BaTiO3–(Na0.5Bi0.5)TiO3 Ceramic Materials Prepared via Multiple Design Strategies with Improved Energy Storage
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Mallikarjuna, K.; Vattikuti, S.V.P.; Altaf, M.; Goud, B.S.; Koyyada, G.; Shim, J. Synergistic enhancement of electrochemical storage using g- C3N4 modified MoO2/MoO3 nanostructure electrodes via thermal decomposition. J. Energy Storage 2024, 96, 112716. [Google Scholar] [CrossRef]
- Chen, L.; Hu, T.F.; Shi, X.M.; Yu, H.F.; Zhang, H.; Wu, J.; Fu, Z.Q.; Qi, H.; Chen, J. Near-zero energy consumption capacitors by controlling inhomogeneous polarization configuration. Adv. Mater. 2024, 36, 10. [Google Scholar] [CrossRef]
- Cui, C.H.; Bai, F.; Yang, Y.N.; Hou, Z.Q.; Sun, Z.; Zhang, T. Ion-exchange-induced phase transition enables an intrinsically air sable hydrogarnet electrolyte for solid-state lithium batteries. Adv. Sci. 2024, 11, 11. [Google Scholar] [CrossRef]
- Duan, J.H.; Wei, K.; Du, Q.B.; Ma, L.Z.; Yu, H.F.; Qi, H.; Tan, Y.C.; Zhong, G.K.; Li, H. High-entropy superparaelectrics with locally diverse ferroic distortion for high-capacitive energy storage. Nat. Commun. 2024, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Y.; Jiang, R.J.; Lan, S.; Liu, S.Z.; Zhou, Z.; Dou, L.; Zhang, M.; Huang, H.; Chen, L.Q.; et al. Enhanced energy storage in antiferroelectrics via antipolar frustration. Nature 2025, 637, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lan, S.; Yang, B.B.; Pan, H.; Liu, Y.Q.; Zhang, Q.H.; Qi, J.L.; Chen, D.; Su, H.; Yi, D.; et al. Ultrahigh energy storage in high-entropy ceramic capacitors with polymorphic relaxor phase. Science 2024, 384, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Pan, H.; Si, W.; Zhang, Q.; Shen, Z.; Yu, Y.; Lan, S.; Meng, F.; Liu, Y.; et al. High-entropy enhanced capacitive energy storage. Nat. Mater. 2022, 21, 1074–1080. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Wang, X.L.; Li, K.; Zhu, Q.F.; Gong, W.P.; Liu, G.; Wang, Q.Y.; Xie, S.X. Structural origin of enhanced storage energy performance and robust mechanical property in A-site disordered high-entropy ceramics. Rare Met. 2025, 44, 551–564. [Google Scholar] [CrossRef]
- Chen, L.; Deng, S.; Liu, H.; Wu, J.; Qi, H.; Chen, J. Giant energy-storage density with ultrahigh efficiency in lead-free relaxors via high-entropy design. Nat. Commun. 2022, 13, 3089. [Google Scholar] [CrossRef]
- Zhong, W.T.; Liu, X.Y.; Zheng, X.T.; Zheng, P.; Wang, J.Q.; Sheng, L.S.; Zheng, L.; Fan, Q.L.; Bai, W.F.; Zhang, Y. Realizing exceptional energy storage performance in tungsten bronze-based ceramics via weakly coupled relaxor and grain boundary reinforcement designs. ACS Appl. Mater. Interfaces 2025, 17, 12375–12383. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Chen, M.; Peng, X.; Wang, B.; Shang, J. Design strategies of perovskite energy-storage dielectrics for next-generation capacitors. J. Eur. Ceram. Soc. 2023, 43, 5713–5747. [Google Scholar] [CrossRef]
- Tang, T.; Liu, J.C.; Liu, D.; Han, Y.; Luan, R.D.; Wang, Q.; Liu, H.; Zhang, B.P.; Zheng, Q.; Deng, S.Q.; et al. Self-generated glass-ceramics-like structure boosts energy storage performance of AgNbO3-based MLCC. Adv. Funct. Mater. 2025, 35, 2425711. [Google Scholar] [CrossRef]
- Tang, T.; Liu, D.; Wang, L.; Li, J.Z.; Zhang, Z.; Zhao, L.; Zhang, B.P.; Zhu, L.F. Ultrahigh energy storage density and efficiency of antiferroelectric AgNbO3-based MLCCs via reducing the off-center cations displacement. Chem. Eng. J. 2025, 503, 158557. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, D.K.; Chen, R.X.; Zhang, K.; Jin, R.Q.; Sun, H.C.; Feng, Y.J.; Wei, X.Y.; Xu, Z.; Xu, R. Enhanced ultra-high efficiency in high-energy-density PbHfO3-based antiferroelectric ceramics through synergistic effect design. Chem. Eng. J. 2024, 496, 154369. [Google Scholar] [CrossRef]
- Wu, L.W.; Cai, Z.M.; Zhu, C.Q.; Feng, P.Z.; Li, L.T.; Wang, X.H. Significantly enhanced dielectric breakdown strength of ferroelectric energy-storage ceramics via grain size uniformity control: Phase-field simulation and experimental realization. Appl. Phys. Lett. 2020, 117, 6. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Yang, Y.; Shi, W.; Huang, Y.; Alikin, D.O.; Shur, V.Y.; Lou, Z.; Zhang, A.; Wei, X.; et al. Enhancing energy storage performance in Na0.5Bi0.5TiO3-based lead-free relaxor ferroelectric ceramics along a stepwise optimization route. J. Mater. Chem. A 2023, 11, 2641–2651. [Google Scholar] [CrossRef]
- Li, D.; Xu, D.; Zhao, W.; Avdeev, M.; Jing, H.; Guo, Y.; Zhou, T.; Liu, W.; Wang, D.; Zhou, D. A high-temperature performing and near-zero energy loss lead-free ceramic capacitor. Energy Environ. Sci. 2023, 16, 4511–4521. [Google Scholar] [CrossRef]
- Cao, W.; Lin, R.; Hou, X.; Li, L.; Li, F.; Bo, D.; Ge, B.; Song, D.; Zhang, J.; Cheng, Z. Interfacial polarization restriction for ultrahigh energy storage density in lead free ceramics. Adv. Funct. Mater. 2023, 33, 2301027. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; Gao, B.; Zhou, C.; Wu, J.; Deng, S.; Liu, H.; Qi, H.; Chen, J. Excellent energy storage and mechanical performance in hetero-structure BaTiO3-based relaxors. Chem. Eng. J. 2023, 452, 139222. [Google Scholar] [CrossRef]
- Lv, J.; Li, Q.; Li, Y.; Tang, M.; Jin, D.; Yan, Y.; Fan, B.; Jin, L.; Liu, G. Significantly improved energy storage performance of NBT-BT based ceramics through domain control and preparation optimization. Chem. Eng. J. 2021, 420, 129900. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Li, G.; Yao, F.Z.; Cheng, S.D.; Wang, Y.F.; Ma, R.; Mi, S.B.; Gu, M.; Wang, K.; Li, J.F.; et al. Simultaneously achieved temperature-insensitive high energy density and efficiency in domain engineered BaTiO3-Bi(Mg0.5Zr0.5)O3 lead-free relaxor ferroelectrics. Nano Energy 2018, 52, 203–210. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, Z.Y.; Deng, W.; Li, K.; Luo, W.Q.; Song, F.S.; Zeng, X.J.; Wang, Z.M.; Li, Y.M. A review: (Bi,Na)TiO3 (BNT)-based energy storage ceramics. J. Mater. 2024, 10, 86–123. [Google Scholar] [CrossRef]
- Zhou, S.; Pu, Y.; Zhang, X.; Shi, Y.; Gao, Z.; Feng, Y.; Shen, G.; Wang, X.; Wang, D. High energy density, temperature stable lead-free ceramics by introducing high entropy perovskite oxide. Chem. Eng. J. 2022, 427, 131684. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, Y.; Liu, X.Y.; Yang, H.B. Bi(Mg2/3Nb1/3)O3 addition inducing high recoverable energy storage density in lead-free 0.65BaTiO3-0.35Bi0.5Na0.5TiO3 bulk ceramics. J. Alloys Compd. 2019, 797, 348–355. [Google Scholar] [CrossRef]
- Dai, Z.; Xie, J.; Fan, X.; Ding, X.; Liu, W.; Zhou, S.; Ren, X. Enhanced energy storage properties and stability of Sr(Sc0.5Nb0.5)O3 modified 0.65BaTiO3-0.35Bi0.5Na0.5TiO3 ceramics. Chem. Eng. J. 2020, 397, 125520. [Google Scholar] [CrossRef]
- Xie, A.; Fu, J.; Zuo, R.; Zhou, C.; Qiao, Z.; Li, T.; Zhang, S. NaNbO3-CaTiO3 lead-free relaxor antiferroelectric ceramics featuring giant energy density, high energy efficiency and power density. Chem. Eng. J. 2022, 429, 132534. [Google Scholar] [CrossRef]
- Ye, W.B.; Zhu, C.H.; Xiao, Y.M.; Bai, X.Z.; Zheng, P.; Zhang, J.J.; Bai, W.F.; Fan, Q.L.; Zheng, L.; Zhang, Y. Remarkable energy-storage performances and excellent stability in CaTiO3-doped BiFeO3-BaTiO3 relaxor ferroelectric ceramics. J. Eur. Ceram. Soc. 2023, 43, 900–908. [Google Scholar] [CrossRef]
- Yang, F.; Pan, Z.; Ling, Z.; Hu, D.; Ding, J.; Li, P.; Liu, J.; Zhai, J. Realizing high comprehensive energy storage performances of BNT-based ceramics for application in pulse power capacitors. J. Eur. Ceram. Soc. 2021, 41, 2548–2558. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhang, X.; Zhang, D.; Gong, W. Simultaneous excellent energy storage density and efficiency under applied low electric field for high entropy relaxor ferroelectric ceramics. Mater. Res. Bull. 2023, 157, 112024. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Pan, Z.; Li, H.; Zhao, J.; Tang, L.; Liu, J.; Li, P.; Xie, H.; Zhai, J. Ultrahigh energy density and efficiency of BaTiO3-based ceramics via multiple design strategies. Chem. Eng. J. 2023, 467, 143395. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Bai, W.; Wu, S.; Zheng, P.; Zhang, J.; Pan, Z.; Zhai, J. Superior energy storage performance in (Bi0.5Na0.5)TiO3-based lead-free relaxor ferroelectrics for dielectric capacitor application via multiscale optimization design. J. Mater. Chem. A 2022, 10, 9535–9546. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous-containing activated carbon derived from natural honeydew peel powers aqueous supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate biopolymer effect on the electrodeposition of manganese dioxide on electrodes for supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Xie, A.; Zuo, R.; Qiao, Z.; Fu, Z.; Hu, T.; Fei, L. NaNbO3-(Bi0.5Li0.5)TiO3 lead-free relaxor ferroelectric capacitors with superior energy-storage performances via multiple synergistic design. Adv. Energy Mater. 2021, 11, 2101378. [Google Scholar] [CrossRef]
- Luo, N.N.; Han, K.; Cabral, M.J.; Liao, X.Z.; Zhang, S.J.; Liao, C.Z.; Zhang, G.Z.; Chen, X.Y.; Feng, Q.; Li, J.F.; et al. Constructing phase boundary in AgNbO3 antiferroelectrics: Pathway simultaneously achieving high energy density and efficiency. Nat. Commun. 2020, 11, 10. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, X.; Sun, J.; Sun, C.C.; Shi, J.P.; Pang, F.H.; Zhou, H.F. Achieving ultrahigh energy storage density and energy efficiency simultaneously in barium titanate based ceramics. Appl. Phys. A-Mater. Sci. Process. 2020, 126, 8. [Google Scholar] [CrossRef]
- Yan, F.; Huang, K.W.; Jiang, T.; Zhou, X.F.; Shi, Y.J.; Ge, G.L.; Shen, B.; Zhai, J.W. Significantly enhanced energy storage density and efficiency of BNT-based perovskite ceramics via A-site defect engineering. Energy Storage Mater. 2020, 30, 392–400. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, Z.; Tang, L.; Shen, Y.; Chen, X.; Li, H.; Li, P.; Zhang, Y.; Liu, J.; Zhai, J. Greatly enhanced discharged energy density and efficiency of BiFeO3-Based ceramics by regulating insulation performance. Mater. Today Phys. 2022, 27, 100821. [Google Scholar] [CrossRef]
- Liu, Z.G.; Li, M.D.; Tang, Z.H.; Tang, X.-G. Enhanced energy storage density and efficiency in lead-free Bi(Mg1/2Hf1/2)O3-modified BaTiO3 ceramics. Chem. Eng. J. 2021, 418, 129379. [Google Scholar] [CrossRef]
- Guan, Z.N.; Yan, Y.; Ma, J.; Pan, T.; Li, X.; Guo, S.; Zhang, J.; Wang, J.; Wang, Y. Significantly enhanced energy storage performance of lead-free BiFeO3-based ceramics via synergic optimization strategy. ACS Appl Mater Interfaces 2022, 14, 44539–44549. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Z.; Zhang, L.Y.; Geng, X.Y.; Zhang, J.; Sun, L.; Wang, R.-X.; Gu, Z.-B.; Zhang, S.-T. Crystal structure, impedance, and multiferroic property of SrZrO3 and MnO2 modified 0.725BiFeO3-0.275BaTiO3 ceramics. Ceram. Int. 2017, 43, 14748–14755. [Google Scholar] [CrossRef]
- Weng, N.; Zhang, J.; Wang, Z.Y.; Wang, H.; Wang, L.; Wang, J.; Wang, Y.J. Moderate electric field driven ultrahigh energy density in BiFeO3-BaTiO3-based ceramics with improved relaxor behavior and breakdown strength. Chem. Eng. J. 2024, 485, 149947. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Du, Y.; Chen, X.; Qin, H.; Wang, J.; Yan, T.; Yu, S.; Hu, Y.; Wang, D. Superior energy storage performance of BiFeO3-BaTiO3-CaHfO3 lead-free ceramics. J. Mater. Chem. A 2024, 12, 5261–5268. [Google Scholar] [CrossRef]
- Montecillo, R.; Chien, R.R.; Chen, C.S.; Wu, P.H.; Tu, C.S.; Feng, K.C. Ultrahigh energy storage in multilayer BiFeO3-BaTiO3-NaTaO3 relaxor ferroelectric ceramics. J. Mater. Chem. A 2024, 12, 30642–30654. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, T.; Fu, Z.; Pan, Z.; Tang, L.; Chen, X.; Li, H.; Hu, J.; Lv, L.; Zhou, Z.; et al. Delayed polarization saturation induced superior energy storage capability of BiFeO3-based ceramics via introduction of non-isovalent ions. Small 2023, 19, 2206840. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Wu, L.Z.; Xu, L.Y.; Li, P.; Wei, T. Superior energy-storage density and ultrahigh efficiency in KNN-based ferroelectric ceramics via high-entropy design. J. Mater. 2025, 11, 100862. [Google Scholar] [CrossRef]
- Zha, J.L.; Yang, Y.L.; Liu, J.X.; Lu, X.M.; Hu, X.L.; Yan, S.; Wu, Z.J.; Zhou, M.; Huang, F.Z.; Ying, X.N.; et al. High energy storage performance of KNN-based relaxor ferroelectrics in multiphase-coexisted superparaelectric state. J. Appl. Phys. 2024, 136, 10. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, S.; Wang, T.; Jing, H.; Guo, Q.; Gao, R.; Diwu, L.; Du, K.; Hu, Y.; Pu, Y. Achieving high overall energy storage performance of KNN-based transparent ceramics by ingenious multiscale designing. J. Mater. Chem. A 2024, 12, 16735–16747. [Google Scholar] [CrossRef]
- Mao, P.; Guo, Y.; Lu, G.; Yan, Q.; Kang, R.; Wang, T.; Xie, B.; Liu, Z.; Zhang, L. Synergistic effect of multi-phase and multi-domain structures induced high energy storage performances under low electric fields in Na0.5Bi0.5TiO3-based lead-free ceramics. Chem. Eng. J. 2023, 472, 144973. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Y.; Shi, P.; Kang, R.; Kang, F.; Qiao, W.; Zhao, J.; Wang, Z.; Yuan, Y.; Lou, X. Ultrahigh energy storage density in (Bi0.5Na0.5)0.65Sr0.35TiO3-based lead-free relaxor ceramics with excellent temperature stability. Nano Energy 2022, 98, 107276. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Y.; Song, B.; Cheng, H.; Fu, Y.; Zheng, M.; Zhu, M. Lead-free multilayer ceramic capacitors with ultra-wide temperature dielectric stability based on multifaceted modification. J. Eur. Ceram. Soc. 2022, 42, 973–980. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Fan, Z.; Huang, Y.; Hu, Y.; Shen, M.; Wang, Z.; He, Y.; Wang, D.; Zhang, Q. Excellent high-temperature dielectric energy storage performance in bilayer nanocomposites with high-entropy ferroelectric oxide fillers. Nat. Commun. 2025, 16, 5570. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Dai, J.; Huang, Y.; Xie, H.; Jiao, Y.; Yue, W.; Huang, F.; Deng, Y.; Wang, D.; Zhang, Q.; et al. Superior energy storage capacity of polymer-based bilayer composites by introducing 2D ferroelectric micro-sheets. Nat. Commun. 2025, 16, 1180. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Peng, Z.; Zhang, X.; Wang, Y.; Chai, Q.; Wu, D.; Liang, P.; Wei, L.; Chao, X.; Yang, Z. Enhanced energy storage performance of BiFeO3-BaTiO3 based ceramics under moderate electric fields via multiple synergistic design. Chem. Eng. J. 2025, 512, 162494. [Google Scholar] [CrossRef]
- Zhang, J.B.; Pu, Y.P.; Hao, Y.X.; Yang, Y.L.; Zhang, L.; Wang, B.; Pan, Q. Realizing excellent energy-storage performance under low electric fields in lead-free BiFeO3-BaTiO3-based ceramics with ultrahigh polarization difference. J. Energy Storage 2025, 105, 114786. [Google Scholar] [CrossRef]
- Kong, X.; Yang, L.T.; Meng, F.Q.; Zhang, T.; Zhang, H.J.; Lin, Y.H.; Huang, H.B.; Zhang, S.J.; Guo, J.M.; Nan, C.W. High-entropy engineered BaTiO3-based ceramic capacitors with greatly enhanced high-temperature energy storage performance. Nat. Commun. 2025, 16, 9. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Q.; Lin, Y.; Li, D.; Yang, H.; Hong, Z.; Ma, Y.; Ma, Y.; Guo, J.; Wang, J. High entropy-driven large capacitive energy storage in BaTiO3-based multilayer ceramic capacitors. Adv. Energy Mater. 2025, 16, e04126. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.M.; Zhang, W.C.; Cheng, X.; Hui, K.Z.; Zhen, Y.C.; Hao, Y.N.; Bi, K.; Guo, L.M.; Zhao, P.Y.; et al. Comprehensively improved energy storage and DC-bias properties in Bi0.5Na0.5TiO3-NaNbO3 based relaxor antiferroelectric. J. Mater. 2025, 11, 8. [Google Scholar]
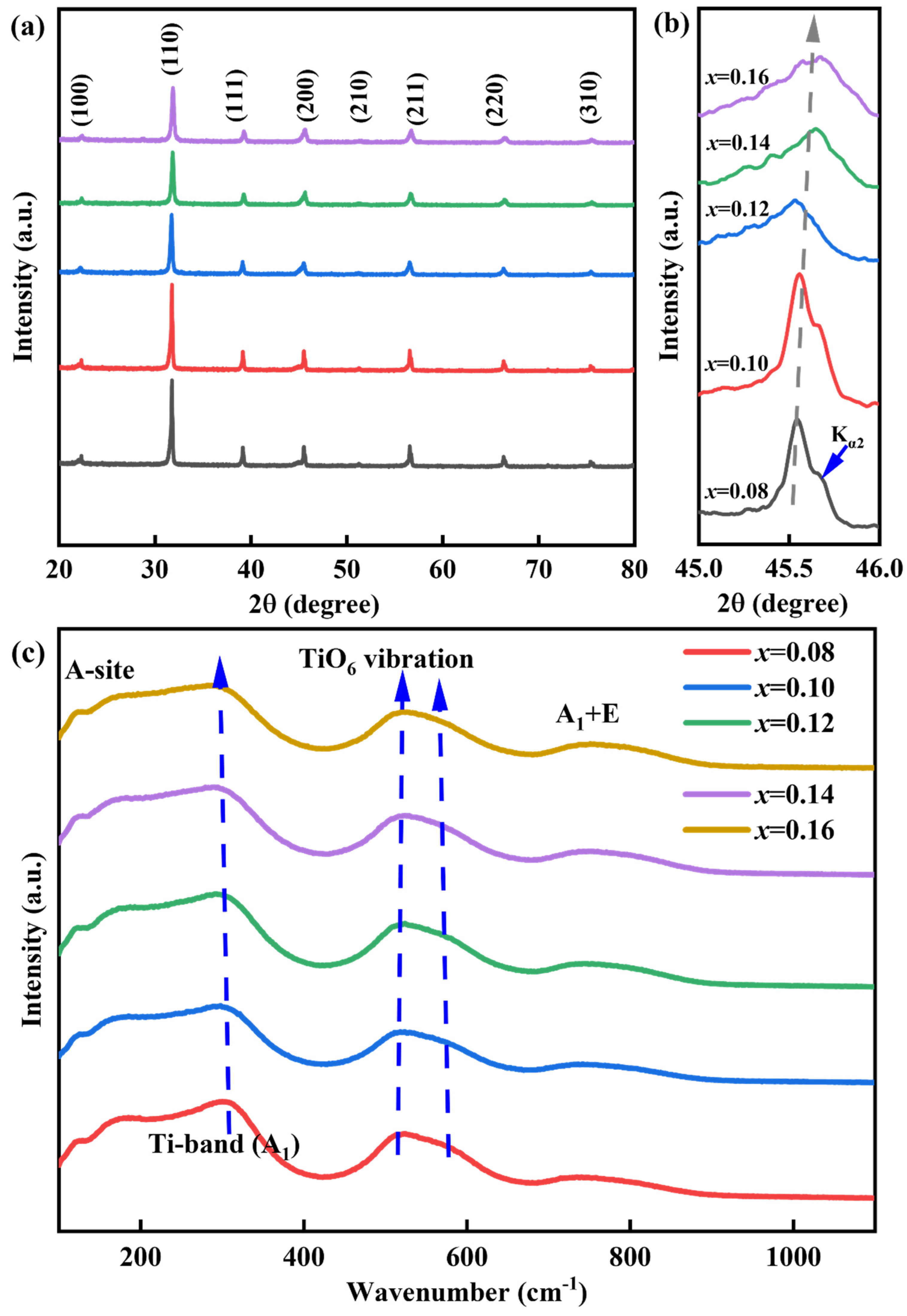

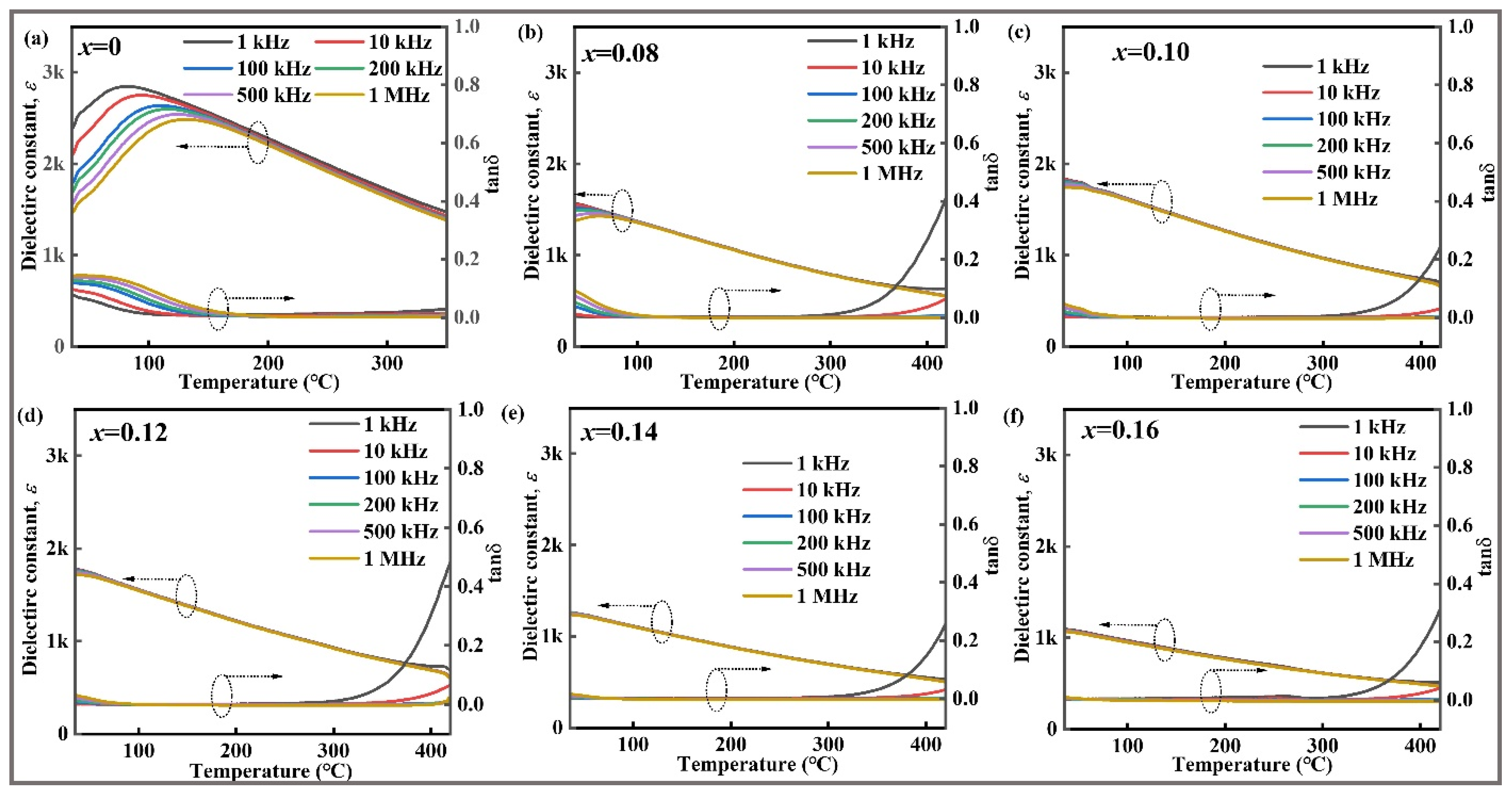
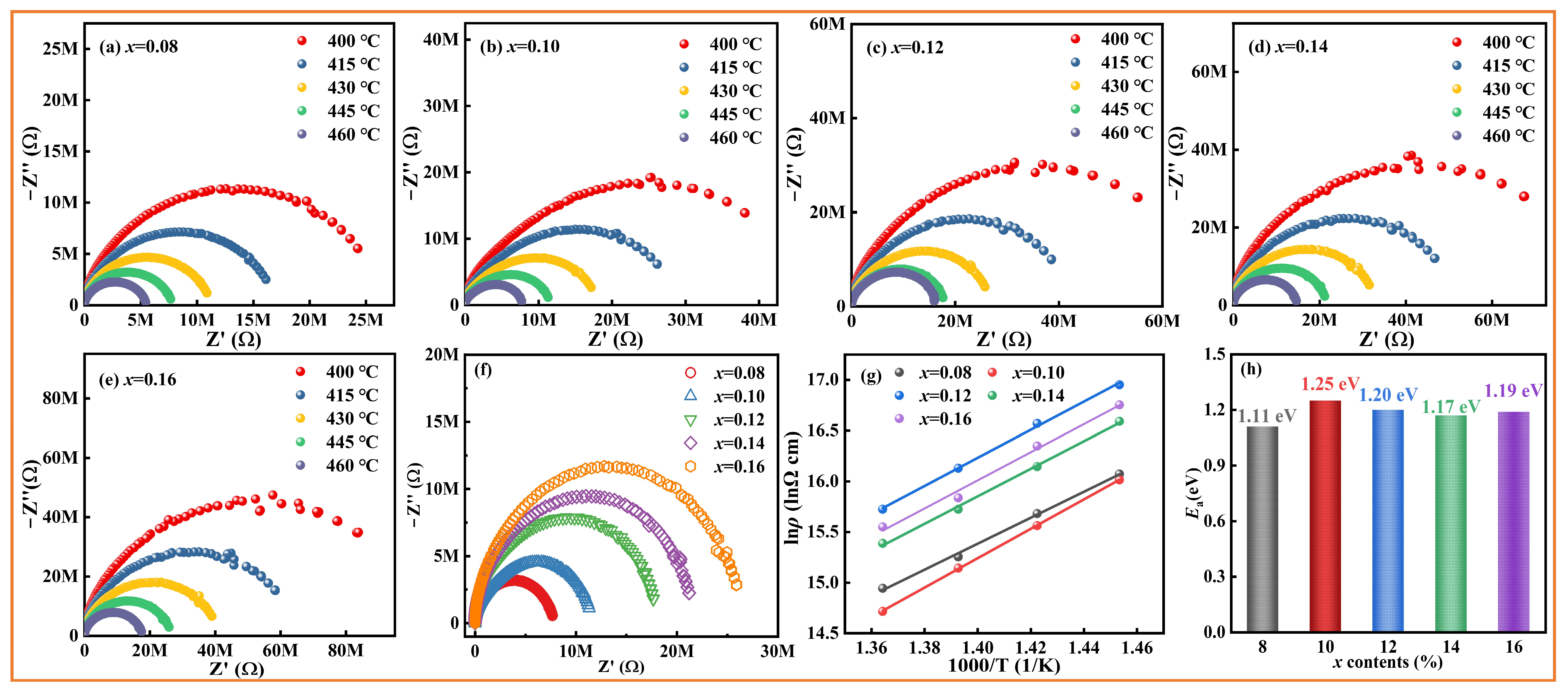

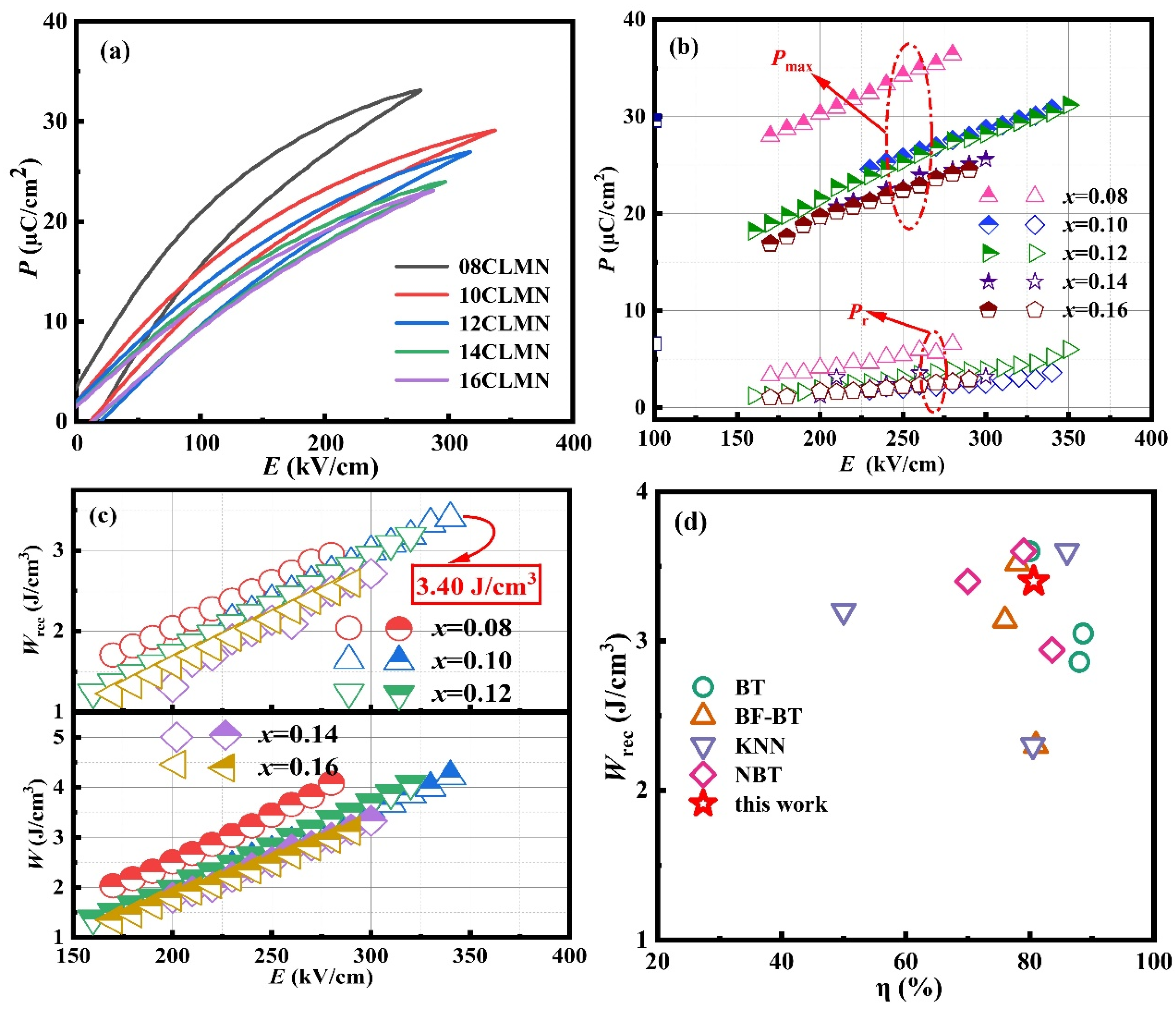

| Composition | Wrec (J cm−3) | η(%) | Eb (kV cm−1) | Refs. |
|---|---|---|---|---|
| BT–NBT–SBMT | 7.12 | 90 | 720 | [30] |
| BT–NBT–CZ | 9.04 | 87.2 | 540 | [19] |
| BT–BMH | 3.38 | 87 | 240 | [39] |
| BT–NBT–BZMASZ | 3.74 | 82.2 | 273 | [23] |
| BT–NBT–BMN | 1.6 | 90.3 | 182 | [24] |
| BF–BT–NNT | 6.1 | 81.4 | 330 | [54] |
| BF–BT–LA | 5.71 | 80.19 | 270 | [55] |
| BCT–BMZ | 10.9 | 93 | 720 | [56] |
| BNBSCTZZTN | 7.3 | 90.6 | 530 | [57] |
| KNN–STZ–BZTN | 11.14 | 87.1 | 750 | [46] |
| NBT–NN | 8.04 | 85 | 630 | [58] |
| BT–NBT–CLMN | 3.40 | 81 | 340 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Guo, J.; Wang, T.; Zhang, J.; Wu, X.; Zhang, X.; Vattikuti, S.V.P.; Ma, Q.; Rosaiah, P.; Zhang, Q. BaTiO3–(Na0.5Bi0.5)TiO3 Ceramic Materials Prepared via Multiple Design Strategies with Improved Energy Storage. Nanomaterials 2025, 15, 1724. https://doi.org/10.3390/nano15221724
Deng J, Guo J, Wang T, Zhang J, Wu X, Zhang X, Vattikuti SVP, Ma Q, Rosaiah P, Zhang Q. BaTiO3–(Na0.5Bi0.5)TiO3 Ceramic Materials Prepared via Multiple Design Strategies with Improved Energy Storage. Nanomaterials. 2025; 15(22):1724. https://doi.org/10.3390/nano15221724
Chicago/Turabian StyleDeng, Jianming, Jingjing Guo, Ting Wang, Jingxiang Zhang, Xu Wu, Xuefeng Zhang, Surya Veerendra Prabhakar Vattikuti, Qing Ma, Pitcheri Rosaiah, and Qingfeng Zhang. 2025. "BaTiO3–(Na0.5Bi0.5)TiO3 Ceramic Materials Prepared via Multiple Design Strategies with Improved Energy Storage" Nanomaterials 15, no. 22: 1724. https://doi.org/10.3390/nano15221724
APA StyleDeng, J., Guo, J., Wang, T., Zhang, J., Wu, X., Zhang, X., Vattikuti, S. V. P., Ma, Q., Rosaiah, P., & Zhang, Q. (2025). BaTiO3–(Na0.5Bi0.5)TiO3 Ceramic Materials Prepared via Multiple Design Strategies with Improved Energy Storage. Nanomaterials, 15(22), 1724. https://doi.org/10.3390/nano15221724







