Dosage of Sulfidized Nano Zero-Valent Iron, Soil Moisture and pH Influences on Fraction of Arsenic and Cadmium in Contaminated Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Preparation and Aging of Soil
2.2. Preparation of Sulfidized Nano Zero-Valent Iron
2.3. Effect of S-nZVI Dosage on Remediation of As and Cd Contaminated Soil
2.4. Effect of Water Content on Remediation of As and Cd-Contaminated Soil
2.5. Effect of pH on Remediation of As and Cd Contaminated Soil
2.6. Methods of Analysis
2.7. Statistical Analysis
3. Results and Discussion
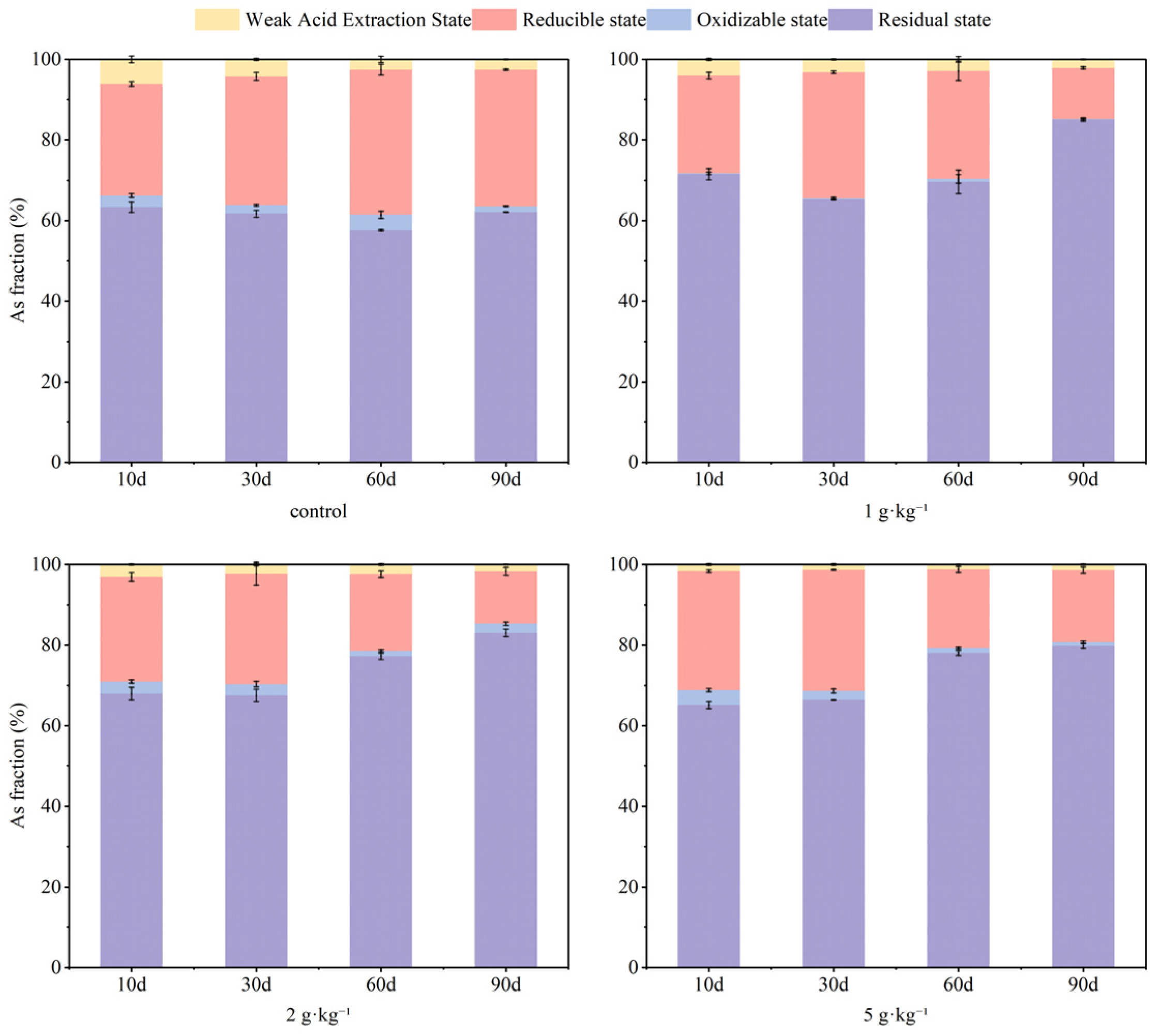
3.1. Effects of S-nZVI Dosage on Soil As and Cd Fraction
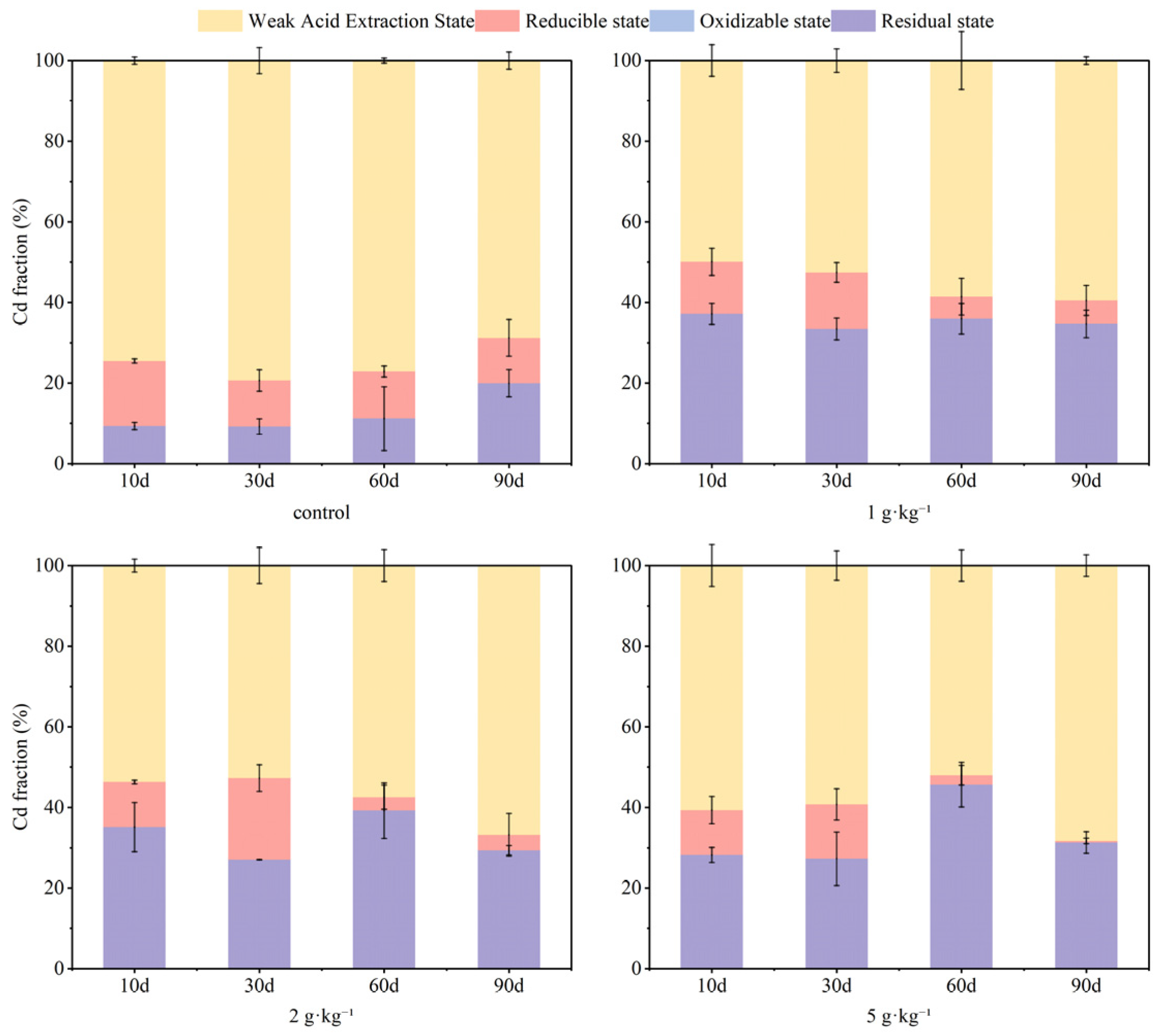
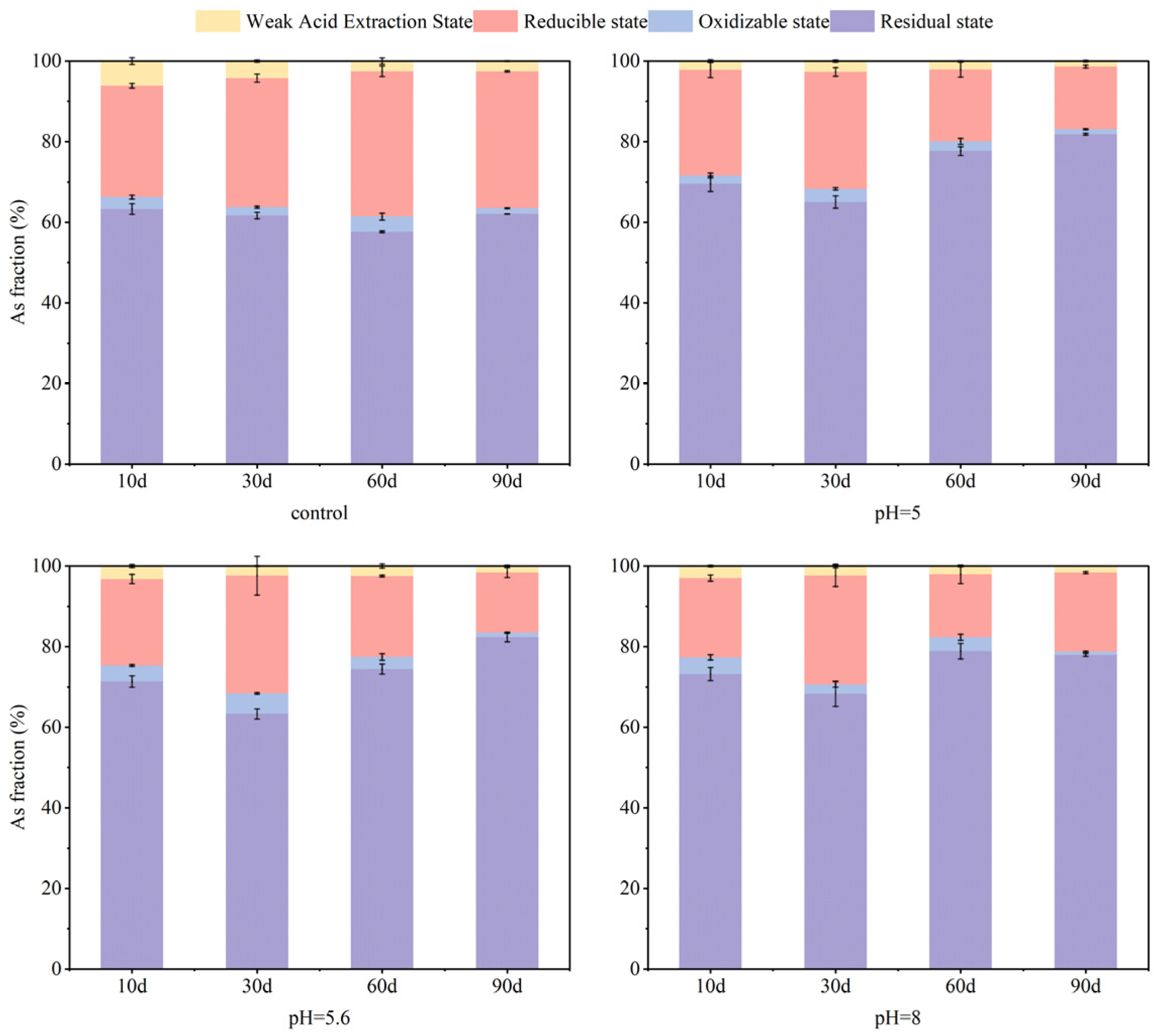
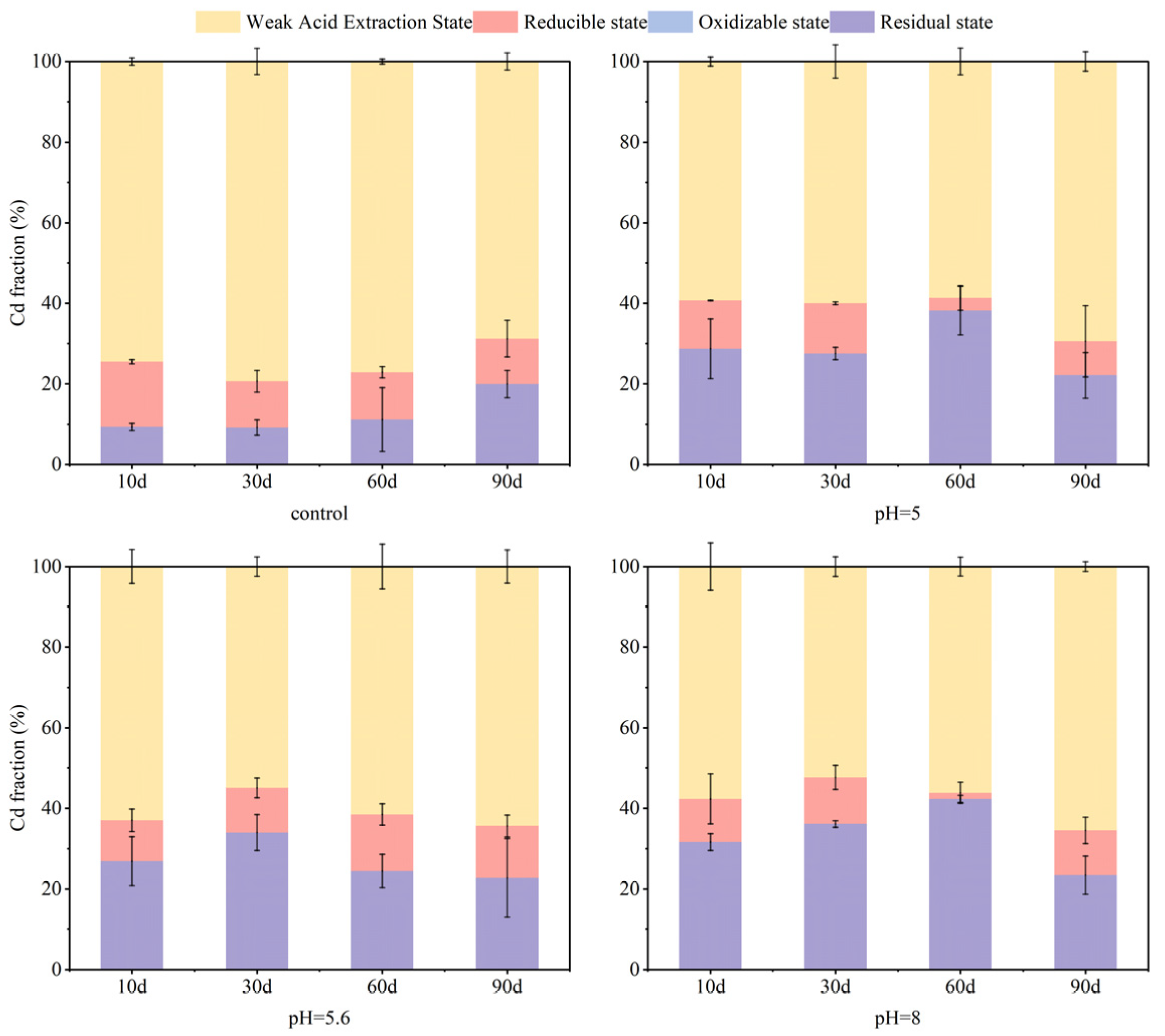
3.2. Effect of Soil Moisture Content on Soil As and Cd Fraction
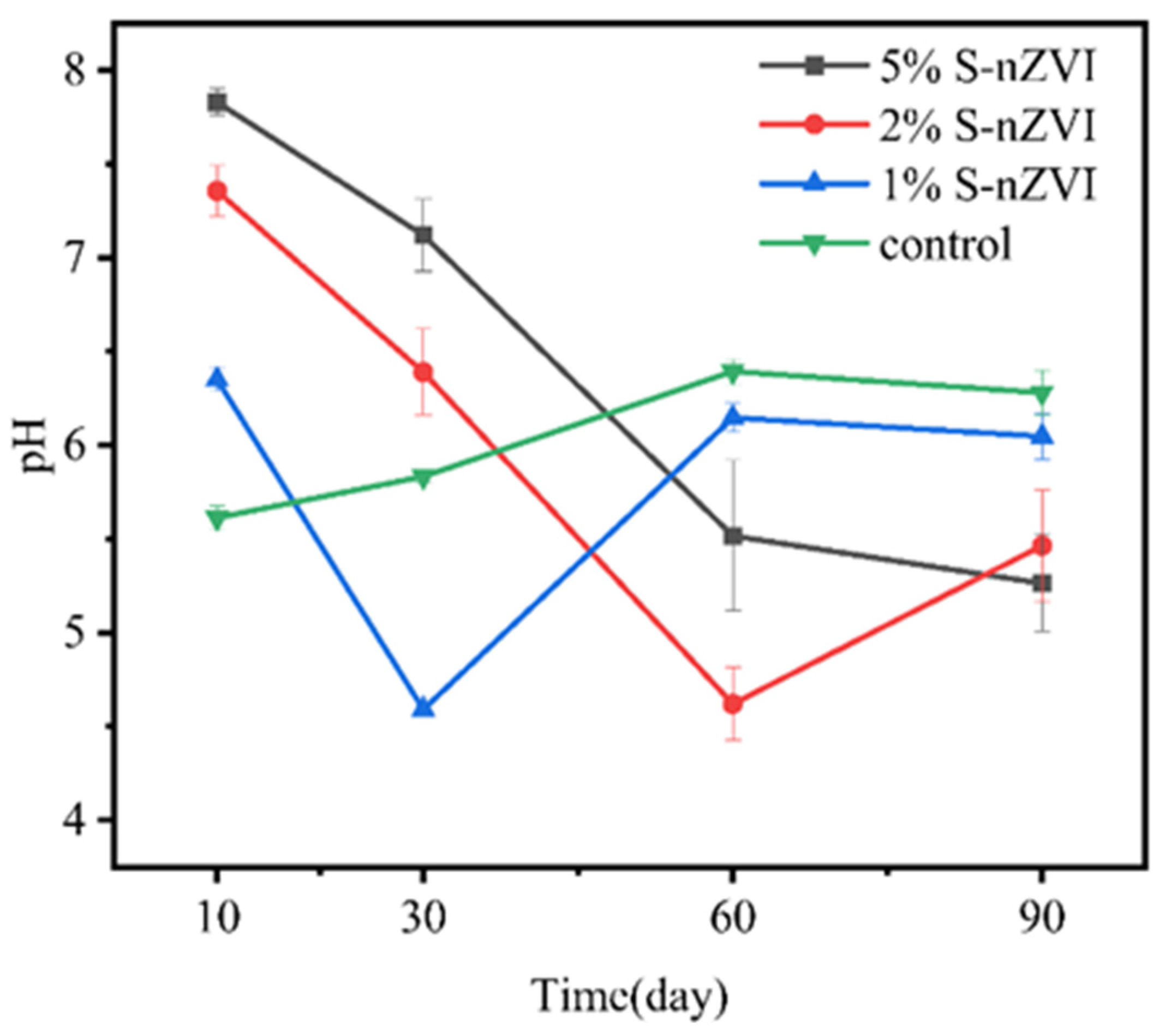
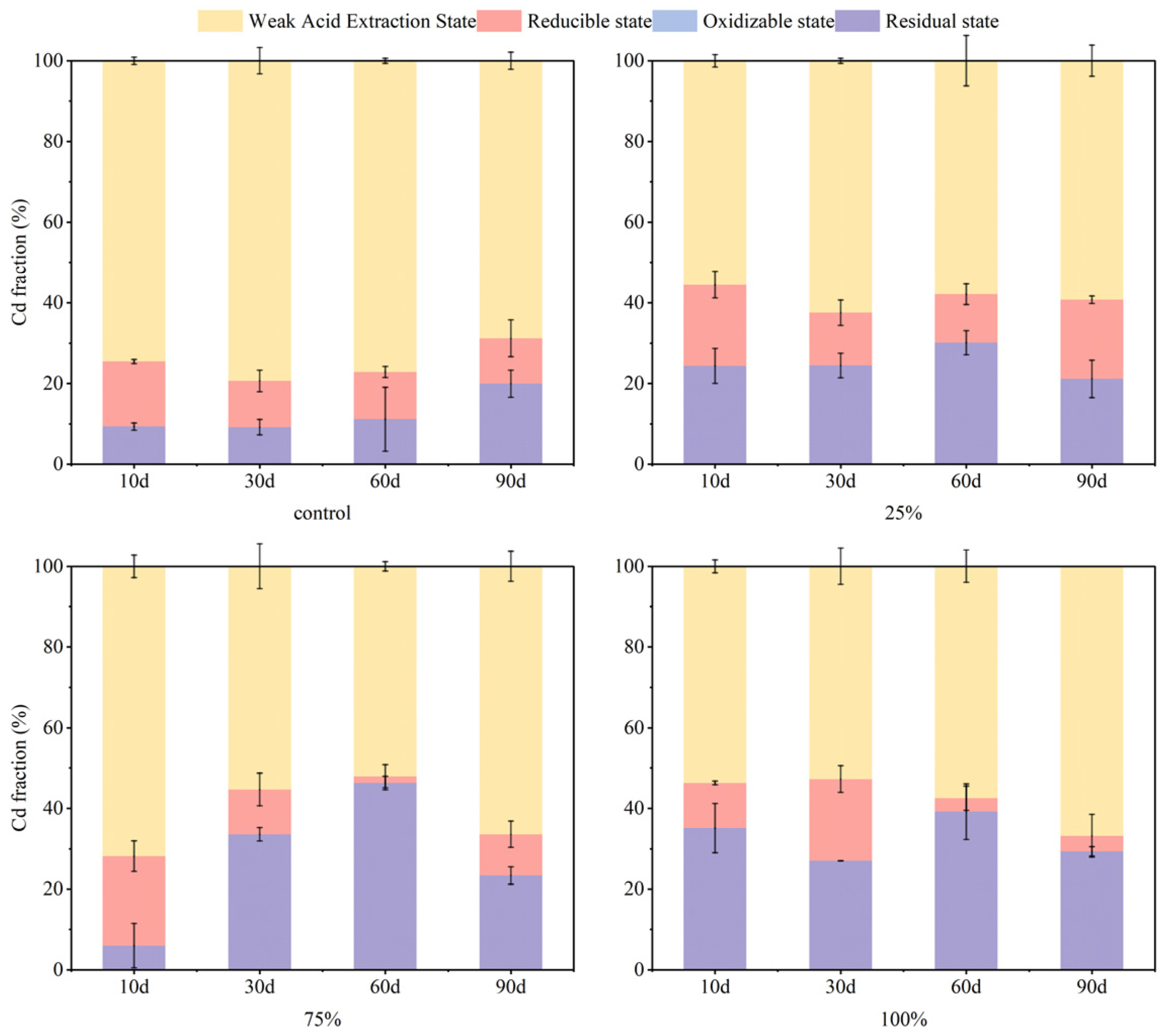
3.3. Effect of pH on Soil As and Cd Fraction
4. Conclusions
- (1)
- S-nZVI dosage exhibited a pronounced dose-dependent effect on both remediation efficiency and long-term stability of As and Cd. Increasing the dosage resulted in a reduction in As content within the weak acid extraction state. During the 90-day experimental time, the concentration of 5 g·kg−1 S-nZVI effectively maintained As content within 1% in this state while promoting the conversion of most reduced state As into more stable residual forms. For Cd, after 60 days, residual state Cd concentrations reached 36%, 39%, and 46% for treatment groups receiving dosages of 1 g·kg−1, 2 g·kg−1, and 5 g·kg−1 respectively, indicating that higher dosages enhanced immobilization effects on Cd. By day 90, although some residual state Cd had transformed back to weak acid extraction states, the higher the dosage and the more obvious transformation, the higher the proportion of residual state Cd. The dosage of 2 g·kg−1 yielded optimal remediation outcomes for both As and Cd.
- (2)
- Soil moisture conditions influenced reduced forms of As and Cd present in soils. Within the first 60 days, S-nZVI markedly decreased mobility for both contaminants. The fixation rates for As remained relatively stable throughout this period. With the extension of the experimental period up to 90 days, the content of residual state As was 83% at 100% water content. For Cd, in the short-term incubation (10–30 days), S-nZVI reduced the weakly acid-extracted state of Cd as the incubation time progressed. Meanwhile, in the period of 60–90 days, an increase was noted alongside a decrease in residual state Cd content. The highest content of residual state Cd was observed at a water content of 100%. The decay of the performance of S-nZVI could be attributed to its chemical interaction with excess water, which led to its aging and reduced capacity for As and Cd.
- (3)
- The application of S-nZVI across different initial pH values (5, 5.6, and 8, respectively) led to significant increases in residual As content, from 62% in untreated soils to 78–82%. After S-nZVI treatment, the content of Cd in the weakly acidic extractive state of the soil was maintained at 64–69% in all cases. Given the strong buffering capacity of soil against pH fluctuations, the final remediation effect of S-nZVI had a limited dependence on the initial pH of the soil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Ma, Z.; Kuijp, T.J.V.D.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, C.; Tu, C.; Zhu, Y. Heavy Metal Pollution in Soils in China: Status and Countermeasures. Ambio 1999, 28, 130–134. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Sun, J.; She, J.; Yin, M.; Fang, F.; Xiao, T.; Song, G.; Liu, J. Geochemical transfer of cadmium in river sediments near a lead-zinc smelter. Ecotoxicol. Environ. Saf. 2020, 196, 110529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Wu, T.; Zhou, T.; Li, Z.; Ouyang, Y.; Jiang, J.; Zhu, D.; Hou, J.; Wang, Z.; Luo, Y.; et al. Geographical variation in arsenic, cadmium, and lead of soils and rice in the major rice producing regions of China. Sci. Total Environ. 2019, 677, 373–381. [Google Scholar] [CrossRef]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-Organic Matter Associations via Coprecipitation versus Adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Zhang, S.; Dai, J.; Chen, H.-P.; Lombi, E.; Howard, D.L.; Van Der Ent, A.; Zhao, F.-J.; Kopittke, P.M. Chemical Speciation and Distribution of Cadmium in Rice Grain and Implications for Bioavailability to Humans. Environ. Sci. Technol. 2020, 54, 12072–12080. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.-G.; Feldmann, J.; et al. Geographical Variation in Total and Inorganic Arsenic Content of Polished (White) Rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Sohn, E. Contamination: The toxic side of rice. Nature 2014, 514, S62–S63. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Kanel, S.R.; Grenèche, J.-M.; Choi, H. Arsenic(V) Removal from Groundwater Using Nano Scale Zero-Valent Iron as a Colloidal Reactive Barrier Material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Pinilla, P.; Alonso, J.; Lobo, M.C. Viability of a nanoremediation process in single or multi-metal(loid) contaminated soils. J. Hazard. Mater. 2017, 321, 812–819. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Liang, L.; Lin, Z.; Su, X.; Zhang, W. Immobilization of cadmium in contaminated soils using sulfidated nanoscale zero-valent iron: Effectiveness and remediation mechanism. J. Hazard. Mater. 2021, 420, 126605. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Z.; Shi, S.; Qi, J.; Cai, S.; Yu, Y.; O’Carroll, D.M.; He, F. Carboxymethyl cellulose stabilized and sulfidated nanoscale zero-valent iron: Characterization and trichloroethene dechlorination. Appl. Catal. B-Environ. 2020, 262, 118303. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Collins, R.N.; Waite, T.D.; Hanna, K. Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review. Environ. Sci. Technol. 2018, 52, 12010–12025. [Google Scholar] [CrossRef]
- Yang, L.; Gao, J.; Liu, Y.; Zhang, Z.; Zou, M.; Liao, Q.; Shang, J. Removal of Methyl Orange from Water Using Sulfur-Modified nZVI Supported on Biochar Composite. Water Air Soil Pollut. 2018, 229, 355. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Yoon, H.; Lee, W.; Kim, E.-j.; Chang, Y.-S. Comparative study of peroxide oxidants activated by nZVI: Removal of 1,4-Dioxane and arsenic(III) in contaminated waters. Chem. Eng. J. 2018, 334, 2511–2519. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Guo, Y.; Lin, Z.; Su, X.; Liu, B. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur. J. Hazard. Mater. 2021, 404, 124057. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Liang, J.; Xue, Y. Ultrasound-assisted electrodeposition synthesis of nZVI-Pd/AC toward reductive degradation of methylene blue. Environ. Sci. Pollut. Res. 2021, 28, 67098–67107. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhu, K.; Wang, X. Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal. J. Taiwan Inst. Chem. Eng. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, J.; Hou, J.; Zeng, R.J.; Xing, B. Degradation of Tetrabromobisphenol A by Sulfidated Nanoscale Zerovalent Iron in a Dynamic Two-Step Anoxic/Oxic Process. Environ. Sci. Technol. 2019, 53, 8105–8114. [Google Scholar] [CrossRef]
- Fan, D.; Lan, Y.; Tratnyek, P.G.; Johnson, R.L.; Filip, J.; O’Carroll, D.M.; Garcia, A.N.; Agrawal, A. Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ. Sci. Technol. 2017, 51, 13070–13085. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Lin, Z.; Tian, C.; Guo, Y. The removal of Cd by sulfidated nanoscale zero-valent iron: The structural, chemical bonding evolution and the reaction kinetics. Chem. Eng. J. 2020, 382, 122933. [Google Scholar] [CrossRef]
- Su, Y.; Adeleye, A.S.; Keller, A.A.; Huang, Y.; Dai, C.; Zhou, X.; Zhang, Y. Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal. Water Res. 2015, 74, 47–57. [Google Scholar] [CrossRef]
- Gallegos, T.J.; Han, Y.-S.; Hayes, K.F. Model Predictions of Realgar Precipitation by Reaction of As(III) with Synthetic Mackinawite Under Anoxic Conditions. Environ. Sci. Technol. 2008, 42, 9338–9343. [Google Scholar] [CrossRef]
- Han, Z.; Salawu, O.A.; Zenobio, J.E.; Zhao, Y.; Adeleye, A.S. Emerging investigator series: Immobilization of arsenic in soil by nanoscale zerovalent iron: Role of sulfidation and application of machine learning. Environ. Sci.-Nano 2021, 8, 619–633. [Google Scholar] [CrossRef]
- Wu, D.; Peng, S.; Yan, K.; Shao, B.; Feng, Y.; Zhang, Y. Enhanced As(III) Sequestration Using Sulfide-Modified Nano-Scale Zero-Valent Iron with a Characteristic Core–Shell Structure: Sulfidation and As Distribution. ACS Sustain. Chem. Eng. 2018, 6, 3039–3048. [Google Scholar] [CrossRef]
- Xie, J.; Wei, H.; Sun, M.; Huang, L.; Zhong, J.; Wu, Y.; Zou, Q.; Chen, Z. The performance and mechanism of sulfidated nano-zero-valent iron for the simultaneous stabilization of arsenic and cadmium. Sci. Total Environ. 2024, 950, 175052. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of Heavy Metal Ions (CuII, CdII, NiII, and PbII) by Broiler Litter-Derived Biochars in Water and Soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef]
- Ainiwaer, M.; Jia, H.; Zhang, T.; Huang, J.; Zhang, N.; Yin, X.; Peng, L.; Li, H.; Zeng, X. Effective co-immobilization of arsenic and cadmium in contaminated soil by sepiolite-modified nano-zero-valent iron and its impact on the soil bacterial community. Sci. Rep. 2024, 14, 26178. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Dai, C.; Zhou, X.; Zhang, W. Sequestration of Cd(II) with nanoscale zero-valent iron (nZVI): Characterization and test in a two-stage system. Chem. Eng. J. 2014, 244, 218–226. [Google Scholar] [CrossRef]
- Hu, Y.-b.; Du, T.; Ma, L.; Feng, X.; Xie, Y.; Fan, X.; Fu, M.-L.; Yuan, B.; Li, X.-y. Insights into the mechanisms of aqueous Cd(II) reduction and adsorption by nanoscale zerovalent iron under different atmosphere conditions. J. Hazard. Mater. 2022, 440, 129766. [Google Scholar] [CrossRef]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Camara, A.Y.; Wang, Q.; Li, H. Water management impacts on the solubility of Cd, Pb, As, and Cr and their uptake by rice in two contaminated paddy soils. Chemosphere 2019, 228, 360–369. [Google Scholar] [CrossRef]
- Regitano, J.B.; Da Rocha, W.S.D.; Alleoni, L.R.F. Soil pH on Mobility of Imazaquin in Oxisols with Positive Balance of Charges. J. Agric. Food Chem. 2005, 53, 4096–4102. [Google Scholar] [CrossRef]
- Li, M.; Li, S. Construction of a Bridging Network Structure by Citric Acid for Environmental Heavy Metal Extraction. ACS Earth Space Chem. 2023, 7, 676–684. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, Y.; Zhao, J.; Li, B.; Li, Y.; Tao, S.; Li, M.; Li, Q.; Xu, Q.; Li, Y.; et al. Enhanced Cd removal from aqueous solution by biologically modified biochar derived from digestion residue of corn straw silage. Sci. Total Environ. 2019, 674, 213–222. [Google Scholar] [CrossRef]
- Mitzia, A.; Vítková, M.; Komárek, M. Assessment of biochar and/or nano zero-valent iron for the stabilisation of Zn, Pb and Cd: A temporal study of solid phase geochemistry under changing soil conditions. Chemosphere 2020, 242, 125248. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, X.; Zhou, J.; Liu, Y.; Li, Y.; Yang, K.; Lou, Z.; Baig, S.A.; Wu, D.; Xu, X. Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 442, 114–123. [Google Scholar] [CrossRef]
- Singh, P.; Pal, P.; Mondal, P.; Saravanan, G.; Nagababu, P.; Majumdar, S.; Labhsetwar, N.; Bhowmick, S. Kinetics and mechanism of arsenic removal using sulfide-modified nanoscale zerovalent iron. Chem. Eng. J. 2021, 412, 128667. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Van Renterghem, W.; Van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Cadmium (Cd2+) removal by nano zerovalent iron: Surface analysis, effects of solution chemistry and surface complexation modeling. Environ. Sci. Pollut. Res. 2013, 20, 6210–6221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.-M.; Gu, Y.; Kopittke, P.M.; Zhao, F.-J.; Wang, P. Iron–Manganese (Oxyhydro)oxides, Rather than Oxidation of Sulfides, Determine Mobilization of Cd during Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Zhang, C.; Li, W.; Chen, C.; Zhang, Z.; Chen, H.; Wang, J.; Li, Y.; Zhang, Y. Nano-FeS incorporated into stable lignin hydrogel: A novel strategy for cadmium removal from soil. Environ. Pollut. 2020, 264, 114739. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, D.; Zhou, Y.; Huang, Y.; Tie, B.; Lei, M. Mechanisms of arsenate and cadmium co-immobilized on ferrihydrite inferred from ternary surface configuration. Chem. Eng. J. 2021, 424, 130410. [Google Scholar] [CrossRef]

| pH | Organic Matter (g·kg−1) | Total Concentration (mg·kg−1) | |
|---|---|---|---|
| As | Cd | ||
| 5.6 | 113.65 | 125.32 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Xie, J.; Wei, H.; Guo, P.; Chen, Z. Dosage of Sulfidized Nano Zero-Valent Iron, Soil Moisture and pH Influences on Fraction of Arsenic and Cadmium in Contaminated Paddy Soil. Nanomaterials 2025, 15, 1768. https://doi.org/10.3390/nano15231768
Wu J, Xie J, Wei H, Guo P, Chen Z. Dosage of Sulfidized Nano Zero-Valent Iron, Soil Moisture and pH Influences on Fraction of Arsenic and Cadmium in Contaminated Paddy Soil. Nanomaterials. 2025; 15(23):1768. https://doi.org/10.3390/nano15231768
Chicago/Turabian StyleWu, Jiabing, Jianxiong Xie, Hang Wei, Pengran Guo, and Zhiliang Chen. 2025. "Dosage of Sulfidized Nano Zero-Valent Iron, Soil Moisture and pH Influences on Fraction of Arsenic and Cadmium in Contaminated Paddy Soil" Nanomaterials 15, no. 23: 1768. https://doi.org/10.3390/nano15231768
APA StyleWu, J., Xie, J., Wei, H., Guo, P., & Chen, Z. (2025). Dosage of Sulfidized Nano Zero-Valent Iron, Soil Moisture and pH Influences on Fraction of Arsenic and Cadmium in Contaminated Paddy Soil. Nanomaterials, 15(23), 1768. https://doi.org/10.3390/nano15231768




