Tunable Luminescence in Sb3+-Doped Cs3LnCl6 Perovskites for Wide-Coverage Emission and Anti-Counterfeiting Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Sb3+-Doped Cs3LnCl6 Perovskite NCs
2.3. Characterizations
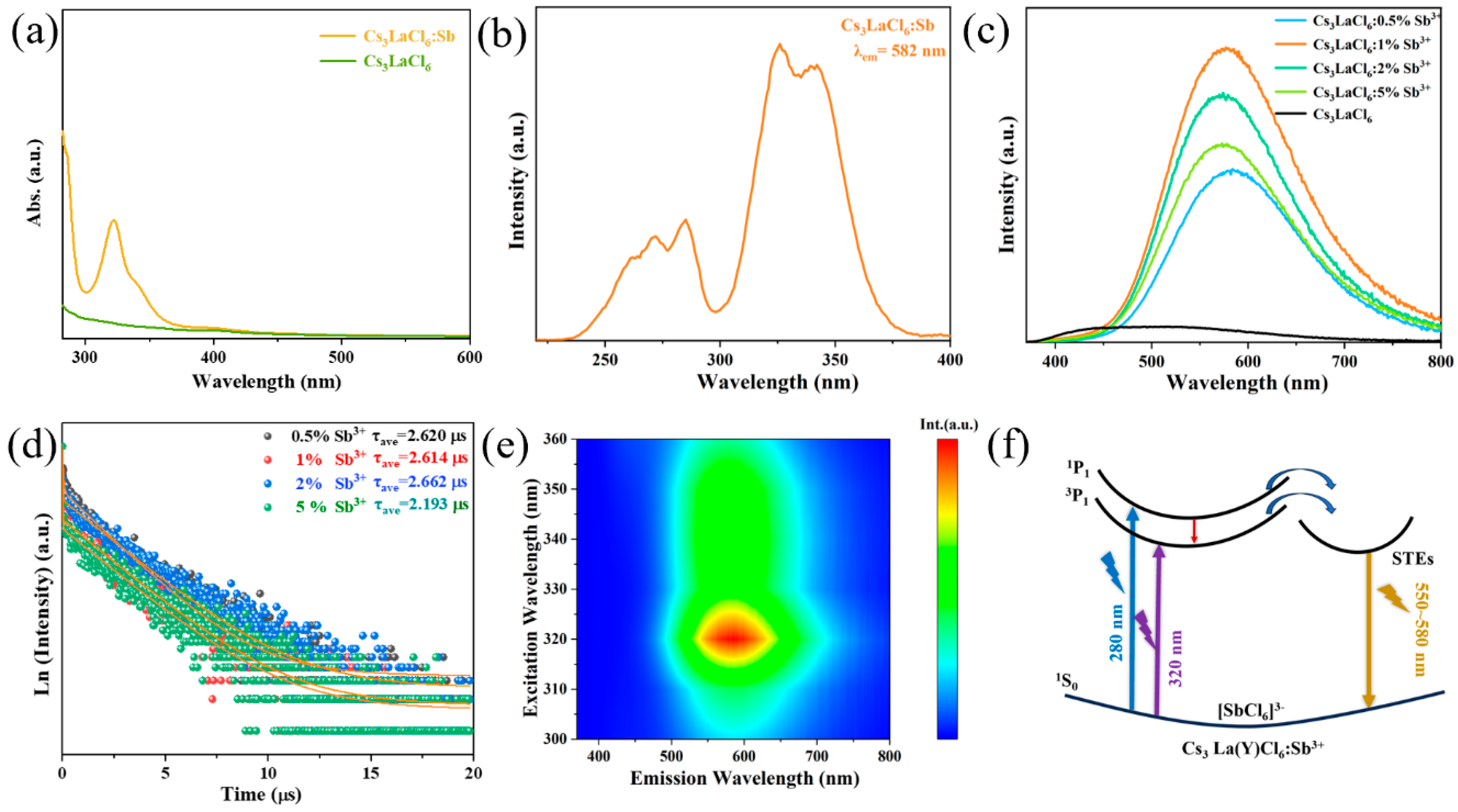
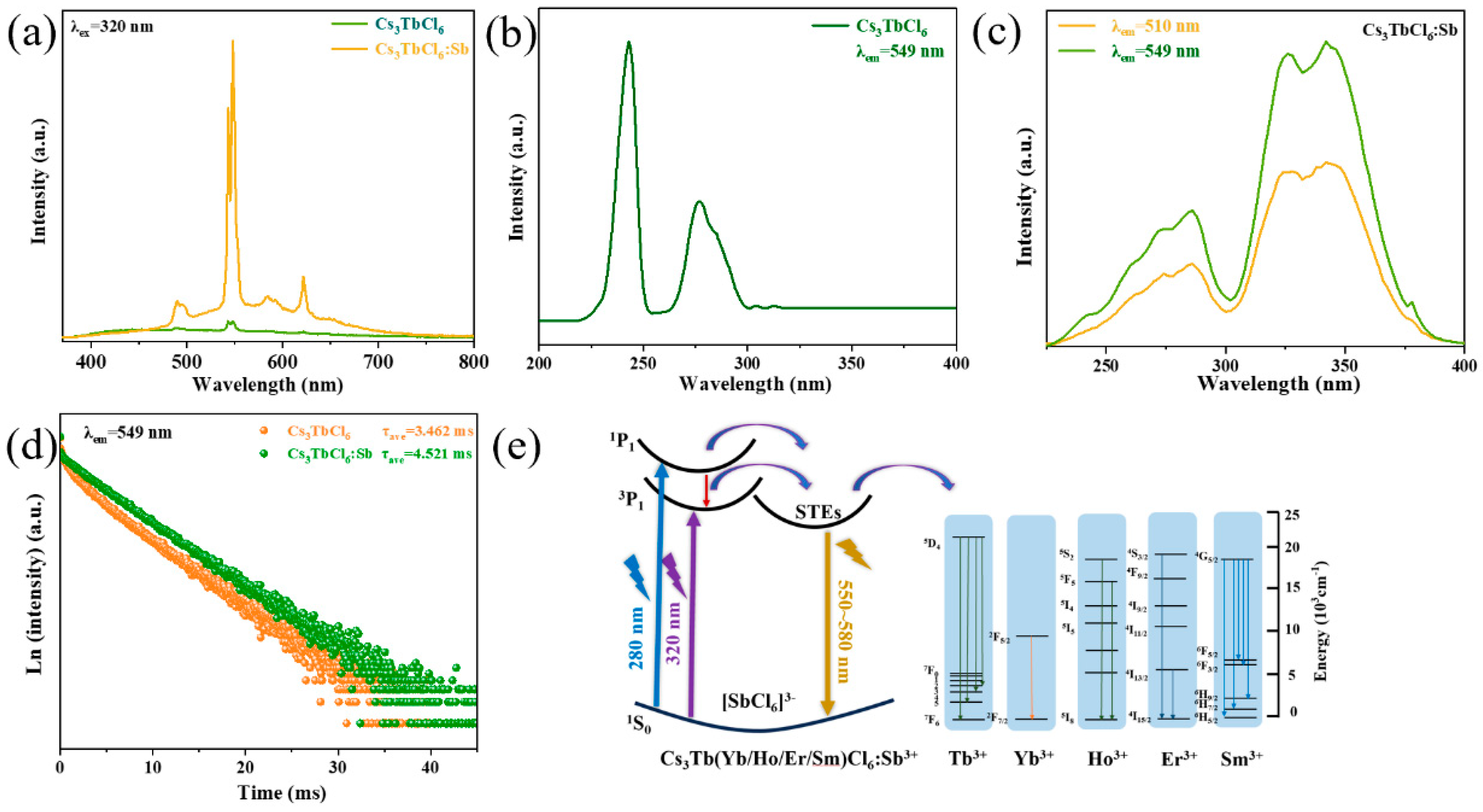
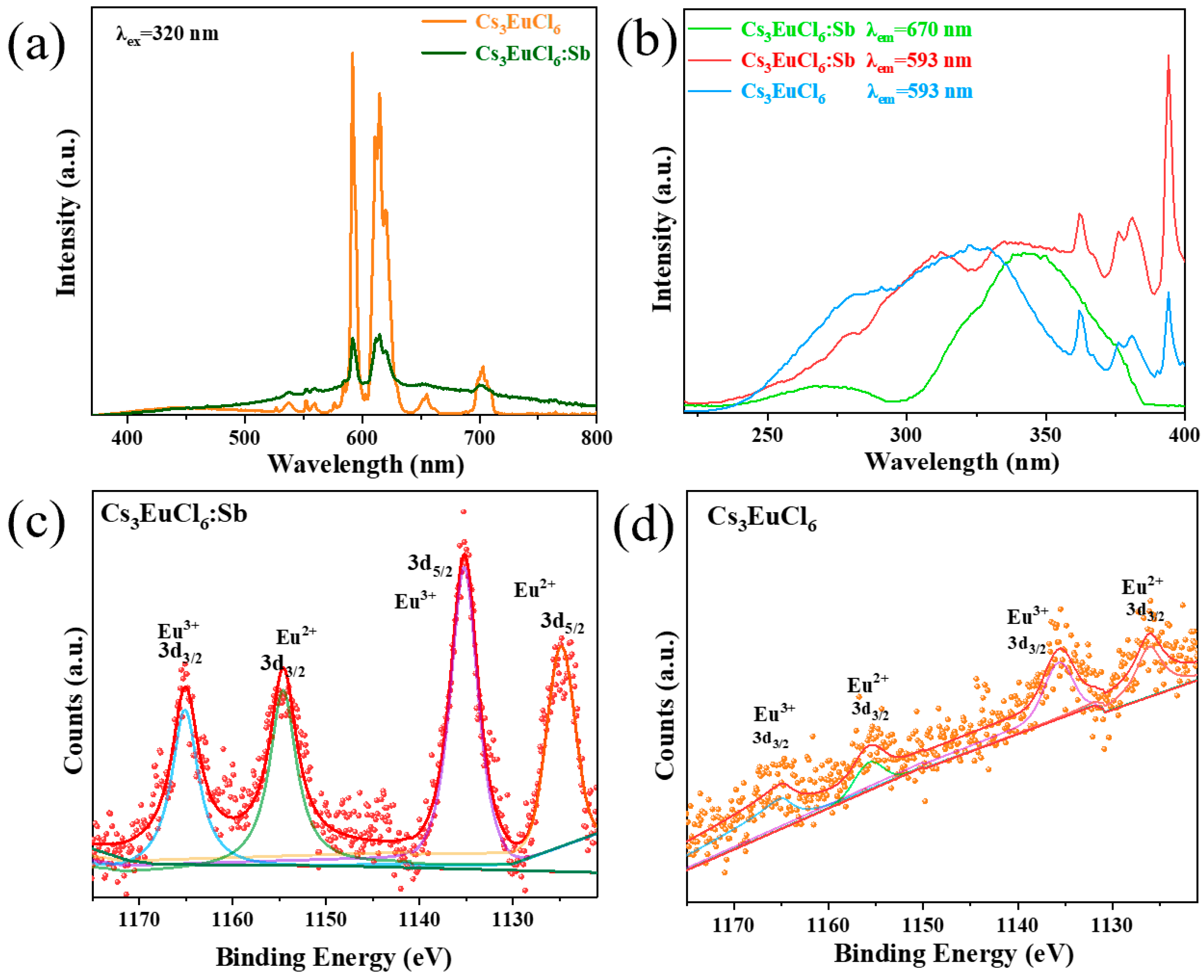
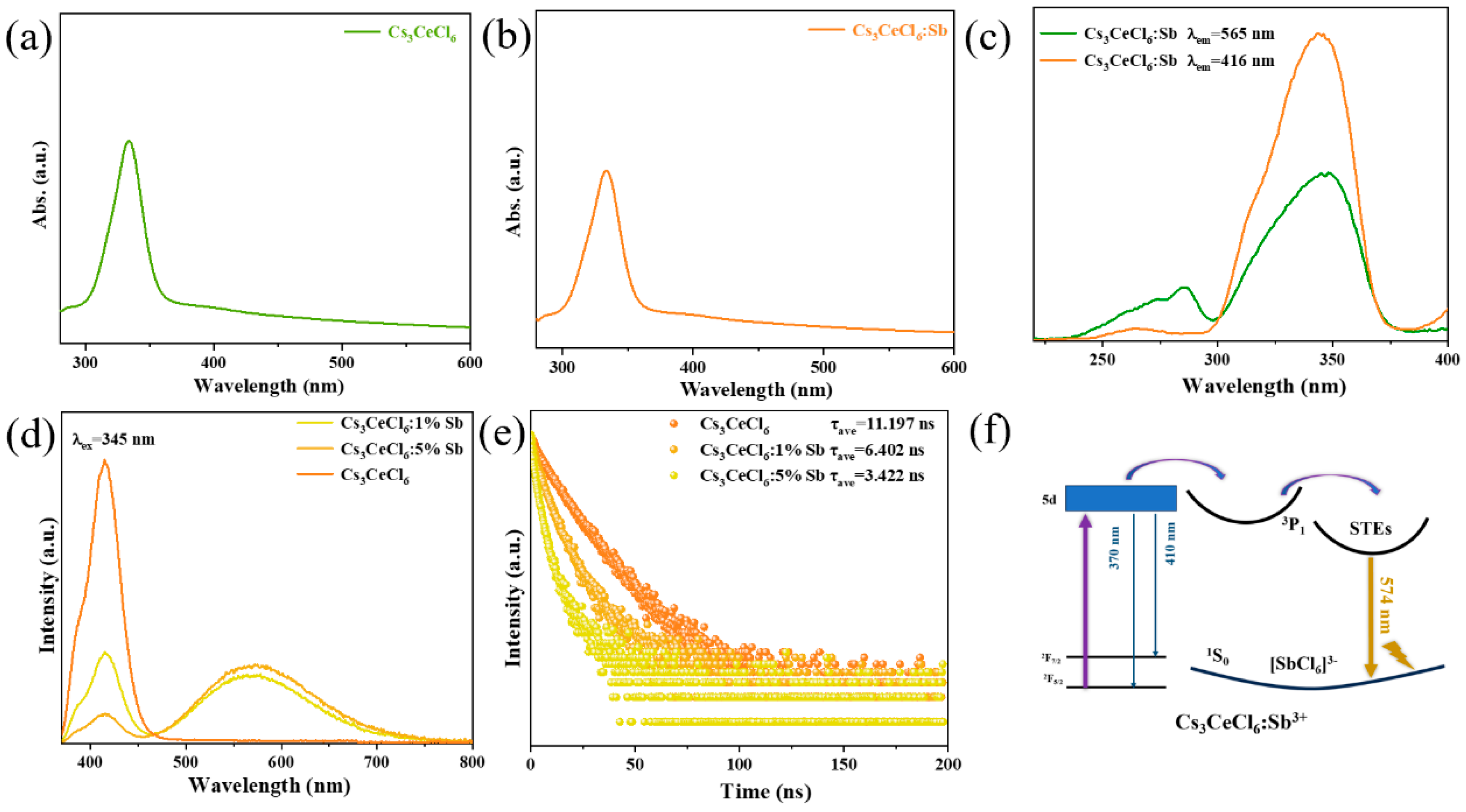
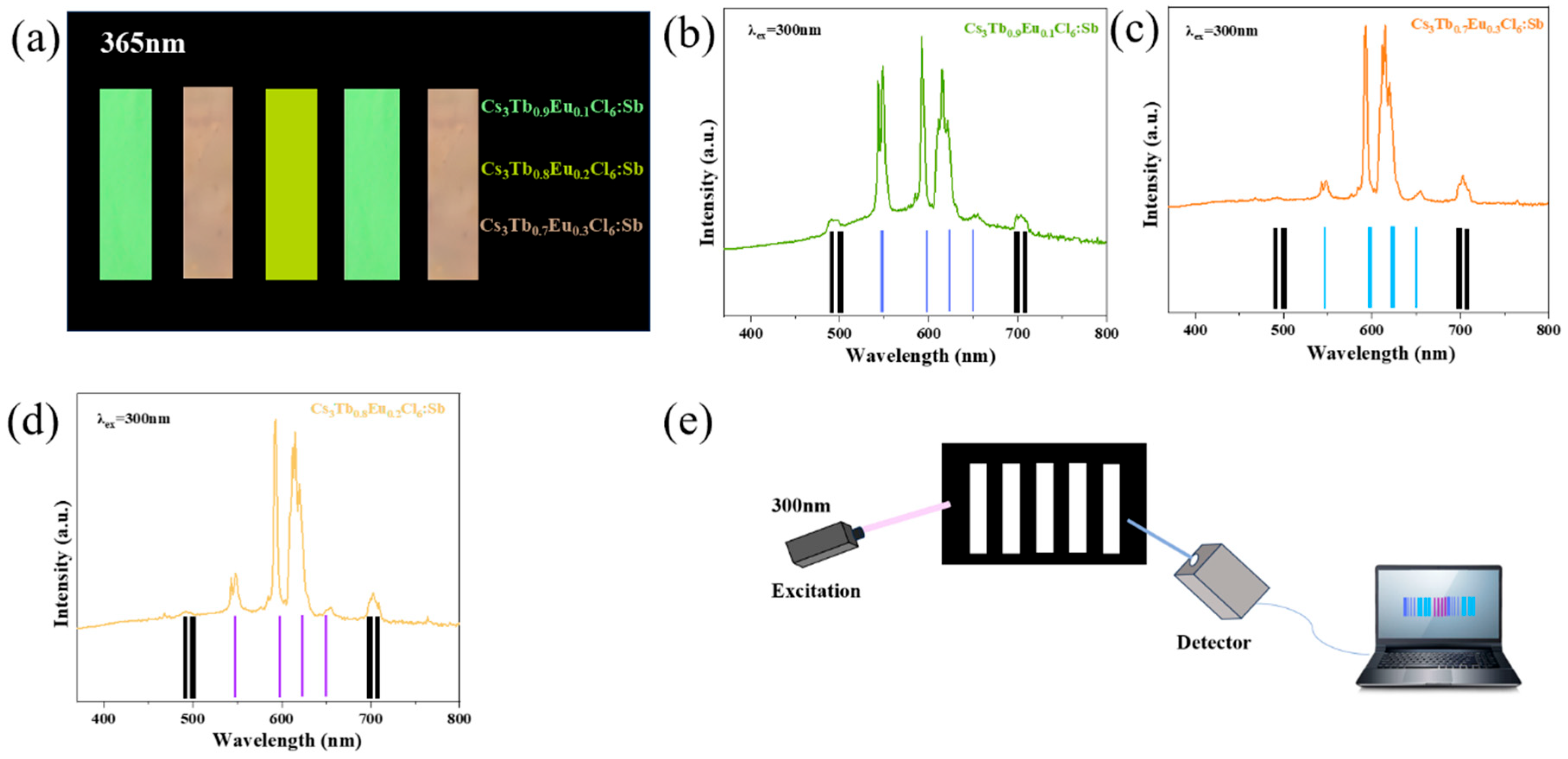
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6, 35685. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Gao, F. Structural and Functional Diversity in Lead-Free Halide Perovskite Materials. Adv. Mater. 2019, 31, 1900326. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, G.K.; Kim, H.J.; Viswanath, N.S.M.; Cho, H.B.; Han, J.H.; Kim, S.M.; Im, W.B. Strategies for Improving Luminescence Efficiencies of Blue-Emitting Metal Halide Perovskites. J. Korean Ceram. Soc. 2021, 58, 28–41. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Zhang, X.; Shen, X.; Lu, M.; Wu, J.; Shi, Z.; Colvin, V.L.; Hu, J.; Bai, X.; et al. Lead-Free Halide Perovskites for Light Emission: Recent Advances and Perspectives. Adv. Sci. 2021, 8, 2003334. [Google Scholar] [CrossRef]
- Sun, S.; Lu, M.; Gao, X.; Shi, Z.; Bai, X.; Yu, W.W.; Zhang, Y. 0D Perovskites: Unique Properties, Synthesis, and Their Applications. Adv. Sci. 2021, 8, 2102689. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, G.; Zhou, X.; Huang, H. Palladium-Catalyzed Regiodivergent Hydroaminocarbonylation of Alkenes to Primary Amides with Ammonium Chloride. Chem. Sci. 2018, 9, 380–386. [Google Scholar] [CrossRef]
- Yan, B.; Ning, B.; Zhang, G.; Zhou, D.; Shi, X.; Wang, C.; Zhao, H. Ultra-Thin GeSe/WS2 Vertical Heterojunction with Excellent Optoelectronic Performances. Adv. Opt. Mater. 2022, 10, 2102413. [Google Scholar] [CrossRef]
- Soni, A.; Ghosal, S.; Kundar, M.; Pati, S.K.; Pal, S.K. Long-Lived Interlayer Excitons in WS2/Ruddlesden–Popper Perovskite van Der Waals Heterostructures. ACS Appl. Mater. Interfaces 2024, 16, 35841–35851. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Saidaminov, M.I.; Haque, M.A.; Alarousu, E.; Sarmah, S.P.; Murali, B.; Dursun, I.; Miao, X.-H.; Abdelhady, A.L.; Wu, T.; et al. Formamidinium Lead Halide Perovskite Crystals with Unprecedented Long Carrier Dynamics and Diffusion Length. ACS Energy Lett. 2016, 1, 32–37. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Wang, C.; Slattum, P.M.; Yang, X.; Zang, L. Thermoactivated Electrical Conductivity in Perylene Diimide Nanofiber Materials. J. Phys. Chem. Lett. 2017, 8, 292–298. [Google Scholar] [CrossRef]
- De Bastiani, M.; Saidaminov, M.I.; Dursun, I.; Sinatra, L.; Peng, W.; Buttner, U.; Mohammed, O.F.; Bakr, O.M. Thermochromic Perovskite Inks for Reversible Smart Window Applications. Chem. Mater. 2017, 29, 3367–3370. [Google Scholar] [CrossRef]
- Cao, L.; Jia, X.; Gan, W.; Ma, C.; Zhang, J.; Lou, B.; Wang, J. Strong Self-Trapped Exciton Emission and Highly Efficient Near-Infrared Luminescence in Sb3+-Yb3+ Co-doped Cs2AgInCl6 Double Perovskite. Adv. Funct. Mater. 2023, 33, 2212135. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, W.; Shi, Y.; Zhang, W.; Yang, H.; Huang, P.; Li, L.; Zhang, Q.; Zheng, W.; Chen, X. Sb3+–Doped 0D Cs3GdCl6 Microcrystals with a near-Unity Photoluminescence Quantum Yield and High Thermal Quenching Resistance for Light-Emitting Application. J. Mater. Chem. C 2024, 12, 5538–5548. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Wen, Z.; Sun, H.; Ji, S.; Meng, X.; Zhang, R.; Jiang, J.; Tang, Z.; Liu, F. Excitation Wavelength-Dependent Fluorescence of a Lanthanide Organic Metal Halide Cluster for Anti-Counterfeiting Applications. Angew. Chem. Int. Ed. 2023, 62, e202316336. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Li, Y.; Gao, Y.; Yu, H.; Cao, Y.; Zhang, X.; Chen, B.; Xu, S. Regulating A-Site Alloying of Te4+-Doped Hafnium-Halide Perovskite for Fluorescence Thermometry Achieving Breakthrough Sensitivity at High Temperatures. Laser Photonics Rev. 2025, 19, 2401620. [Google Scholar] [CrossRef]
- Wei, J.-H.; Luo, J.-B.; Liao, J.-F.; Ou, W.-T.; Kuang, D.-B. Te4+-Doped Cs2InCl5·H2O Single Crystals for Remote Optical Thermometry. Sci. China Mater. 2022, 65, 764–772. [Google Scholar] [CrossRef]
- Gong, Z.; Zheng, W.; Huang, P.; Cheng, X.; Zhang, W.; Zhang, M.; Han, S.; Chen, X. Highly Efficient Sb3+ Emitters in 0D Cesium Indium Chloride Nanocrystals with Switchable Photoluminescence through Water-Triggered Structural Transformation. Nano Today 2022, 44, 101460. [Google Scholar] [CrossRef]
- Zhang, G.; Dang, P.; Tian, L.; Yang, W.; Cheng, Z.; Lian, H.; Lin, J. Boosting Energy Transfer from Self-Trapped Exciton to Er3+ Through Sb3+ Doping in Cs2 Na(Lu/Er)Cl6 Double Perovskites. Adv. Opt. Mater. 2023, 11, 2202369. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, Y.; Han, P.; Li, C.; Wu, T.; Ding, Z.; Liu, R.; Zhang, R.; Luo, C.; Li, H.; et al. Sb-Doped Cs3TbCl6 Nanocrystals for Highly Efficient Narrow-Band Green Emission and X-Ray Imaging. Adv. Mater. 2024, 36, 2302140. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, Y.; Jiang, X.; Molokeev, M.S.; Lin, Z.; Xia, Z. Sb3+ Dopant and Halogen Substitution Triggered Highly Efficient and Tunable Emission in Lead-Free Metal Halide Single Crystals. Chem. Mater. 2020, 32, 5327–5334. [Google Scholar] [CrossRef]
- Sun, L.; Dong, B.; Sun, J.; Wang, Y.; Wang, Y.; Hu, S.; Zhou, B.; Bai, X.; Xu, L.; Zhou, D.; et al. Efficient and Stable Multicolor Emissions of the Coumarin-Modified Cs3LnCl6 Lead-Free Perovskite Nanocrystals and LED Application. Adv. Mater. 2024, 36, 2310065. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-Y.; Choi, Y.; Song, Y.; Eom, N.S.A.; Kim, S.; Cho, H.-B.; Myung, N.V.; Choa, Y.-H. A Noble Gas Sensor Platform: Linear Dense Assemblies of Single-Walled Carbon Nanotubes (LACNTs) in a Multi-Layered Ceramic/Metal Electrode System (MLES). J. Mater. Chem. C 2018, 6, 972–979. [Google Scholar] [CrossRef]
- Zhu, D.; Zaffalon, M.L.; Zito, J.; Cova, F.; Meinardi, F.; De Trizio, L.; Infante, I.; Brovelli, S.; Manna, L. Sb-Doped Metal Halide Nanocrystals: A 0D versus 3D Comparison. ACS Energy Lett. 2021, 6, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sun, M.; Zhang, S.; Zhang, H.; Shi, X.; Ye, S.; Huang, B.; Du, Y.; Yan, C. Rare-Earth-Based Perovskite Cs2AgScCl6:Bi for Strong Full Visible Spectrum Emission. Adv. Funct. Mater. 2022, 32, 2204780. [Google Scholar] [CrossRef]
- Arfin, H.; Nag, A. Origin of Luminescence in Sb3+- and Bi3+-Doped Cs2SnCl6 Perovskites: Excited State Relaxation and Spin–Orbit Coupling. J. Phys. Chem. Lett. 2021, 12, 10002–10008. [Google Scholar] [CrossRef]
- Nie, J.; Ying, W.; Cao, R.; Liu, S.; Qiu, S.; Liao, C.; Yun, X.; Lan, B.; Wang, J. Modulation of the Near-Infrared-I and -II Luminescence of Thulium-Incorporated Lead-Free Double Perovskites. Inorg. Chem. Front. 2024, 11, 6960–6969. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Li, C.; Han, P.; Li, H.; Zhao, K. Tb3+ and Bi3+ Co-Doping of Lead-Free Cs2NaInCl6 Double Perovskite Nanocrystals for Tailoring Optical Properties. Nanomaterials 2023, 13, 549. [Google Scholar] [CrossRef]
- Song, R.; Xu, S.; Li, Y.; Gao, Y.; Yu, H.; Cao, Y.; Zhang, X.; Chen, B. Designing Multi-Mode Optical Thermometers via Sb3+/Er3+ Co-Doped Cs2NaInCl6 Lead-Free Double Perovskite Microcrystals. J. Alloys Compd. 2023, 961, 171126. [Google Scholar] [CrossRef]
- Wei, J.-H.; Liao, J.-F.; Zhou, L.; Luo, J.-B.; Wang, X.-D.; Kuang, D.-B. Indium-Antimony-Halide Single Crystals for High-Efficiency White-Light Emission and Anti-Counterfeiting. Sci. Adv. 2021, 7, eabg3989. [Google Scholar] [CrossRef]
- Huang, H.; Fu, Z.; Yin, S.; Chen, D.; Xie, A. Tunable Emission in Sb3+/Eu3+ Codoped Rare-Earth-Based Cs2NaTbCl6 Double Perovskite and Its LED Applications. Inorg. Chem. 2025, 64, 9287–9293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, Y.; Chang, J.; Song, R.; Li, Y.; Liu, B.; Chen, B.; Xu, S. A Three-Mode Optical Thermometer Utilizing Cs2NaInCl6: Sb3+/Tb3+ Lead-Free Double Perovskite Microcrystals for Lubricating Oil Temperature Monitoring. J. Alloys Compd. 2024, 1009, 177030. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Zhang, S.; Zhu, X.; Zou, B.; Zeng, R. Effective Energy Transfer Boosts Emission of Rare-Earth Double Perovskites: The Bridge Role of Sb(III) Doping. J. Phys. Chem. Lett. 2023, 14, 7108–7117. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Wang, T.; Yuan, J.; Han, L.; Song, H.; Yao, S.; Meng, B.; Wang, T.; Xu, X. X-Ray Real-Time Imaging and Delayed Imaging from Lead-Free Perovskite Scintillators. Adv. Opt. Mater. 2024, 12, 2401455. [Google Scholar] [CrossRef]
- Liu, Y.; Rong, X.; Li, M.; Molokeev, M.S.; Zhao, J.; Xia, Z. Incorporating Rare-Earth Terbium(III) Ions into Cs2AgInCl6:Bi Nanocrystals toward Tunable Photoluminescence. Angew. Chem. Int. Ed. 2020, 59, 11634–11640. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Gao, Y.; Cao, Y.; Zhang, X.; Yu, H.; Wang, Y.; Liu, T.; Chen, B. Thermochromic Cs2NaInCl6:Sb3+/Ln3+ (Ln = Er, Dy, Tb, Ho) Phosphors for Multifunctional Applications. Ceram. Int. 2024, 50, 13378–13387. [Google Scholar] [CrossRef]
- Samanta, T.; Viswanath, N.S.M.; Kim, H.W.; Jang, S.W.; Han, J.H.; Cho, S.B.; Im, W.B. Demystifying Role of Local Distortion in Emission Colors Tuning of Lead-Free Zero-Dimensional Metal Halide Nanocrystals. Chem. Eng. J. 2024, 484, 149697. [Google Scholar] [CrossRef]
- Nakano, H.; Ozono, K.; Hayashi, H.; Fujihara, S. Synthesis and Luminescent Properties of a New Eu3+-Doped Li1+x (Ta1-zNbz)1-xTixO3 Red Phosphor. J. Am. Ceram. Soc. 2012, 95, 2795–2797. [Google Scholar] [CrossRef]
- Walsh, K.M.; Pressler, K.; Crane, M.J.; Gamelin, D.R. Ferromagnetism and Spin-Polarized Luminescence in Lead-Free CsEuCl3 Perovskite Nanocrystals and Thin Films. ACS Nano 2022, 16, 2569–2576. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Li, Y.; Fu, X.; Feng, J.; Zhang, H. Efficient Multi-Luminescence Covering the Visible to Near-Infrared Range in Antimony and Lanthanide Co-Doped Indium-Based Zero-Dimensional Perovskites Nanocrystals. Adv. Opt. Mater. 2023, 11, 2300429. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Z.; Liu, X.; Zhou, X.; Zhang, J.; Li, W. Bright Blue Light-Emitting Doped Cesium Bromide Nanocrystals: Alternatives of Lead-Free Perovskite Nanocrystals for White LEDs. Adv. Opt. Mater. 2019, 7, 1900108. [Google Scholar] [CrossRef]
- Lee, M.; Chung, H.; Hong, S.V.; Woo, H.Y.; Chae, J.-Y.; Yoon, T.Y.; Diroll, B.T.; Paik, T. Dynamically Tunable Multicolor Emissions from Zero-Dimensional Cs3 LnCl6 (Ln: Europium and Terbium) Nanocrystals with Wide Color Gamut. Nanoscale 2023, 15, 1513–1521. [Google Scholar] [CrossRef]
- Ullah Khan, W.; Zhou, L.; Li, X.; Zhou, W.; Khan, D.; Niaz, S.-I.; Wu, M. Single Phase White LED Phosphor Ca3YAl3B4O15:Ce3+,Tb3+,Sm3+ with Superior Performance: Color-Tunable and Energy Transfer Study. Chem. Eng. J. 2021, 410, 128455. [Google Scholar] [CrossRef]
- Zhang, K.; Li, T.; Cheng, H.; Zhu, C. Photoluminescence in Rare-Earth Based Halide Double Perovskite Cs2NaRECl6 (RE = Ce, Eu, Y, Lu) Microcrystals. Ceram. Int. 2024, 50, 1867–1873. [Google Scholar] [CrossRef]
- Chen, C.; Chen, R.; Gao, R.; Zheng, J.; Xiang, J.; Guo, C. Highly Efficient and Stable Narrow Band Green Emitting Phosphor of Sb3+/Ce3+ Sensitized Cs2 NaTbCl6 for WLED. Laser Photonics Rev. 2025, 19, 2401158. [Google Scholar] [CrossRef]
- Cui, E.; Yuan, X.; Tang, L.; Yang, L.; Yang, X.; Liao, X.; Tang, J.; Zhao, Y.; Sun, W.; Liu, K.; et al. Eu3+, Tb3+ Doping Induced Tunable Luminescence of Cs2AgInCl6 Double Perovskite Nanocrystals and Its Mechanism. Appl. Surf. Sci. 2023, 609, 155472. [Google Scholar] [CrossRef]
- S, R. Exploring the Structural and Optical Properties of Eu3+, Sb3+ Co-Doping Effect on Cs2NaInCl6 Double Perovskites for LEDs Applications. Solid State Commun. 2023, 376, 115375. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhao, W.; Liang, L.; Bun Pun, E.Y.; Lin, H. Kinetics-Tunable Photochromic Platform in Perovskites for Advanced Dynamic Information Encryption. Chem. Eng. J. 2025, 503, 158416. [Google Scholar] [CrossRef]
- Pei, K.; Wu, J.; Zhao, M.; Feng, X.; Li, Y.; Ma, Y.; Li, H.; Zhai, T. Polarized Emission of Lanthanide Metal–Organic Framework (Ln-MOF) Crystals for High-Capacity Photonic Barcodes. Adv. Opt. Mater. 2022, 10, 2102143. [Google Scholar] [CrossRef]
- Li, J.; Sun, R.; Chen, J.; Su, Q.; Xie, Y.; Sun, G.; Zhang, H.; Sun, L. Heterostructured Lanthanide Perovskite Nanoparticles with High Quantum Yield and Water Stability for Optical Information Storage. Adv. Opt. Mater. 2025, 13, 2403340. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, L.; Xu, S.; Cao, Y.; Zhang, X.; Yu, H.; Gao, Y.; Chen, B. Tunable Luminescence in Sb3+-Doped Cs3LnCl6 Perovskites for Wide-Coverage Emission and Anti-Counterfeiting Applications. Nanomaterials 2025, 15, 1790. https://doi.org/10.3390/nano15231790
Zhang L, Chen L, Xu S, Cao Y, Zhang X, Yu H, Gao Y, Chen B. Tunable Luminescence in Sb3+-Doped Cs3LnCl6 Perovskites for Wide-Coverage Emission and Anti-Counterfeiting Applications. Nanomaterials. 2025; 15(23):1790. https://doi.org/10.3390/nano15231790
Chicago/Turabian StyleZhang, Lianao, Le Chen, Sai Xu, Yongze Cao, Xizhen Zhang, Hongquan Yu, Yuefeng Gao, and Baojiu Chen. 2025. "Tunable Luminescence in Sb3+-Doped Cs3LnCl6 Perovskites for Wide-Coverage Emission and Anti-Counterfeiting Applications" Nanomaterials 15, no. 23: 1790. https://doi.org/10.3390/nano15231790
APA StyleZhang, L., Chen, L., Xu, S., Cao, Y., Zhang, X., Yu, H., Gao, Y., & Chen, B. (2025). Tunable Luminescence in Sb3+-Doped Cs3LnCl6 Perovskites for Wide-Coverage Emission and Anti-Counterfeiting Applications. Nanomaterials, 15(23), 1790. https://doi.org/10.3390/nano15231790





