Piotrowski, W.; Ristić, Z.; Periša, J.; Evans, I.; Mitrić, J.; Đorđević, V.; Romčević, N.; et al. Mn5+-activated Ca6Ba(PO4)4O near-infrared phosphor and its application in luminescence thermometry. Light Sci. Appl. 2022, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nie, J.; Liu, S.; Qiu, J. Structural variation and near infrared luminescence in Mn5+-doped M2SiO4 (M = Ba, Sr, Ca) phosphors by cation substitution. J. Mater. Sci. Mater. Electron. 2018, 29, 6419–6427. [Google Scholar] [CrossRef]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Thomas, J.M. Nanoporous solids as receptacles and catalysts for unusual conversions of organic compounds. Solid State Sci. 2006, 8, 326–331. [Google Scholar] [CrossRef]
- Chen, N.Y.; Garwood, W.E. Some catalytic properties of ZSM-5, a new shape selective zeolite. J. Catal. 1978, 52, 453–458. [Google Scholar] [CrossRef]
- Shahid, R.; Murugavel, S. Synthesis and characterization of olivine phosphate cathode material with different particle sizes for rechargeable lithium-ion batteries. Mater. Chem. Phys. 2013, 140, 659–664. [Google Scholar] [CrossRef]
- Jin, B.; Sun, G.; Liang, J.; Gu, H.-B. Physicochemical properties of lithium iron phosphate carbon as lithium polymer battery cathodes. Int. J. Energy Res. 2013, 37, 500–509. [Google Scholar] [CrossRef]
- Anseán, D.; González, M.; Viera, J.C.; García, V.M.; Blanco, C.; Valledor, M. Fast charging technique for high power lithium iron phosphate batteries: A cycle life analysis. J. Power Sources 2013, 239, 9–15. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Z.; Qing, F.; Hong, Y.; Zhang, X. Applications of calcium phosphate nanoparticles in porous hard tissue engineering scaffolds. Nano 2012, 7, 1230004. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Heinemann, S.; Heinemann, C.; Wenisch, S.; Alt, V.; Worch, H.; Hanke, T. Calcium phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomater. 2013, 9, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Saviuc-Paval, A.M.; Victor Sandu, A.; Marcel Popa, I.; Anca Sandu, I.C.; Petru Bertea, A.; Sandu, I. Colorimetric and microscopic study of the thermal behavior of new ceramic pigments. Microsc. Res. Tech. 2013, 76, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Laha, S.; Sharma, R.; Bhat, S.V.; Reddy, M.L.P.; Gopalakrishnan, J.; Natarajan, S. Ba3(P1-xMnxO4)2: Blue/green inorganic materials based on tetrahedral Mn(V). Bull. Mater. Sci. 2011, 34, 1257–1262. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Luo, W.-Q.; Chen, Y.-X. Study on Co-KZr2(PO4)3-type crystalline purple ceramic pigments. Appl. Mech. Mater. 2010, 34–35, 790–794. [Google Scholar] [CrossRef]
- Llusar, M.; Zielinska, A.; Tena, M.A.; Badenes, J.A.; Monrós, G. Blue-violet ceramic pigments based on Co and Mg Co2-xMgxP2O7 diphosphates. J. Eur. Ceram. Soc. 2010, 30, 1887–1896. [Google Scholar] [CrossRef]
- Onoda, H.; Tange, K.; Tanaka, I. Influence of lanthanum addition on preparation and powder properties of cobalt phosphates. J. Mater. Sci. 2008, 43, 5483–5488. [Google Scholar] [CrossRef]
- Brunold, T.C.; Güdel, H.U.; Kück, S.; Huber, G. Excited state properties of ferrate (VI) doped crystals of K2SO4 and K2CrO4. J. Lumin. 1997, 65, 293–301. [Google Scholar] [CrossRef]
- Kuck, S.; Petermann, K.; Pohlmann, U.; Huber, G. Near-infrared emission of Cr4+-doped garnets: Lifetimes, quantum efficiencies, and emission cross sections. Phys. Rev. B Condens. Matter 1995, 51, 17323–17331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, J.; Ozarowski, A.; Sleight, A.W.; Subramanian, M.A. Intense turquoise and green colors in brownmillerite-type oxides based on Mn5+ in Ba2In2−xMnxO5+x. Inorg. Chem. 2013, 52, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lachwa, H.; Reinen, D. Color and electronic structure of manganese(V) and manganese(VI) in tetrahedral oxo coordination. A spectroscopic investigation. Inorg. Chem. 1989, 28, 1044–1053. [Google Scholar] [CrossRef]
- Albrecht, C.; Cohen, S.; Mayer, I.; Reinen, D. The structure of Sr2(VO4)Cl and Sr2(CrO4)Cl and spectroscopic properties of Mn5+-and Cr5+-doped Sr2(VO4)Cl. J. Solid State Chem. 1993, 107, 218–228. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, Y.Z.; Wang, C.M. Prediction of apatite lattice constants from their constituent elemental radii and artificial intelligence methods. Biomaterials 2024, 25, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Chernorukov, N.G.; Knyazev, A.V.; Bulanov, E.N. Phase transitions and thermal expansion of apatite-structured compounds. Inorg. Mater. 2011, 47, 172–177. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Maczka, M.; Bulanov, E.N.; Ptak, M.; Belopolskaya, S.S. High-temperature thermal and X-ray diffraction studies, and room-temperature spectroscopic investigation of some inorganic pigments. Dye. Pigment. 2011, 91, 286–293. [Google Scholar] [CrossRef]
- Johnson, P.D.; Prener, J.S.; Kingsley, J.D. Apatite: Origin of blue color. Science 1964, 141, 1179–1180. [Google Scholar] [CrossRef]
- Reinen, D.; Lachwa, H.; Allmann, R. Colour and constitution for MnV in tetrahedral oxygen coordination. An EPR and ligand field spectroscopic investigation of MnV in apatite phases and the structure of Ba5(MnO4)3Cl. Z. Anorg. Allg. Chem. 1986, 542, 71–88. (In German) [Google Scholar] [CrossRef]
- Medina, E.A.; Li, J.; Stalick, J.K.; Subramanian, M.A. Intense turquoise colors of apatite-type compounds with Mn5+ in tetrahedral coordination. Solid State Sci. 2016, 52, 97–105. [Google Scholar] [CrossRef]
- Laha, S.; Tamilarasan, S.; Natarajan, S.; Gopalakrishnan, J. Stabilization of a tetrahedral (Mn5+O4) chromophore in ternary barium oxides as a strategy toward development of new turquoise/green-colored pigments. Inorg. Chem. 2016, 55, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Sim, G.E.; Ock, J.Y.; Son, J.H.; Hasegawa, T.; Toda, K.; Bae, D.S. Discovery of novel inorganic Mn5+-doped sky-blue pigments based on Ca6BaP4O17: Crystal structure, optical and color properties, and color durability. Dye. Pigment. 2017, 139, 344–348. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Jiang, P.; Liu, L.; Cui, K.; Cao, W. Synthesis and optical properties of intense blue colors oxides based on Mn5+ in tetrahedral sites in Ba7Al2-xMnxO10+y. Ceram. Int. 2021, 47, 686–691. [Google Scholar] [CrossRef]
- Dardenne, K.; Vivien, D.; Huguenin, D. Color of Mn(V)-substituted apatites A10((B,Mn)O4)6F2, A = Ba, Sr, Ca; B = P,V. J. Solid State Chem. 1999, 146, 464–472. [Google Scholar] [CrossRef]
- Kettler, W.; Binder, M.; Franz, W.; Gabel, P.; Gauss, S.; Wilker, G.; Hempelmann, U.; Henning, R.; Kremitzl, H.-J.; Weixel, S. Colour Technology of Coatings; Vincentz Network: Hanovra, Germany, 2016. [Google Scholar]
- Wood, C.A.; Attridge, G.G.; Jacobson, R.E.; Pointer, M.R. Minimum perceptible differences in the colour reproduction of photographic prints. J. Photogr. Sci. 1991, 39, 119–127. [Google Scholar] [CrossRef]
- Hazenkamp, M.F.; Gudel, H.U.; Kuck, S.; Huber, G.; Rauw, W.; Reinen, D. Excited state absorption and laser potential of Mn5+-doped Li3PO4. Chem. Phys. Lett. 1997, 265, 264–270. [Google Scholar] [CrossRef]
- Kück, S.; Schepler, K.L.; Chai, B.H.T. Evaluation of Mn5+-doped Sr5(VO4)3F as a laser material based on excited-state absorption and stimulated-emission measurements. J. Opt. Soc. Am. B 1997, 14, 957–963. [Google Scholar] [CrossRef]
- Herren, M.; Riedener, T.; Gudel, H.U.; Albrecht, C.; Kaschuba, U.; Reinen, D. Near-infrared luminescence of manganate(V)-doped phosphates and vanadates. J. Lumin. 1992, 53, 452–456. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Cormier, G.; Bettinelli, M.; Moncorge, R.; Manaa, H. Near-infrared intraconfigurational luminescence spectroscopy of the Mn5+ (3d2) ion in Ca2PO4Cl, Sr5(PO4)3Cl, Ca2VO4Cl and Sr2VO4Cl. J. Lumin. 1992, 54, 1–11. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Cormier, G.; Morrison, C.A.; Moncorge, R. Crystal-field analyis of Mn5+ (3d2) in Sr5(PO4)3Cl. Opt. Mater. 1992, 1, 209–216. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Zhou, X.; Delaey, M.; Wang, M.; Fu, R.; Lei, S.; Vrielinck, H.; Poelman, D. Achieving high quantum efficiency in Mn5+ activated phosphors for NIR-II deep bioimaging application. Laser Photonics Rev. 2024, 18, 2400781. [Google Scholar] [CrossRef]
- Piotrowski, W.M.; Marin, R.; Szymczak, M.; Martín Rodríguez, E.; Ortgies, D.H.; Rodríguez-Sevilla, P.; Dramićanin, M.D.; Jaque, D.; Marciniak, L. Mn5+ lifetime-based thermal imaging in the optical transparency windows through skin-mimicking tissue phantom. Adv. Opt. Mater. 2023, 11, 2202366. [Google Scholar] [CrossRef]
- Dramićanin, M.D. Trends in luminescence thermometry. J. Appl. Phys. 2020, 128, 040902. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef]
- Đačanin Far, L.; Dramićanin, M.D. Luminescence Thermometry with Nanoparticles: A Review. Nanomaterials 2023, 13, 2904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).