Atomic Layer Deposition for Perovskite Solar Cells: Interface Engineering, Stability Enhancement, and Future Prospects
Abstract
1. Introduction
2. Device Structures and Challenges of PSCs
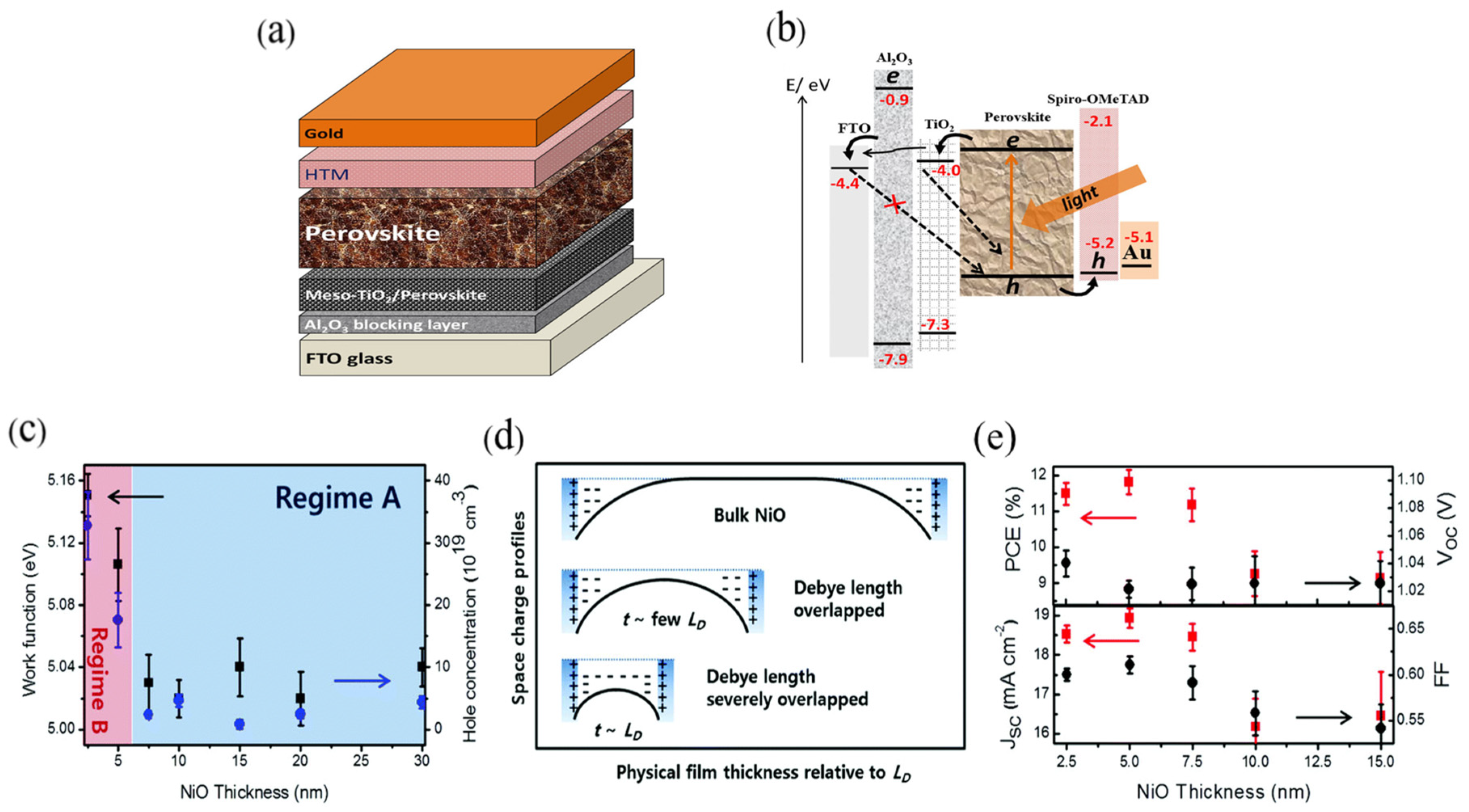
2.1. Device Structures of PSCs

2.2. Challenges of PSCs
3. Fundamentals of ALD
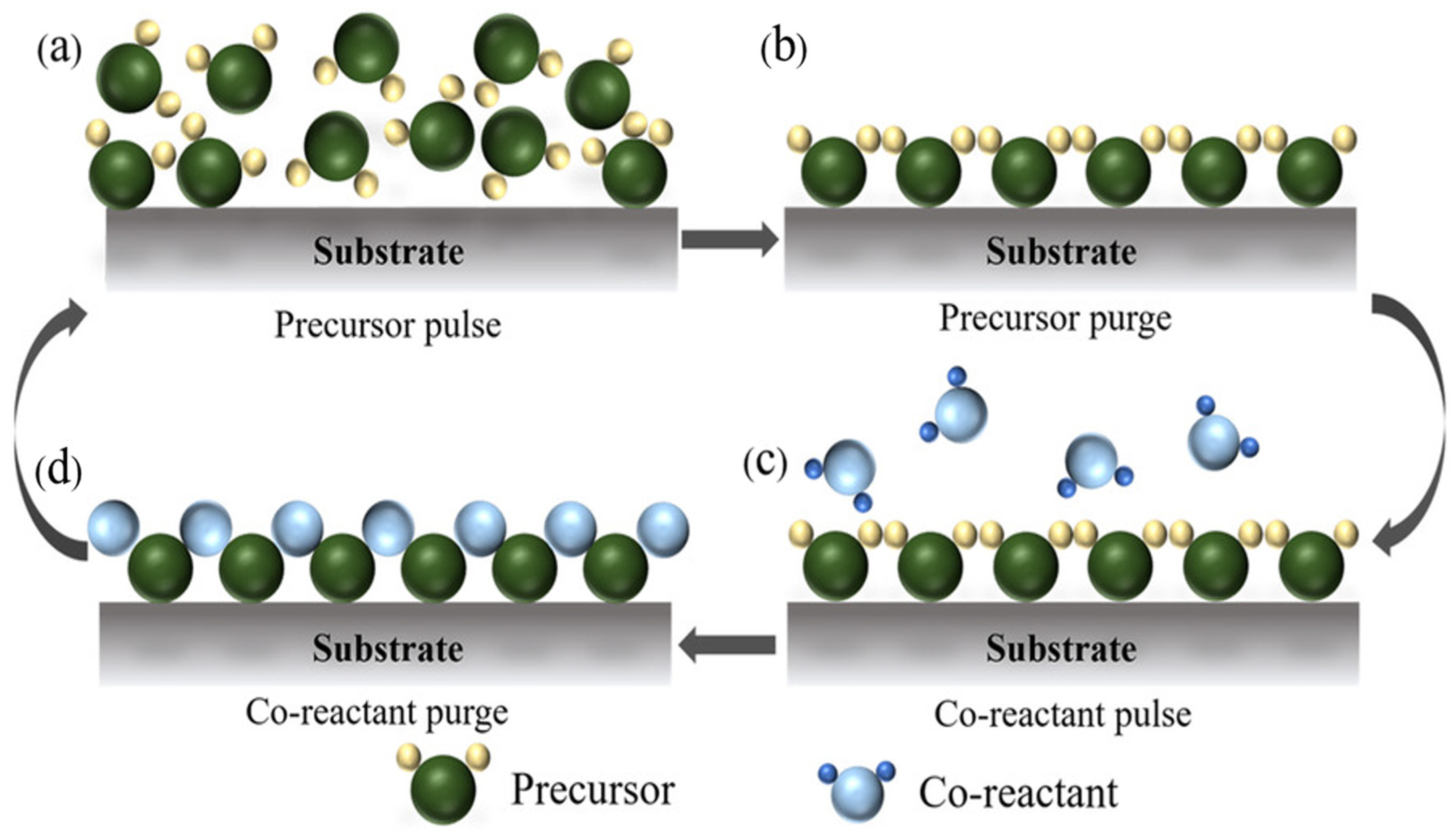
3.1. Basic Principles of ALD and Its Variants
3.2. Advantages of ALD
| Method | Application | Compatibility with Perovskites | Characteristics | Refs. |
|---|---|---|---|---|
| Sol–gel method | TiO2, ZnO, NiO, etc. | Pinholes can induce leakage current and oxygen vacancies; surface roughness inhomogeneity may lead to short circuits | Simple operation; highly sensitive to environmental conditions; poor reproducibility | [28,29,90,126,127,128,129,130] |
| Sputtering | NiO, ZnO, ITO, SnO2, etc. | Potential damage to the perovskite layer due to ion bombardment | Uniform deposition; strong film adhesion; relatively high cost | [103,131,132,133,134,135,136,137] |
| Thermal Evaporation | MoOx, CuI, CdS, CO, etc. | Vacuum-induced thermal stress can cause severe degradation of organic halides; annealing temperature of materials is limited | Fast deposition rate; suitable for low-melting-point materials; difficult to deposit high-melting-point oxides; good film uniformity | [87,103,135,138,139,140,141,142] |
| Chemical Vapor Deposition | CuO, TiO2, ZnO, etc. | High deposition temperature is unfavorable for thermally sensitive perovskite materials | Low cost, high scalability, and fast deposition rate with good step coverage; however, the high deposition temperature may impair other solar cell components, and introduce impurities during the process | [103,143,144,145,146,147] |
| Atomic Layer Deposition | TiO2, SnO2, Al2O3, VOx, etc. | Slow deposition rate; vacuum environment increases cost | Enables conformal growth with uniform and pinhole-free films; precise thickness control via self-limiting reactions; allows low-temperature synthesis compatible with thermally unstable PSCs; uniform interfaces facilitate carrier dynamics regulation | [28,92,93,148,149] |
| Spray Pyrolysis | TiO2, NiO, SnO2, CuCrO2, CuI, etc. | High temperature is unfavorable for thermally sensitive perovskite materials; prone to pinhole formation, leading to carrier recombination and short circuits | Low cost and simple operation; suitable for large-scale applications, but process parameters are difficult to control precisely; pinholes may remain on the film surface | [28,150,151,152,153,154,155] |
| Spin Coating | SnO2, ZnO, CuI, etc. | Requires high annealing temperature, which is unfavorable for thermally sensitive perovskite materials; irregular surface prone to pinhole formation, leading to carrier recombination and short circuits | Not suitable for large-area deposition; limited control over film thickness and uniformity on rough surfaces; high annealing temperature required | [20,92,129,132,138,156,157,158] |
4. ALD Applications in PSCs
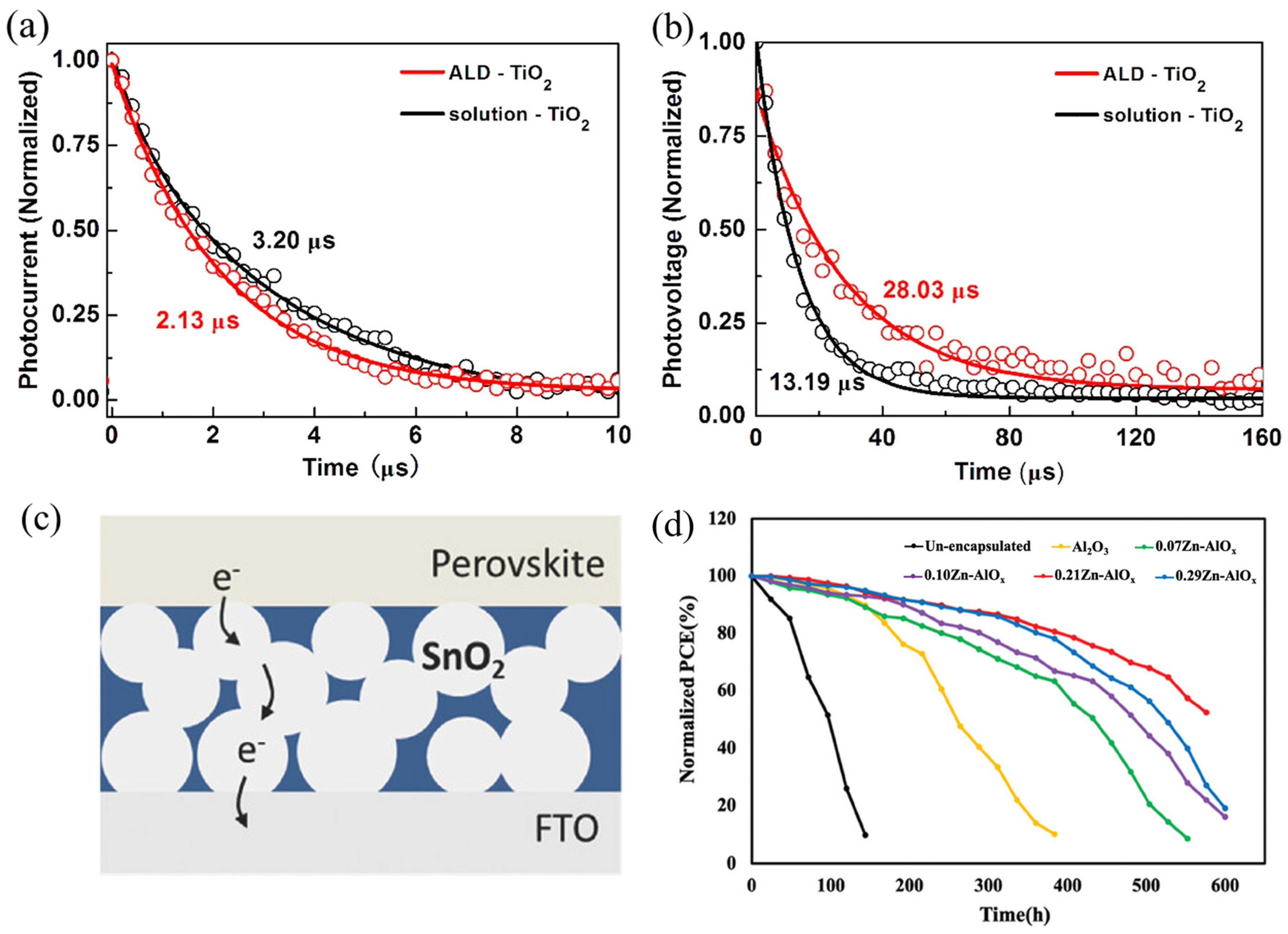
4.1. Low-Temperature Synthesis
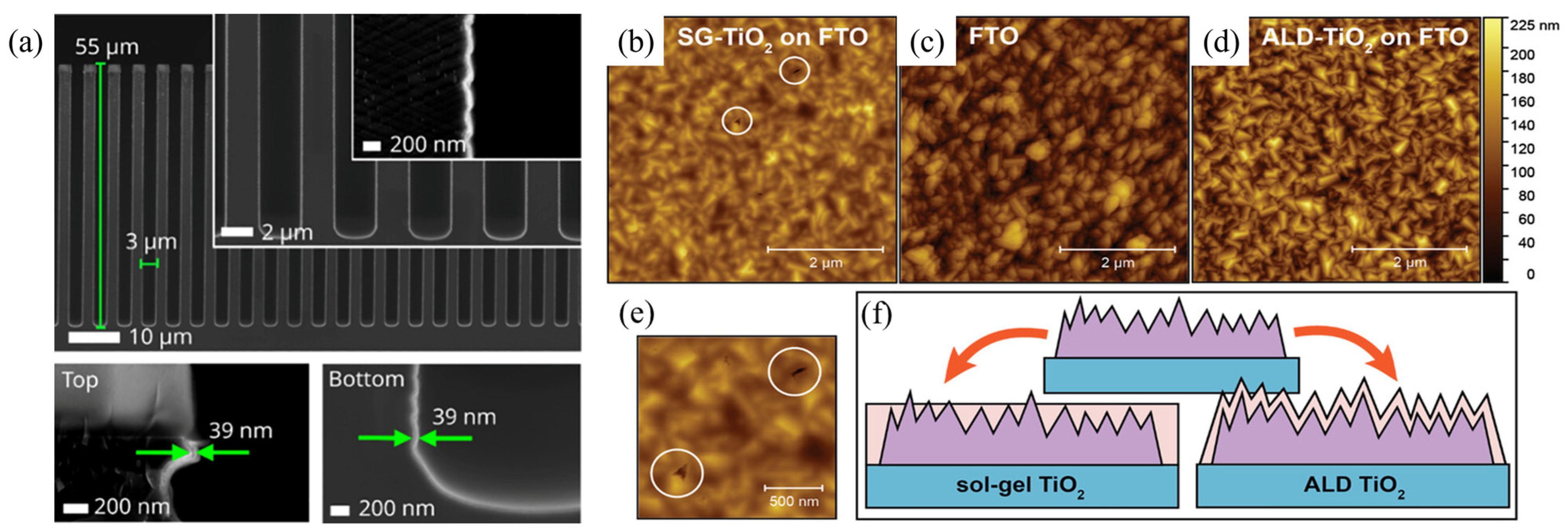
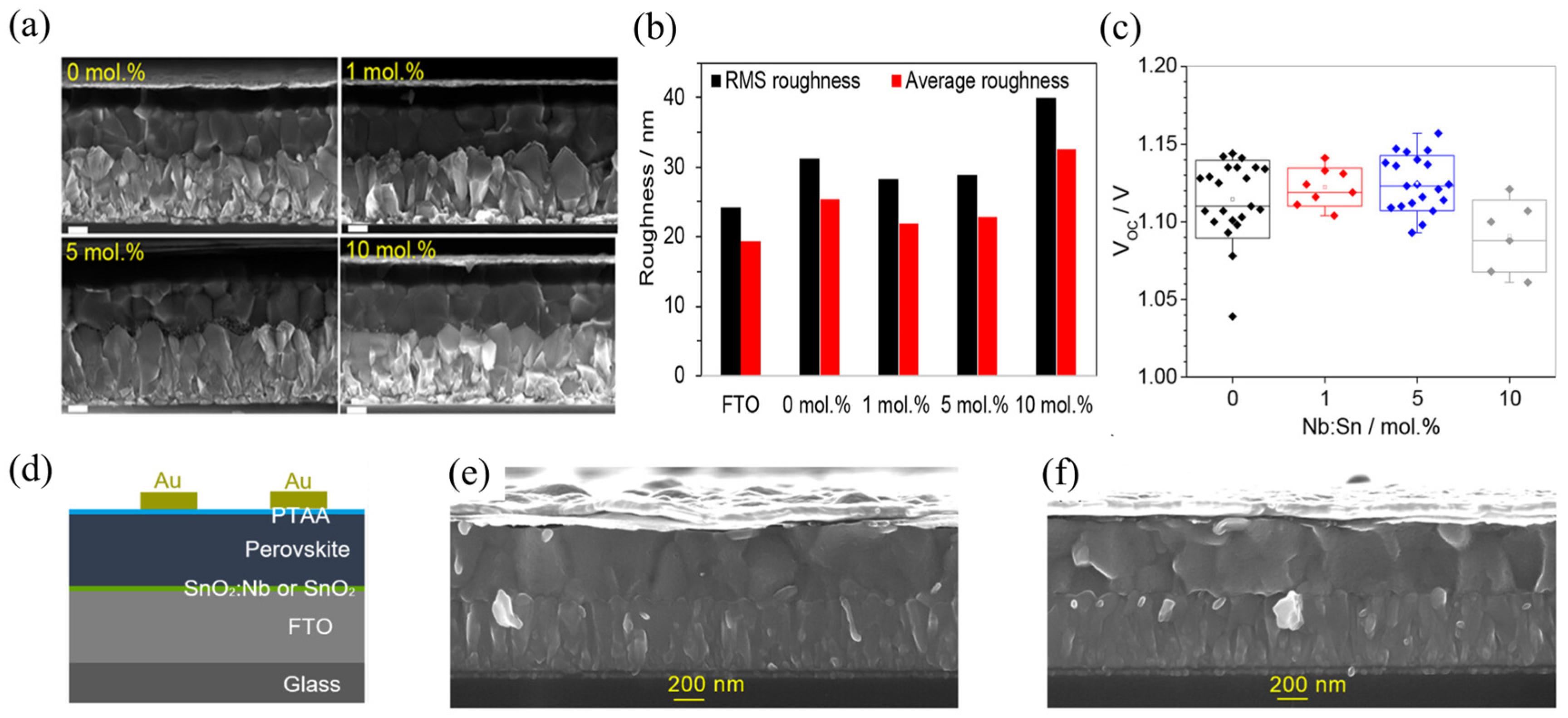
4.2. Thickness and Composition Control
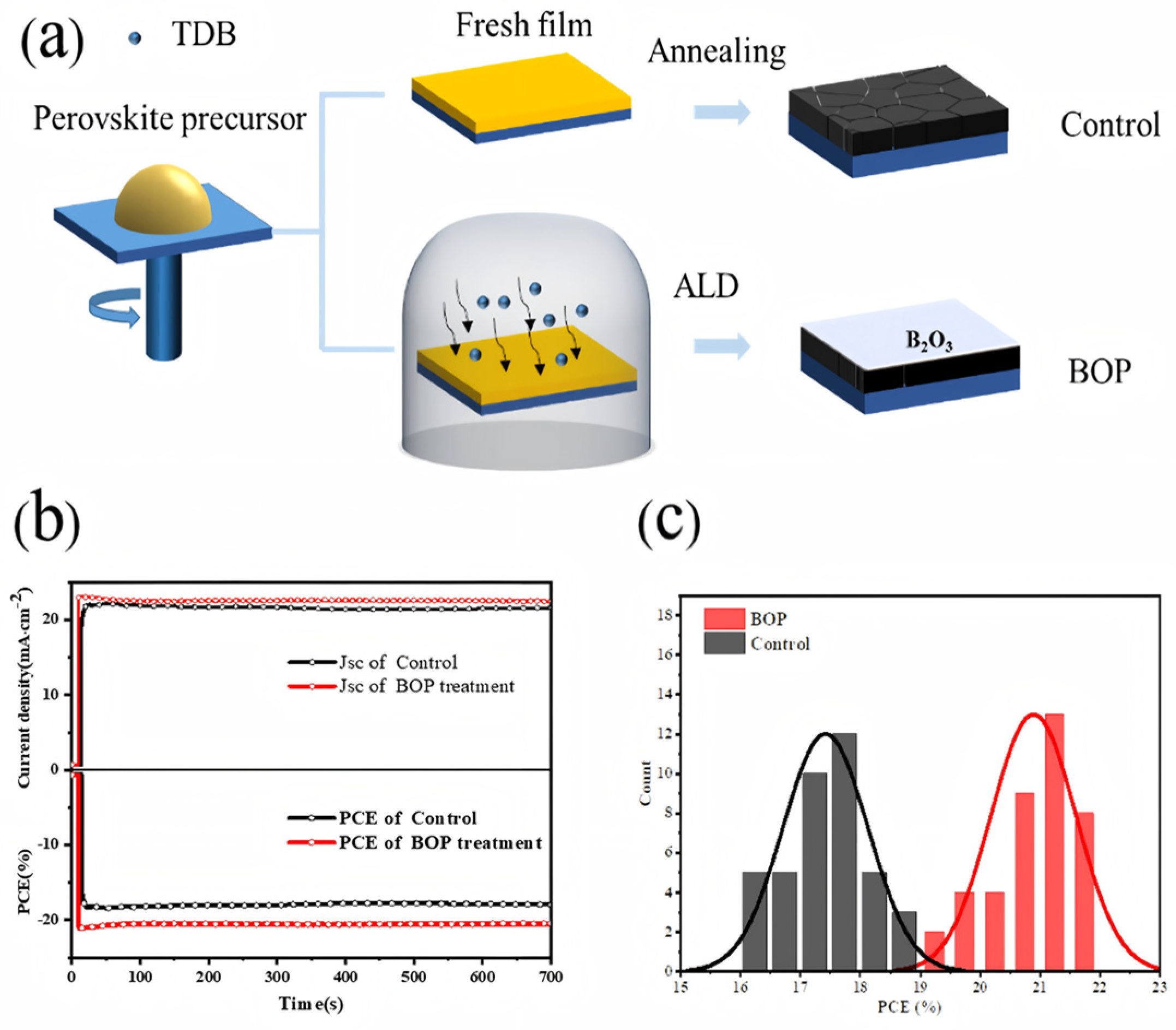
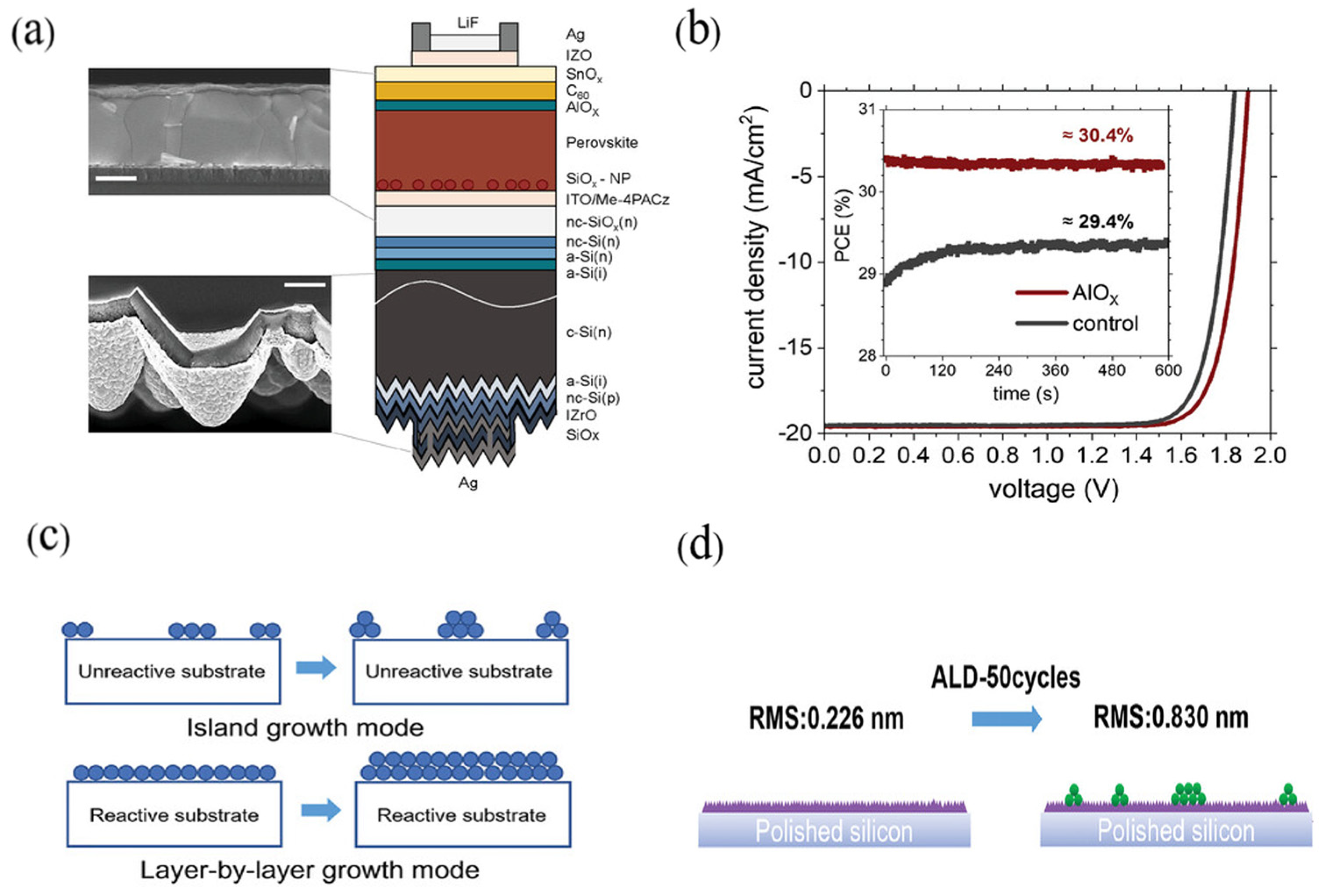
4.3. Defect Passivation
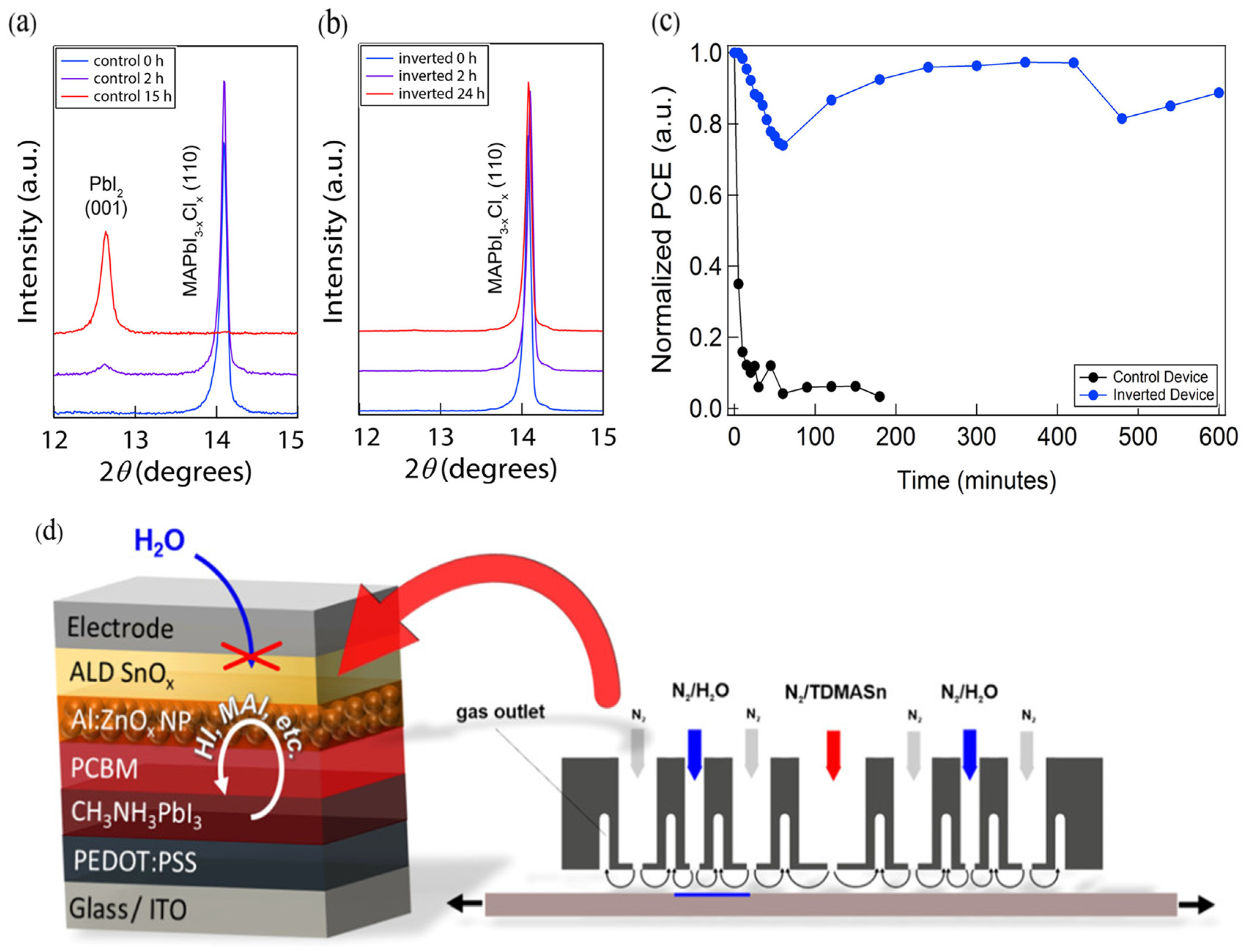
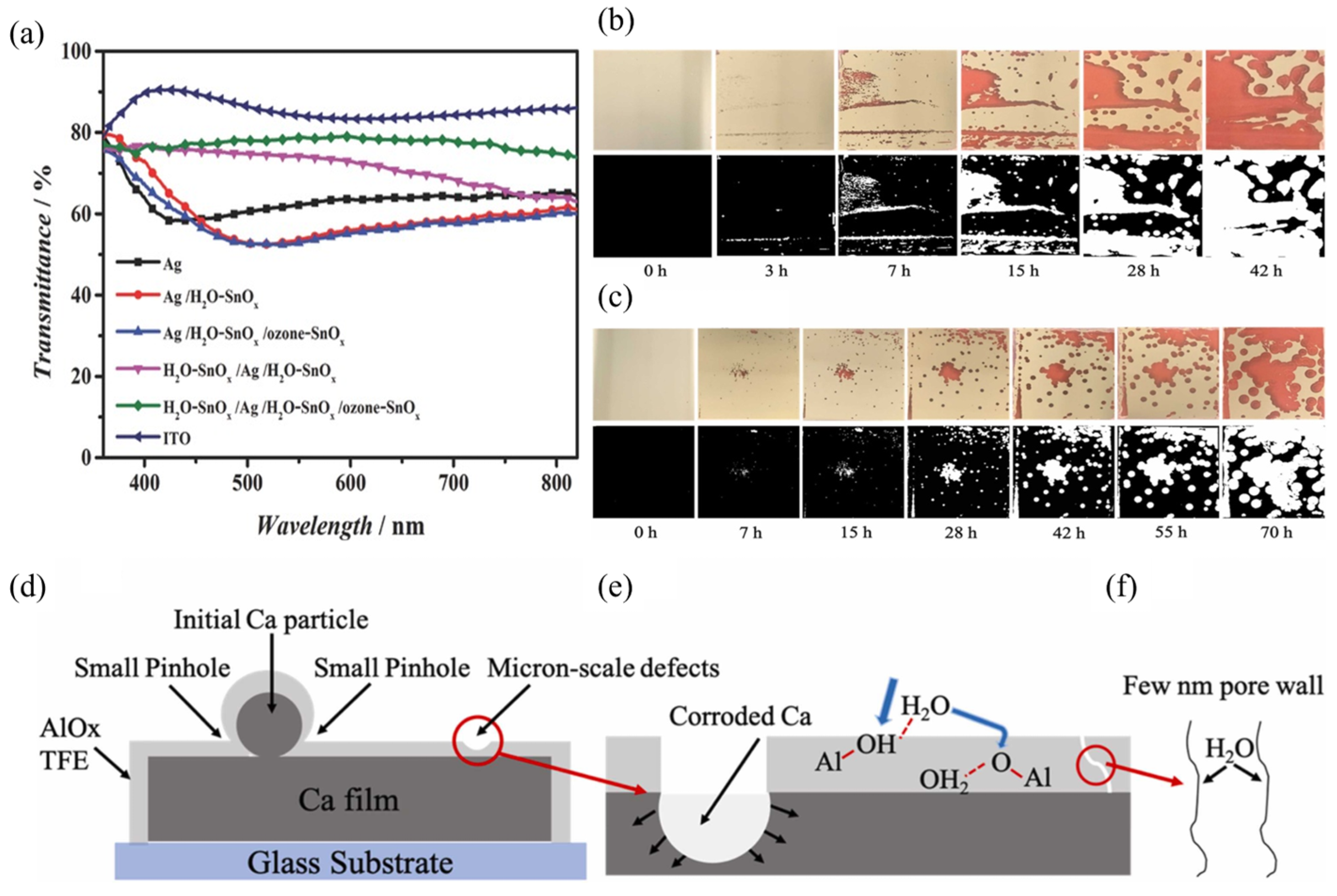
4.4. Isolation and Encapsulation
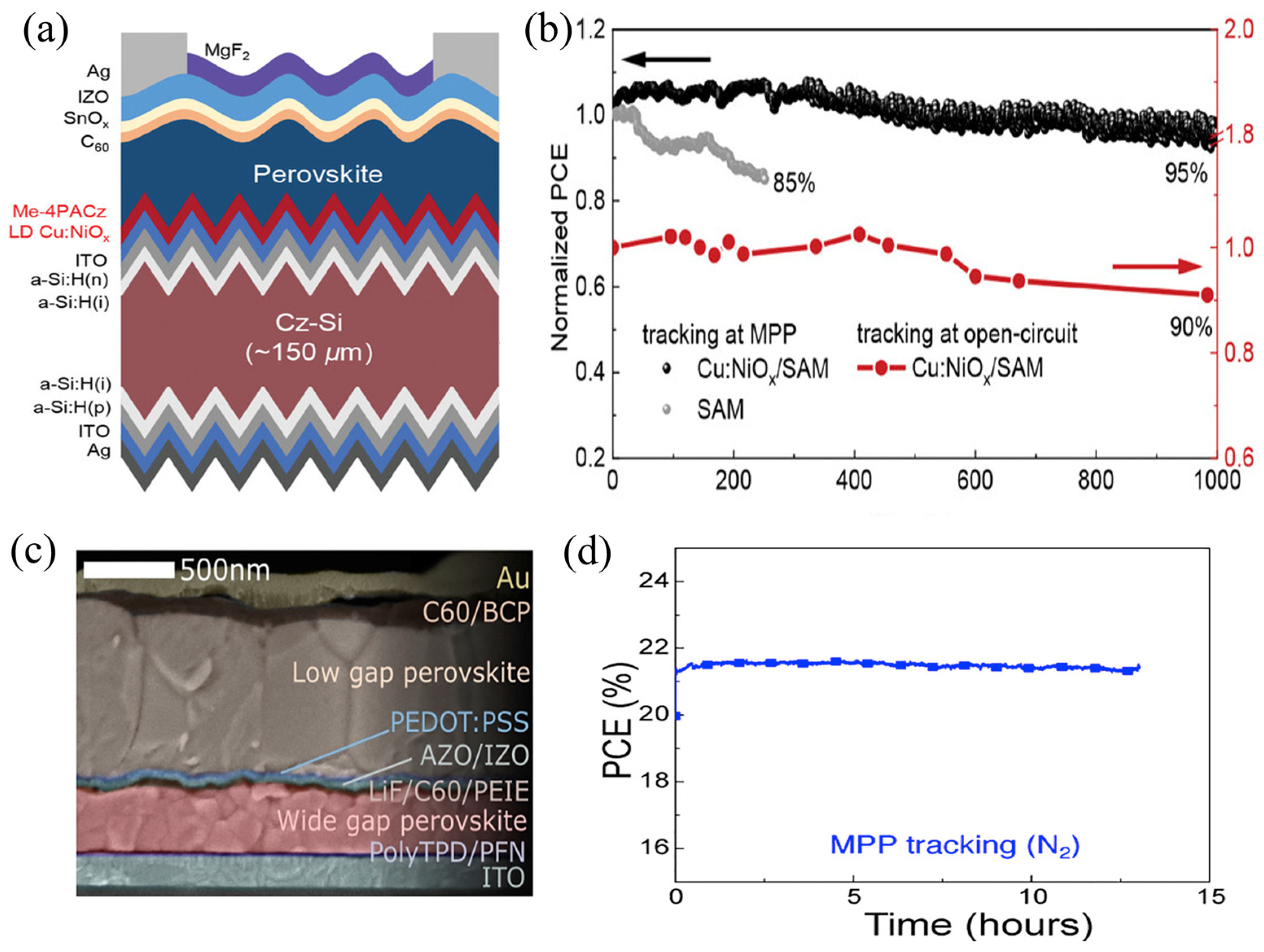
4.5. Tandem Solar Cells
5. Limitations and Challenges
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, Z.; Zheng, D.; Zhao, S.; Wang, L.; Wu, S.; Liu, L.; Li, Z.; Zhang, L.; Dong, Q.; Wang, H.; et al. Designing Heterovalent Substitution with Antioxidant Attribute for High-Performance Sn-Pb Alloyed Perovskite Solar Cells. Adv. Funct. Mater. 2023, 33, 2214983. [Google Scholar] [CrossRef]
- Min, J.; Choi, Y.; Kim, D.; Park, T. Beyond Imperfections: Exploring Defects for Breakthroughs in Perovskite Solar Cell Research. Adv. Energy Mater. 2023, 14, 2302659. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, X.; Lin, R.; Luo, X.; Liu, Z.; Zhou, K.; Xiong, S.; Bao, Q.; Chen, G.; et al. Flexible all-perovskite tandem solar cells approaching 25% efficiency with molecule-bridged hole-selective contact. Nat. Energy 2022, 7, 708–717. [Google Scholar] [CrossRef]
- Hu, S.; Otsuka, K.; Murdey, R.; Nakamura, T.; Truong, M.A.; Yamada, T.; Handa, T.; Matsuda, K.; Nakano, K.; Sato, A.; et al. Optimized carrier extraction at interfaces for 23.6% efficient tin–lead perovskite solar cells. Energy Environ. Sci. 2022, 15, 2096–2107. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, P.; Chen, R.; Du, L.; Shao, X.; Zhang, X.; Zheng, Y.; Jia, N.; Fang, Z.; Ma, L.; et al. Heteroepitaxy with PbSe Nanocrystals Enables Highly Stable Wide-Bandgap Perovskite Solar Cells. Adv. Energy Mater. 2025, 15, 2501312. [Google Scholar] [CrossRef]
- Mo, H.; Wang, L.; Li, Y.; Zhu, T.; Sergeev, A.; Wang, J.; He, Y.; Ren, Z.; Rehman, A.U.; Ali, M.U.; et al. Multifunctional Universal Additive for Stable and Efficient Inverted Perovskite Solar Cells. Adv. Energy Mater. 2025, 15, 2500088. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, Z.; Xu, H.; Li, Y.; Gao, J.; Xiang, F.; Meng, H.; Chen, Y.; Liu, T.; Wu, X.; et al. Boosting Tin Perovskite Solar Cell Performance via Light-Induced Interface Doping. Nano Lett. 2025, 25, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Li, X.; Xiong, Y.; Shen, G.; Zhang, W.; Huang, Y.; Jiang, Q.; Ng, X.R.; Luo, Y.; Zheng, J.; et al. Self-assembled monolayers (SAMs) in inverted perovskite solar cells and their tandem photovoltaics application. Interdiscip. Mater. 2024, 3, 203–244. [Google Scholar] [CrossRef]
- Asgarimoghaddam, H.; Chen, Q.; Ye, F.; Shahin, A.; Song, B.; Musselman, K.P. Zinc Aluminum Oxide Encapsulation Layers for Perovskite Solar Cells Deposited Using Spatial Atomic Layer Deposition. Small Methods 2024, 8, 2300995. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Zeng, Z.; Liu, M.; Du, T.; Huang, X.; Bi, L.; Wang, J.; Jiang, W.; An, Y.; et al. Regulating the crystallization of mixed-halide perovskites by cation alloying for perovskite–organic tandem solar cells. Energy Environ. Sci. 2024, 17, 9580–9589. [Google Scholar] [CrossRef]
- Er-raji, O.; Mahmoud, M.A.A.; Fischer, O.; Ramadan, A.J.; Bogachuk, D.; Reinholdt, A.; Schmitt, A.; Kore, B.P.; Gries, T.W.; Musiienko, A.; et al. Tailoring perovskite crystallization and interfacial passivation in efficient, fully textured perovskite silicon tandem solar cells. Joule 2024, 8, 2811–2833. [Google Scholar] [CrossRef]
- Kang, J.; Bi, H.; Guo, M.; Guo, Y.; Zou, H.; Han, G.; Hou, W. Modifying the buried interface with azodicarbonamide for high-efficiency MA-free perovskite solar cells. Mater. Today Energy 2022, 31, 101227. [Google Scholar] [CrossRef]
- Yu, W.; Zou, Y.; Wang, H.; Qi, S.; Wu, C.; Guo, X.; Liu, Y.; Chen, Z.; Qu, B.; Xiao, L. Breaking the bottleneck of lead-free perovskite solar cells through dimensionality modulation. Chem. Soc. Rev. 2024, 53, 1769–1788. [Google Scholar] [CrossRef]
- Heydarian, M.; Heydarian, M.; Schygulla, P.; Reichmuth, S.K.; Bett, A.J.; Hohl-Ebinger, J.; Schindler, F.; Hermle, M.; Schubert, M.C.; Schulze, P.S.C.; et al. Recent progress in monolithic two-terminal perovskite-based triple-junction solar cells. Energy Environ. Sci. 2023, 17, 1781–1818. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, R.; Wei, M.; Yin, M.; Wu, P.; Li, M.; Li, L.; Wang, Y.; Chen, G.; Carnevali, V.; et al. All-perovskite tandem solar cells achieving >29% efficiency with improved (100) orientation in wide-bandgap perovskites. Nat. Mater. 2025, 24, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bi, E.; Ono, L.K.; Li, D.; Bakr, O.M.; Yan, Y.; Qi, Y. Passivation strategies for enhancing device performance of perovskite solar cells. Nano Energy 2023, 115, 108731. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, G.; Hong, R.; Liang, Z.; Kirchartz, T. Quantifying Efficiency Limitations in All-Inorganic Halide Perovskite Solar Cells. Adv. Mater. 2022, 34, 2108132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, J.; Li, K.; Zhang, R.; Qin, Z.; Zhang, Y.; Wu, B.; Ma, Q.; Zhang, Y.; Zhang, W. Controllable Crystallization of Perovskite Films during the Blade-Coating Fabrication Process for Efficient and Stable Solar Cells. Coatings 2024, 14, 1113. [Google Scholar] [CrossRef]
- Parida, B.; Singh, A.; Kalathil Soopy, A.K.; Sangaraju, S.; Sundaray, M.; Mishra, S.; Liu, S.; Najar, A. Recent Developments in Upscalable Printing Techniques for Perovskite Solar Cells. Adv. Sci. 2022, 9, e2200308. [Google Scholar] [CrossRef]
- Han, J.; Park, K.; Tan, S.; Vaynzof, Y.; Xue, J.; Diau, E.W.-G.; Bawendi, M.G.; Lee, J.-W.; Jeon, I. Perovskite solar cells. Nat. Rev. Methods Primers 2025, 5, 3. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, H.; Xiao, C.; Jia, X.; Guo, R.; Chen, J.; Guo, X.; Luo, R.; Wang, X.; Li, M.; et al. Enhancing the efficiency and longevity of inverted perovskite solar cells with antimony-doped tin oxides. Nat. Energy 2024, 9, 308–315. [Google Scholar] [CrossRef]
- Ou, Y.; Huang, H.; Shi, H.; Li, Z.; Chen, Z.; Mateen, M.; Lu, Z.; Chi, D.; Huang, S. Collaborative interfacial modification and surficial passivation for high-efficiency MA-free wide-bandgap perovskite solar cells. Chem. Eng. J. 2023, 469, 143860. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Chen, Y.; Zhao, H.; Wei, Y.; Zhou, R.; Wan, H.; Deng, J.; Fan, D.; Luo, Y.; et al. Multifunctional amine salt additive for enhancing the performance of perovskite and perovskite/silicon tandem solar cells. Chem. Eng. J. 2025, 515, 163967. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Seo, G.; Paek, S.; Cho, K.T.; Huckaba, A.J.; Calizzi, M.; Choi, D.-w.; Park, J.-S.; Lee, D.; et al. Efficient Planar Perovskite Solar Cells Using Passivated Tin Oxide as an Electron Transport Layer. Adv. Sci. 2018, 5, 1800130. [Google Scholar] [CrossRef]
- Xia, Z.; Rozyyev, V.; Mane, A.U.; Elam, J.W.; Darling, S.B. Surface Zeta Potential of ALD-Grown Metal-Oxide Films. Langmuir 2021, 37, 11618–11624. [Google Scholar] [CrossRef]
- Zhang, J.; Hultqvist, A.; Zhang, T.; Jiang, L.; Ruan, C.; Yang, L.; Cheng, Y.; Edoff, M.; Johansson, E.M.J. Al2O3 Underlayer Prepared by Atomic Layer Deposition for Efficient Perovskite Solar Cells. ChemSusChem 2017, 10, 3810–3817. [Google Scholar] [CrossRef]
- Suchikova, Y.; Nazarovets, S.; Konuhova, M.; Popov, A.I. Binary Oxide Ceramics (TiO2, ZnO, Al2O3, SiO2, CeO2, Fe2O3, and WO3) for Solar Cell Applications: A Comparative and Bibliometric Analysis. Ceramics 2025, 8, 119. [Google Scholar] [CrossRef]
- Yasmeen, S.; Ryu, S.W.; Lee, S.-H.; Lee, H.-B.-R. Atomic Layer Deposition Beyond Thin Film Deposition Technology. Adv. Mater. Technol. 2023, 8, 2200876. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Provost, M.; Yaiche, A.; Medina Flechas, J.P.; Harada, N.; Ory, D.; Schneider, N. Innovative Nb-Doped SnO2 Electron Transport Layers Prepared by Atomic Layer Deposition for Enhanced Perovskite Solar Cells. ACS Appl. Energy Mater. 2025, 8, 5421–5430. [Google Scholar] [CrossRef]
- Chen, D.; Su, A.; Li, X.; Pang, S.; Zhu, W.; Xi, H.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Efficient planar perovskite solar cells with low-temperature atomic layer deposited TiO2 electron transport layer and interfacial modifier. Sol. Energy 2019, 188, 239–246. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, Z.; Fan, S.; Feng, X.; Sun, X.; Tang, J.; Yan, L.; Wang, Z.; Li, Z.; Cui, X.; et al. Enhanced Light Transmittance of Electron Transport Layer through Bilayer SnO2 for High-Performance Semitransparent Perovskite Solar Cells. ChemSusChem 2025, 18, e202402582. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wen, Z.; Odabasi, O.; Achamyeleh, E.; Sun, K.; Ahmadi, E. Investigation of ALD HfSiOx as gate dielectric on β-Ga2O3 (001). Appl. Phys. Lett. 2024, 124, 132103. [Google Scholar] [CrossRef]
- Paghi, A.; Battisti, S.; Tortorella, S.; De Simoni, G.; Giazotto, F. Cryogenic behavior of high-permittivity gate dielectrics: The impact of atomic layer deposition temperature and the lithographic patterning method. J. Appl. Phys. 2025, 137, 044103. [Google Scholar] [CrossRef]
- Jung, D.; Jang, Y.; Sultane, P.R.; Bielawski, C.W.; Oh, J. Energy band offsets of BeO dielectrics grown via atomic-layer deposition on β-Ga2O3 substrates. J. Alloys Compd. 2022, 922, 166197. [Google Scholar] [CrossRef]
- Son, K.; Kim, G.; Jeong, D.; Kim, S.-H.; Park, Y.-B. Effects of the TiN diffusion barrier and post-annealing between Ru and SiO2 films on the interfacial adhesion energy for advanced interconnections. Jpn. J. Appl. Phys. 2025, 64, 03SP46. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, M.; Kotsugi, Y.; Cheon, T.; Mohapatra, D.; Jang, Y.; Bae, J.S.; Hong, T.E.; Ramesh, R.; An, K.S.; et al. Atomic Layer Deposited RuO2 Diffusion Barrier for Next Generation Ru-Interconnects. Adv. Funct. Mater. 2022, 32, 2206667. [Google Scholar] [CrossRef]
- Im, B.; Mun, K.; Kim, S.; Kim, S.-H. Seedless electrodeposition of Cu thin films on ALD Ru diffusion barriers with different electrical properties. Electrochim. Acta 2023, 443, 141971. [Google Scholar] [CrossRef]
- Deijkers, J.H.; de Jong, A.A.; Mattinen, M.J.; Schulpen, J.J.P.M.; Verheijen, M.A.; Sprey, H.; Maes, J.W.; Kessels, W.M.M.; Bol, A.A.; Mackus, A.J.M. MoS2 Synthesized by Atomic Layer Deposition as Cu Diffusion Barrier. Adv. Mater. Interfaces 2023, 10, 2202426. [Google Scholar] [CrossRef]
- Roozeboom, F.; van den Bruele, F.; Creyghton, Y.; Poodt, P.; Kessels, W.M.M. Cyclic Etch/Passivation-Deposition as an All-Spatial Concept toward High-Rate Room Temperature Atomic Layer Etching. ECS J. Solid State Sci. Technol. 2015, 4, N5067. [Google Scholar] [CrossRef]
- Kumakura, S.; Sasagawa, H.; Nishizuka, T.; Kihara, Y.; Honda, M. Novel technology of high-aspect-ratio etch utilizing coverage-controllable atomic layer deposition. Jpn. J. Appl. Phys. 2022, 61, SI1015. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Mallick, B.C.; Gandomi, Y.A.; Huang, Y.-C.; Fu, C.-C.; Juang, R.-S.; Chang, J.-K. Improvement on high-temperature electrochemical performance of lithium-ion pouch cells by spatial atomic layer deposition. Electrochim. Acta 2022, 423, 140605. [Google Scholar] [CrossRef]
- Su, J.; Tsuruoka, T.; Tsujita, T.; Inatomi, Y.; Terabe, K. Nitrogen Plasma Enhanced Low Temperature Atomic Layer Deposition of Magnesium Phosphorus Oxynitride (MgPON) Solid-State Electrolytes. Angew. Chem. Int. Ed. 2023, 62, e202217203. [Google Scholar] [CrossRef]
- Gomez, O.J.; Antar, A.; Hall, A.T.; Tapia-Aracayo, L.; Seo, J.; Kim, N.; Sun, Z.; Lim, R.; Chen, F.; Li, Y.; et al. A facile synthesis of bulk LiPON in solution for solid-state electrolytes. J. Mater. Chem. A 2025, 3, 28368–28376. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wang, C.-C.; Cheng, C.-H.; Lu, Y.-L.; Lin, S.-H.; Granwehr, J.; Windmüller, A.; Tsai, C.-L.; Eichel, R.-A.; Chiu, K.-F. Enhanced stability and high rate capability of garnet solid-state electrolyte interface through integration of nanoscale Li4Ti5O12 for Li battery applications. J. Power Sources 2025, 652, 237593. [Google Scholar] [CrossRef]
- Jiang, X.; Shan, B.; Ma, G.; Xu, Y.; Yang, X.; Zhou, W.; Li, C.; Yang, F.; Chen, R. Scalable deposition of SnO2 ETL via SALD for large-area inverted perovskite solar modules. Chem. Eng. J. 2025, 505, 159629. [Google Scholar] [CrossRef]
- Porcar, S.; Schmickler, M.; Okcu, H.; Obenlüneschloß, J.; d’Ercole, S.; Gabalda, L.C.; Galarreta-Rodriguez, I.; Rubio-Zuazo, J.; Cuadra, J.G.; Lahlahi, A.; et al. Low-temperature, high-throughput spatial atomic layer deposition of NiOx nanocrystalline thin films from [Ni(ipki)2]. Appl. Surf. Sci. Adv. 2025, 29, 100836. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Geng, X.-P.; Huang, P.-H.; Wu, W.-Y.; Zhao, M.-J.; Zhang, X.-Y.; Huang, Q.-H.; Su, Z.-B.; Chen, Z.-R.; Lien, S.-Y.; et al. High doping efficiency Al-doped ZnO films prepared by co-injection spatial atomic layer deposition. J. Alloys Compd. 2021, 884, 161025. [Google Scholar] [CrossRef]
- Katsouras, I.; Frijters, C.; Poodt, P.; Gelinck, G.; Kronemeijer, A.J. Large-area spatial atomic layer deposition of amorphous oxide semiconductors at atmospheric pressure. J. Soc. Inf. Disp. 2019, 27, 304–312. [Google Scholar] [CrossRef]
- Samaneh Bahrani, S.; Ali Khodadadi, A.; Mortazavi, Y. Selective atomic layer deposition of NiO on MoOx/γ-Al2O3 to enhance the active catalytic phase formation for hydrodesulfurization of dibenzothiophene. Appl. Surf. Sci. 2023, 625, 157141. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yoo, K.S.; Kim, D.; Kim, J.-M.; Park, J.-S. Advanced atmospheric-pressure spatial atomic layer deposition for OLED encapsulation: Controlling growth dynamics for superior film performance. Chem. Eng. J. 2024, 503, 158424. [Google Scholar] [CrossRef]
- Gregory, G.; Luderer, C.; Ali, H.; Sakthivel, T.S.; Jurca, T.; Bivour, M.; Seal, S.; Davis, K.O. Spatial Atomic Layer Deposition of Molybdenum Oxide for Industrial Solar Cells. Adv. Mater. Interfaces 2020, 7, 2000895. [Google Scholar] [CrossRef]
- Lee, W.-B.; Kim, M.-S.; Lee, C.-H.; Lim, J.H.; Park, J.-S. Stress-Relief Island Design of Island-Bridge Structured InZnO TFT for Form-Free Display Applications Using Spatial ALD. ACS Appl. Electron. Mater. 2025, 7, 6680–6688. [Google Scholar] [CrossRef]
- Chen, R.; Cao, K.; Wen, Y.; Yang, F.; Wang, J.; Liu, X.; Shan, B. Atomic layer deposition in advanced display technologies: From photoluminescence to encapsulation. Int. J. Extrem. Manuf. 2024, 6, 022003. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Ren, J.; Wu, Z.; Duan, Y. Innovative stress-release method for low-stress flexible Al2O3 encapsulation films in OLED applications. npj Flex. Electron. 2025, 9, 1–10. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Fang, K.; Jiang, W.; Duan, Y. An Electrode Based on Ca:Au Alloy and Atomic Layer Deposition for a Transparent Flexible OLED Device. J. Electron. Mater. 2024, 53, 2955–2964. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yoo, K.S.; Kim, D.-G.; Park, C.-K.; Park, J.-S. Compositionally engineered amorphous InZnO thin-film transistor with high mobility and stability via atmospheric pressure spatial atomic layer deposition. J. Ind. Eng. Chem. 2024, 140, 269–276. [Google Scholar] [CrossRef]
- Zhang, W.; Shrestha, S.; Parajuli, S.; Maskey, B.B.; Park, J.; Yang, H.; Jung, Y.; Cho, G. Tuning the charge carrier polarity of roll-to-roll gravure printed carbon nanotube-based thin film transistors by an atomic layer deposited alumina nanolayer. Nanoscale Adv. 2023, 5, 3879–3886. [Google Scholar] [CrossRef]
- Alakoski, E.; Kinnunen, S.; Tewari, G.C.; Julin, J.; Soininen, A.J.; Karppinen, M. Toward roll-to-roll ALD of thermoelectric Al-doped ZnO thin films on flexible nanostructured PET membranes. Appl. Phys. Lett. 2025, 126. [Google Scholar] [CrossRef]
- Bihari, N.; Rauha, I.T.S.; Marin, G.; Ekstrum, C.; de Alwis, C.; Mayville, P.J.; Savin, H.; Karppinen, M.; Pearce, J.M. Understanding the multilevel phenomena that enables inorganic atomic layer deposition to provide barrier coatings for highly-porous 3-D printed plastic in vacuums. Surf. Coat. Technol. 2023, 462, 129475. [Google Scholar] [CrossRef]
- Niemiec, M.; Bayansal, F.; Bhosale, T.; Suib, S.; Biyikli, N.; Kim, K. Failure behavior of polymer microelectrode arrays encapsulated with conventional ALD and 3D-ALI barriers. Front. Bioeng. Biotechnol. 2025, 13, 1622927. [Google Scholar] [CrossRef]
- Mariello, M.; von Allmen, M.; Wu, K.; Van Gompel, M.; Lacour, S.P.; Leterrier, Y. Hermetic, Hybrid Multilayer, Sub-5µm-Thick Encapsulations Prepared with Vapor-Phase Infiltration of Metal Oxides in Conformal Polymers for Flexible Bioelectronics. Adv. Funct. Mater. 2024, 34, 2403973. [Google Scholar] [CrossRef]
- Schultheiss, A.; Sekkat, A.; Nguyen, V.H.; Carella, A.; Benayad, A.; Revaux, A.; Demadrille, R.; Muñoz-Rojas, D.; Simonato, J.-P. High performance encapsulation of transparent conductive polymers by spatial atomic layer deposition. Synth. Met. 2021, 284, 116995. [Google Scholar] [CrossRef]
- Sharif, R.; Khalid, A.; Ahmad, S.W.; Rehman, A.; Qutab, H.G.; Akhtar, H.H.; Mahmood, K.; Afzal, S.; Saleem, F. A comprehensive review of the current progresses and material advances in perovskite solar cells. Nanoscale Adv. 2023, 5, 3803–3833. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, M.; Heydarian, M.; Bett, A.J.; Bivour, M.; Schindler, F.; Hermle, M.; Schubert, M.C.; Schulze, P.S.C.; Borchert, J.; Glunz, S.W. Monolithic Two-Terminal Perovskite/Perovskite/Silicon Triple-Junction Solar Cells with Open Circuit Voltage >2.8 V. ACS Energy Lett. 2023, 8, 4186–4192. [Google Scholar] [CrossRef]
- Li, F.; Wu, D.; Shang, L.; Xia, R.; Zhang, H.; Huang, Z.; Gong, J.; Mao, L.; Zhang, H.; Sun, Y.; et al. Highly Efficient Monolithic Perovskite/Perovskite/Silicon Triple-Junction Solar Cells. Adv. Mater. 2024, 36, e2311595. [Google Scholar] [CrossRef]
- Dong, Y.; Yuan, L.; Tang, L.; Zhang, Z.; Zou, S.; Chen, J.; Cui, X.; Li, N.; Shi, L.; Yan, K. Surface Passivation and Energy Alignment Modulation of n–i–p Perovskite Solar Cells with Self-Assembled Molecule. Small 2025, 21, 2412628. [Google Scholar] [CrossRef]
- Wang, L.; Song, Q.; Pei, F.; Chen, Y.; Dou, J.; Wang, H.; Shi, C.; Zhang, X.; Fan, R.; Zhou, W.; et al. Strain Modulation for Light-Stable n–i–p Perovskite/Silicon Tandem Solar Cells. Adv. Mater. 2022, 34, e2201315. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Kalasariya, N.; Dally, P.; Deger, C.; Yavuz, I.; Razzaq, A.; Vishal, B.; Prasetio, A.; Utomo, D.S.; et al. Mitigating Buried-Interface Energy Losses through Multifunctional Ligands in n–i–p Perovskite/Silicon Tandem Solar Cells. ACS Energy Lett. 2024, 9, 4633–4644. [Google Scholar] [CrossRef]
- Homnan, S.; Malison, P.; Amratisha, K.; Kanjanaboos, P.; Wongratanaphisan, D.; Sagawa, T.; Ruankham, P. Low-temperature processable Sn-doped ZnO films as electron transporting layers for perovskite solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 27279–27289. [Google Scholar] [CrossRef]
- Jin, T.-Y.; Li, W.; Li, Y.-Q.; Luo, Y.-X.; Shen, Y.; Cheng, L.-P.; Tang, J.-X. High-Performance Flexible Perovskite Solar Cells Enabled by Low-Temperature ALD-Assisted Surface Passivation. Adv. Opt. Mater. 2018, 6, 1801153. [Google Scholar] [CrossRef]
- Huang, Z.; Ge, X.; Liu, Z.; Shi, B.; Wang, P.; Zhao, Y.; Zhang, X. Highly Efficient Blade-Coated 1.67 eV p–i–n Perovskite Solar Cells Enabled by a Hybrid Self-Assembled Monolayer and Surface Passivation. ACS Appl. Energy Mater. 2024, 7, 11683–11690. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, H.; Lu, Z.; Xia, H.; Wang, H.-L. Interface engineering using a neutral carbolong complex for efficient and stable p–i–n perovskite solar cells. J. Mater. Chem. C 2023, 11, 2480–2483. [Google Scholar] [CrossRef]
- Nyiekaa, E.A.; Aika, T.A.; Orukpe, P.E.; Akhabue, C.E.; Danladi, E. Development on inverted perovskite solar cells: A review. Heliyon 2024, 10, e24689. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Oveisi, H. A high-performance perovskite solar cell with a designed nanoarchitecture and modified mesoporous titania electron transport layer by zinc nanoparticles impurity. Mater. Sci. Eng. B 2023, 296, 116608. [Google Scholar] [CrossRef]
- Kim, M.; Choi, I.-w.; Choi, S.J.; Song, J.W.; Mo, S.-I.; An, J.-H.; Jo, Y.; Ahn, S.; Ahn, S.K.; Kim, G.-H.; et al. Enhanced electrical properties of Li-salts doped mesoporous TiO2 in perovskite solar cells. Joule 2021, 5, 659–672. [Google Scholar] [CrossRef]
- Saliba, M.; Correa-Baena, J.-P.; Wolff, C.M.; Stolterfoht, M.; Phung, N.; Albrecht, S.; Neher, D.; Abate, A. How to Make over 20% Efficient Perovskite Solar Cells in Regular (n–i–p) and Inverted (p–i–n) Architectures. Chem. Mater. 2018, 30, 4193–4201. [Google Scholar] [CrossRef]
- Brinkmann, K.O.; Wang, P.; Lang, F.; Li, W.; Guo, X.; Zimmermann, F.; Olthof, S.; Neher, D.; Hou, Y.; Stolterfoht, M.; et al. Perovskite–organic tandem solar cells. Nat. Rev. Mater. 2024, 9, 202–217. [Google Scholar] [CrossRef]
- Shishido, H.; Sato, R.; Ieki, D.; Matsuo, G.; Saito, K.; Konagai, M.; Ishikawa, R. High-Efficiency Perovskite/Silicon Tandem Solar Cells with Flexibility. Sol. RRL 2025, 9, 202400899. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, S.; Wang, J.; Lu, J.; Zheng, X.; Lu, S.; Yue, S.; Liu, K.; Wang, Z.; Qu, S. Facet orientation control of tin-lead perovskite for efficient all-perovskite tandem solar cells. J. Mater. Sci. Technol. 2024, 213, 118–124. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, H.; Jin, K.; Xiao, Z.; Du, X.; Yan, K.; Hao, F.; Bao, Q.; Yi, C.; Liu, F.; et al. 4-Terminal Inorganic Perovskite/Organic Tandem Solar Cells Offer 22% Efficiency. Nano-Micro Lett. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Zhao, D.; Yip, H.L.; Ho-Baillie, A. Perovskite-Based Tandem Solar Cells. Sol. RRL 2024, 8, 2400755. [Google Scholar] [CrossRef]
- Fang, Z.; Zeng, Q.; Zuo, C.; Zhang, L.; Xiao, H.; Cheng, M.; Hao, F.; Bao, Q.; Zhang, L.; Yuan, Y.; et al. Perovskite-based tandem solar cells. Sci. Bull. 2021, 66, 621–636. [Google Scholar] [CrossRef]
- Warby, J.; Shah, S.; Thiesbrummel, J.; Gutierrez-Partida, E.; Lai, H.; Alebachew, B.; Grischek, M.; Yang, F.; Lang, F.; Albrecht, S.; et al. Mismatch of Quasi–Fermi Level Splitting and Voc in Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2303135. [Google Scholar] [CrossRef]
- Yang, Q.; Bittkau, K.; Klingebiel, B.; Kirchartz, T.; Rau, U.; Ding, K. Toward the working mechanisms of tin oxide as buffer layer in perovskite/silicon tandem solar cells. Appl. Phys. Rev. 2025, 12, 021403. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Bai, L.; Malouangou, D.M.; Matondo, J.T.; Pan, J.; Dai, S.; Cai, M.; Liu, X.; Guli, M. Research progress of atomic layer deposition technology to improve the long-term stability of perovskite solar cells. J. Mater. Chem. C 2022, 10, 819–839. [Google Scholar] [CrossRef]
- Asare, G.K.; Adu, J.S.; Shin, B.; Fermin, D.J.; Park, H.H. Perspective: Atomic Layer Deposition Strategies for Surface Passivation of Metal-Halide Perovskite Absorbers. Electron. Mater. Lett. 2025, 21, 331–336. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, Z.; Ma, S.; Guo, S.; Wu, J.; Zhang, Y.; Cao, B.; Dong, J. Novel Strategy for High Efficient and Stable Perovskite Solar Cells through Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2024, 16, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Puliparambil Thilakan, A.; Hsu, W.-H.; Yang, Y.-W.; Wang, Y.-T.; Yabushita, A.; Luo, C.-W.; Chen, M.-H. Investigations of Carrier Transport Mechanisms in Perovskite Solar Cells with Different Types of ZnO Layer. Sol. RRL 2023, 7, 2300314. [Google Scholar] [CrossRef]
- Ren, N.; Zhu, C.; Li, R.; Mazumdar, S.; Sun, C.; Chen, B.; Xu, Q.; Wang, P.; Shi, B.; Huang, Q.; et al. 50 °C low-temperature ALD SnO2 driven by H2O2 for efficient perovskite and perovskite/silicon tandem solar cells. Appl. Phys. Lett. 2022, 121, 033502. [Google Scholar] [CrossRef]
- Erdenebileg, E.; Wang, H.; Li, J.; Singh, N.; Dewi, H.A.; Tiwari, N.; Mathews, N.; Mhaisalkar, S.; Bruno, A. Low-Temperature Atomic Layer Deposited Electron Transport Layers for Co-Evaporated Perovskite Solar Cells. Sol. RRL 2022, 6, 2100842. [Google Scholar] [CrossRef]
- Seo, S.; Park, I.J.; Kim, M.; Lee, S.; Bae, C.; Jung, H.S.; Park, N.-G.; Kim, J.Y.; Shin, H. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic–inorganic hybrid perovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef]
- Yu, B.; Tang, F.; Yang, Y.; Huang, J.; Wu, S.; Lu, F.; Duan, W.; Lambertz, A.; Ding, K.; Mai, Y. Impermeable Atomic Layer Deposition for Sputtering Buffer Layer in Efficient Semi-Transparent and Tandem Solar Cells via Activating Unreactive Substrate. Adv. Mater. 2023, 35, 2202447. [Google Scholar] [CrossRef] [PubMed]
- Artuk, K.; Turkay, D.; Mensi, M.D.; Steele, J.A.; Jacobs, D.A.; Othman, M.; Yu Chin, X.; Moon, S.-J.; Tiwari, A.N.; Hessler-Wyser, A.; et al. A Universal Perovskite/C60 Interface Modification via Atomic Layer Deposited Aluminum Oxide for Perovskite Solar Cells and Perovskite–Silicon Tandems. Adv. Mater. 2024, 36, 2311745. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xu, D.; Ma, Q.; Gao, K.; Zhang, C.; Xing, C.; Wang, S.; Shi, W.; Wang, X.; Li, K.; et al. Atomic Layer Deposited ZnO–SnO2 Electron Transport Bilayer for Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2023, 7, 2201026. [Google Scholar] [CrossRef]
- Puurunen, R.L. A Short History of Atomic Layer Deposition: Tuomo Suntola’s Atomic Layer Epitaxy. Chem. Vap. Depos. 2014, 20, 332–344. [Google Scholar] [CrossRef]
- Cho, J.W.; Song, M.S.; Choi, I.H.; Go, K.J.; Han, J.; Lee, T.Y.; An, C.; Choi, H.J.; Sohn, C.; Park, M.H.; et al. Atomic Layer Deposition of Epitaxial Ferroelectric Hf0.5Zr0.5O2 Thin Films. Adv. Funct. Mater. 2024, 34, 2314396. [Google Scholar] [CrossRef]
- Li, J.; Chai, G.; Wang, X. Atomic layer deposition of thin films: From a chemistry perspective. Int. J. Extrem. Manuf. 2023, 5, 032003. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, L.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Atomic layer deposition to heterostructures for application in gas sensors. Int. J. Extrem. Manuf. 2023, 5, 022008. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Ai, W.; Chen, J.; Lv, Z.; Wang, B.; Yang, M.; Luo, F.; Wu, J. Vertically grown metal nanosheets integrated with atomic-layer-deposited dielectrics for transistors with subnanometre capacitance-equivalent thicknesses. Nat. Electron. 2024, 7, 662–670. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, L.; Ding, Y.; Xiao, H.; Shi, Q.; Li, X.; Li, A.; Fang, G. Atomic layer deposition meets metal–organic frameworks. Prog. Mater. Sci. 2023, 138, 101159. [Google Scholar] [CrossRef]
- Oke, J.A.; Jen, T.-C. Atomic layer deposition and other thin film deposition techniques: From principles to film properties. J. Mater. Res. Technol. 2022, 21, 2481–2514. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Mukherjee, R.; Sukotjo, C.; Diwekar, U.; Takoudis, C. Recent Advances in Theoretical Development of Thermal Atomic Layer Deposition: A Review. Nanomaterials 2022, 12, 831. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical Vapour Deposition (CVD): Advances, Technology and Applications, Series in Materials Science and Engineering; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Park, H.; Hwang, J.H.; Oh, S.H.; Ryu, J.J.; Jeon, K.; Kang, M.; Chai, H.-J.; Ham, A.; Kim, G.H.; Kang, K.; et al. Direct Growth of Bi2SeO5 Thin Films for High-k Dielectrics via Atomic Layer Deposition. ACS Nano 2024, 18, 22071–22079. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Tian, Y.; Wang, Y.; Peng, L.; Pei, C.; Wang, T.; Gong, J. Deposition of uniform films on complex 3D objects by atomic layer deposition for plasma etch resistant coatings. Natl. Sci. Rev. 2025, 12, nwaf247. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.W.; Jeng, H.T.; Perng, T.P. Formation Mechanism and Bandgap Reduction of GaN–ZnO Solid-Solution Thin Films Fabricated by Nanolamination of Atomic Layer Deposition. Adv. Mater. 2022, 35, e2207849. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Du, M.; Li, P.; Cui, S.; Liu, Y.; Wei, H.; Peng, M.; Zheng, X. Growth of AlxGa1-xN Thin Films with Controllable Composition and Optical Bandgap on 4H-SiC by Sub-Cycle Incomplete Reaction Plasma Enhanced Atomic Layer Deposition. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Shahin, A.; Saini, A.; Kim, N.Y.; Musselman, K.P. Tailoring CuOx Films via Open-Air Spatial Atomic Layer Deposition and Their Application in Room-Temperature Humidity Sensing. Small 2025, 21, e06835. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, S.; Sajavaara, T. Spatial ALD of Al2O3 and ZnO using heavy water. Surf. Coat. Technol. 2022, 441, 128456. [Google Scholar] [CrossRef]
- Wu, W.-B.; Hsu, C.-H.; Yue, X.-X.; Zhang, W.-Z.; Zhang, J.; Zhang, X.-Y.; Gao, P.; Wu, W.-Y.; Wuu, D.-S.; Lai, F.-M.; et al. Low temperature (002)-oriented zinc oxide films prepared using ozone-based spatial atomic layer deposition. Ceram. Int. 2024, 50, 26770–26779. [Google Scholar] [CrossRef]
- Sekkat, A.; Weber, M.; López-Sánchez, J.; Rabat, H.; Hong, D.; Rubio-Zuazo, J.; Bellet, D.; Chichignoud, G.; Kaminski-Cachopo, A.; Muñoz-Rojas, D. Selective spatial atomic layer deposition of Cu, Cu2O, and CuO thin films in the open air: Reality or fiction? Mater. Today Chem. 2023, 29, 101431. [Google Scholar] [CrossRef]
- Asgarimoghaddam, H.; Chen, Q.; Ye, F.; Shahin, A.; Song, B.; Musselman, K.P. Effect of different oxygen precursors on alumina deposited using a spatial atomic layer deposition system for thin-film encapsulation of perovskite solar cells. Nanotechnology 2024, 35, 095401. [Google Scholar] [CrossRef]
- Poodt, P.; Knaapen, R.; Illiberi, A.; Roozeboom, F.; van Asten, A. Low temperature and roll-to-roll spatial atomic layer deposition for flexible electronics. J. Vac. Sci. Technol. A 2011, 30, 01A142. [Google Scholar] [CrossRef]
- Yoo, K.S.; Lee, C.-H.; Kim, D.-G.; Choi, S.-H.; Lee, W.-B.; Park, C.-K.; Park, J.-S. High mobility and productivity of flexible In2O3 thin-film transistors on polyimide substrates via atmospheric pressure spatial atomic layer deposition. Appl. Surf. Sci. 2024, 646, 158950. [Google Scholar] [CrossRef]
- Li, Y.; Cao, K.; Xiong, Y.f.; Yang, H.; Zhang, Y.; Lin, Y.; Zhou, B.; Huang, J.; Chen, R. Composite Encapsulation Films with Ultrahigh Barrier Performance for Improving the Reliability of Blue Organic Light-Emitting Diodes. Adv. Mater. Interfaces 2020, 7, 2000237. [Google Scholar] [CrossRef]
- Choi, H.; Shin, S.; Choi, Y.; Choi, Y.; Kim, J.; Kim, S.; Kim, H.; Park, J.; Chung, S.C.; Jeon, H.; et al. 71.1: High Throughput and Scalable Spatial Atomic Layer Deposition of Al2O3as a Moisture Barrier for Flexible OLED Display. SID Symp. Dig. Tech. Pap. 2015, 46, 1043–1046. [Google Scholar] [CrossRef]
- Asgarimoghaddam, H.; Chen, Q.; Ye, F.; Shahin, A.; Marchione, O.A.C.; Song, B.; Musselman, K.P. Spatial atomic layer deposition of nitrogen-doped alumina thin films for high-performance perovskite solar cell encapsulation. Nano Energy 2024, 127, 109782. [Google Scholar] [CrossRef]
- Zimmermann, E.; Wong, K.K.; Seewald, T.; Kalb, J.; Linke, J.; Hahn, G.; Schmidt-Mende, L. Controlled Crystallinity of TiO2 Layers Grown by Atmospheric Pressure Spatial Atomic Layer Deposition and their Impact on Perovskite Solar Cell Efficiency. Int. J. Photoenergy 2022, 2022, 1172871. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, H.; Zhang, K.; Qin, C.; Liu, J.; Peng, W.; Islam, A.; Bi, E.; Ye, F.; et al. Highly compact TiO2 layer for efficient hole-blocking in perovskite solar cells. Appl. Phys. Express 2014, 7, 052301. [Google Scholar] [CrossRef]
- Wack, S.; Lunca Popa, P.; Adjeroud, N.; Vergne, C.; Leturcq, R. Two-Step Approach for Conformal Chemical Vapor-Phase Deposition of Ultra-Thin Conductive Silver Films. ACS Appl. Mater. Interfaces 2020, 12, 36329–36338. [Google Scholar] [CrossRef]
- Park, H.H. Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells. Nanomaterials 2021, 11, 88. [Google Scholar] [CrossRef]
- Sukharevska, N.; Bederak, D.; Dirin, D.; Kovalenko, M.; Loi, M.A. Improved Reproducibility of PbS Colloidal Quantum Dots Solar Cells Using Atomic Layer–Deposited TiO2. Energy Technol. 2020, 8, 1900887. [Google Scholar] [CrossRef] [PubMed]
- Choolakkal, A.H.; Mpofu, P.; Niiranen, P.; Birch, J.; Pedersen, H. Using a Heavy Inert Diffusion Additive for Superconformal Atomic Layer Deposition. J. Phys. Chem. Lett. 2025, 16, 2369–2372. [Google Scholar] [CrossRef]
- Arshad, Z.; Shakir, S.; Khoja, A.H.; Javed, A.H.; Anwar, M.; Rehman, A.; Javaid, R.; Qazi, U.Y.; Farrukh, S. Performance Analysis of Calcium-Doped Titania (TiO2) as an Effective Electron Transport Layer (ETL) for Perovskite Solar Cells. Energies 2022, 15, 1408. [Google Scholar] [CrossRef]
- Chantana, J.; Hirayama, T.; Ding, C.; Kawano, Y.; Shen, Q.; Yoshino, K.; Hayase, S.; Minemoto, T. Micro-scale current path distributions of Zn1-Mg O-coated SnO2:F transparent electrodes prepared by sol-gel and sputtering methods in perovskite solar cells. Thin Solid Film. 2019, 669, 455–460. [Google Scholar] [CrossRef]
- Bilandi, N.; Verma, H.K.; Dhir, R. Novel transportation layers of NiO and ZnO for the fabrication of perovskite solar cell for e-monitoring of healthcare. J. Sol-Gel Sci. Technol. 2020, 95, 300–307. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhang, Y.; Tang, Y.; Zhang, Z.; Li, H.; Wu, Y.; Li, Y.; Liu, X.; Wang, H. Decreased Accumulation of SnO2 Particles Results from Sodium Citrate Dispersant Assisted Chemical Bath Deposition for High Quality Perovskite Solar Cells. Sol. RRL 2024, 8, 2400020. [Google Scholar] [CrossRef]
- Rajabi, N.; Wojcik, P.M.; Khanal, L.R.; Qiang, Y.; McIlroy, D.N. A comparison of the morphological and electrical properties of sol-gel dip coating and atomic layer deposition of ZnO on 3D nanospring mats. Mater. Res. Express 2019, 6, 035902. [Google Scholar] [CrossRef]
- Bush, K.A.; Bailie, C.D.; Chen, Y.; Bowring, A.R.; Wang, W.; Ma, W.; Leijtens, T.; Moghadam, F.; McGehee, M.D. Thermal and Environmental Stability of Semi-Transparent Perovskite Solar Cells for Tandems Enabled by a Solution-Processed Nanoparticle Buffer Layer and Sputtered ITO Electrode. Adv. Mater. 2016, 28, 3937–3943. [Google Scholar] [CrossRef]
- Mallik, N.; Hajhemati, J.; Frégnaux, M.; Coutancier, D.; Toby, A.; Zhang, S.-T.; Hartmann, C.; Hüsam, E.; Saleh, A.; Vincent, T.; et al. Interface defect formation for atomic layer deposition of SnO2 on metal halide perovskites. Nano Energy 2024, 126, 109582. [Google Scholar] [CrossRef]
- Thote, A.; Jeon, I.; Lin, H.-S.; Manzhos, S.; Nakagawa, T.; Suh, D.; Hwang, J.; Kashiwagi, M.; Shiomi, J.; Maruyama, S.; et al. High-Working-Pressure Sputtering of ZnO for Stable and Efficient Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 1, 389–396. [Google Scholar] [CrossRef]
- Peng, Z.; Jin, L.; Zuo, Z.; Qi, Q.; Hou, S.; Fu, Y.; Zou, D. Isolating the Oxygen Adsorption Defects on Sputtered Tin Oxide for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 23518–23526. [Google Scholar] [CrossRef]
- Nakamura, M.; Lin, C.C.; Nishiyama, C.; Tada, K.; Bessho, T.; Segawa, H. Semi-transparent Perovskite Solar Cells for Four-Terminal Perovskite/CIGS Tandem Solar Cells. ACS Appl. Energy Mater. 2022, 5, 8103–8111. [Google Scholar] [CrossRef]
- Liu, K.; Chen, B.; Yu, Z.J.; Wu, Y.; Huang, Z.; Jia, X.; Li, C.; Spronk, D.; Wang, Z.; Wang, Z.; et al. Reducing sputter induced stress and damage for efficient perovskite/silicon tandem solar cells. J. Mater. Chem. A 2021, 10, 1343–1349. [Google Scholar] [CrossRef]
- Lee, Y.; Seok, S.I. Sputtered Nickel Oxide Hole Transporting Layers for Perovskite Solar Cells. Korean J. Chem. Eng. 2024, 41, 3669–3676. [Google Scholar] [CrossRef]
- Kroll, M.; Öz, S.D.; Zhang, Z.; Ji, R.; Schramm, T.; Antrack, T.; Vaynzof, Y.; Olthof, S.; Leo, K. Insights into the evaporation behaviour of FAI: Material degradation and consequences for perovskite solar cells. Sustain. Energy Fuels 2022, 6, 3230–3239. [Google Scholar] [CrossRef]
- Shi, B.; Jia, J.; Feng, X.; Ma, G.; Wu, Y.; Cao, B. Thermal evaporated CuI film thickness-dependent performance of perovskite solar cells. Vacuum 2021, 187, 110076. [Google Scholar] [CrossRef]
- Said, A.A.; Aydin, E.; Ugur, E.; Xu, Z.; Deger, C.; Vishal, B.; Vlk, A.; Dally, P.; Yildirim, B.K.; Azmi, R.; et al. Sublimed C60 for efficient and repeatable perovskite-based solar cells. Nat. Commun. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Sun, B.; Tan, X.; Ye, H.; Tu, Y.; Shi, T.; Tang, Z.; Liao, G. Fully low-temperature processed carbon-based perovskite solar cells using thermally evaporated cadmium sulfide as efficient electron transport layer. Org. Electron. 2019, 74, 152–160. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.S.; Park, J.; Lee, J.W.; Kim, K. Opportunities and Challenges for Perovskite Solar Cells Based on Vacuum Thermal Evaporation. Adv. Mater. Technol. 2022, 8, 2200928. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Sun, J.; Hu, M.; Chen, J.; Duan, B.; Hu, S.; Hou, P.; Tan, W.L.; Ku, Z.; et al. Low Pressure Chemical Vapor Deposited Perovskite Enables all Vacuum-Processed Monolithic Perovskite-Silicon Tandem Solar Cells. Adv. Energy Mater. 2025, 15, 2405377. [Google Scholar] [CrossRef]
- Qiu, L.; He, S.; Jiang, Y.; Qi, Y. Metal halide perovskite solar cells by modified chemical vapor deposition. J. Mater. Chem. A 2021, 9, 22759–22780. [Google Scholar] [CrossRef]
- Eom, T.; Kim, S.; Agbenyeke, R.E.; Jung, H.; Shin, S.M.; Lee, Y.K.; Kim, C.G.; Chung, T.M.; Jeon, N.J.; Park, H.H.; et al. Buffer Layers: Copper Oxide Buffer Layers by Pulsed-Chemical Vapor Deposition for Semitransparent Perovskite Solar Cells (Adv. Mater. Interfaces 1/2021). Adv. Mater. Interfaces 2021, 8, 2001482. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Zhang, Z.; Briscoe, J.; Warwick, M.E.A.; Li, H.; Hu, P. Aerosol assisted chemical vapour deposition of conformal ZnO compact layers for efficient electron transport in perovskite solar cells. Mater. Lett. 2018, 217, 251–254. [Google Scholar] [CrossRef]
- Idígoras, J.; Contreras-Bernal, L.; Cave, J.M.; Courtier, N.E.; Barranco, Á.; Borras, A.; Sánchez-Valencia, J.R.; Anta, J.A.; Walker, A.B. The Role of Surface Recombination on the Performance of Perovskite Solar Cells: Effect of Morphology and Crystalline Phase of TiO2 Contact. Adv. Mater. Interfaces 2018, 5, 1801076. [Google Scholar] [CrossRef]
- Cho, Y.J.; Jeong, M.J.; Park, J.H.; Hu, W.; Lim, J.; Chang, H.S. Charge Transporting Materials Grown by Atomic Layer Deposition in Perovskite Solar Cells. Energies 2021, 14, 1156. [Google Scholar] [CrossRef]
- Raiford, J.A.; Belisle, R.A.; Bush, K.A.; Prasanna, R.; Palmstrom, A.F.; McGehee, M.D.; Bent, S.F. Atomic layer deposition of vanadium oxide to reduce parasitic absorption and improve stability in n–i–p perovskite solar cells for tandems†. Sustain. Energy Fuels 2019, 3, 1517–1525. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Hossain, M.I.; Visal, S.; Kaneko, T.; Qarony, W.; Umezu, S.; Tomita, K.; Iwamori, S.; Knipp, D.; Tsang, Y.H.; et al. Spray Pyrolyzed TiO2 Embedded Multi-Layer Front Contact Design for High-Efficiency Perovskite Solar Cells. Nano-Micro Lett. 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Nukunudompanich, M.; Suzuki, K.; Kameda, K.; Manzhos, S.; Ihara, M. Nano-scale smooth surface of the compact-TiO2 layer via spray pyrolysis for controlling the grain size of the perovskite layer in perovskite solar cells. RSC Adv. 2023, 13, 27686–27695. [Google Scholar] [CrossRef]
- Hu, C.; Yang, S. Ultrasonic Spray Pyrolysis Deposition of NiO Thin Film for Efficient Perovskite Solar Cell. ECS Meet. Abstr. 2020, MA2019-01, 2211. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, Y.; Zhang, Z.; Rong, Y.; Mei, A.; Tian, C.; Zhang, J.; Zhang, Y.; Jin, Y.; Han, H.; et al. Efficient hole-conductor-free printable mesoscopic perovskite solar cells based on SnO2 compact layer. Electrochim. Acta 2018, 263, 134–139. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Liu, C.; Shan, Y.; Li, F.; Sun, L. Spray Pyrolysis Deposition of CuCrO2 Films as Promising Inorganic Hole Transport Layers for Highly Efficient Perovskite Solar Cells. Energy Technol. 2022, 10, 2200518. [Google Scholar] [CrossRef]
- Prakash, K.; Prabakaran, S.; Harish, S.; Navaneethan, M. Interfacial charge transport of Ag2+-decorated CuI thin film for solar cell application. J. Mater. Sci. Mater. Electron. 2022, 33, 8586–8593. [Google Scholar] [CrossRef]
- Ren, H.; Zou, X.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile Solution Spin-Coating SnO2 Thin Film Covering Cracks of TiO2 Hole Blocking Layer for Perovskite Solar Cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef]
- Dehghan, M.; Behjat, A. Deposition of zinc oxide as an electron transport layer in planar perovskite solar cells by spray and SILAR methods comparable with spin coating†. RSC Adv. 2019, 9, 20917–20924. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, W.; Jiang, Z.; Zhang, Y.; Ni, C. CuI/Spiro-OMeTAD Double-Layer Hole Transport Layer to Improve Photovoltaic Performance of Perovskite Solar Cells. Coatings 2021, 11, 978. [Google Scholar] [CrossRef]
- Chen, M.; Nijboer, M.P.; Kovalgin, A.Y.; Nijmeijer, A.; Roozeboom, F.; Luiten-Olieman, M.W.J. Atmospheric-pressure atomic layer deposition: Recent applications and new emerging applications in high-porosity/3D materials. Dalton Trans. 2023, 52, 10254–10277. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Muñoz-Rojas, D.; Nelson, S.F.; Illiberi, A.; Poodt, P.; Roozeboom, F.; MacManus-Driscoll, J.L. Research Update: Atmospheric pressure spatial atomic layer deposition of ZnO thin films: Reactors, doping, and devices. APL Mater. 2015, 3, 040701. [Google Scholar] [CrossRef]
- Grau-Luque, E.; Guc, M.; Becerril-Romero, I.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Bolt, P.; Van den Bruele, F.; Ruhle, U. Thickness evaluation of AlOx barrier layers for encapsulation of flexible PV modules in industrial environments by normal reflectance and machine learning. Prog. Photovolt. 2021, 30, 229–239. [Google Scholar] [CrossRef]
- Ameen, M.; Beeker, I.; Haverkate, L.; Anothumakkool, B.; Grob, F.; Hermes, D.; Huijssen, N.; Khandan del, S.; Roozeboom, F.; Unnikrishnan, S. Next-Generation Li-ion Batteries Made with Spatial Atomic Layer Deposition as an Enabling Technology. ECS Meet. Abstr. 2020, MA2020-02, 1695. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, B.; Hui, W.; Su, Z.; Wang, H.; Zhang, Q.; Gao, K.; Zhang, X.; Li, B.-h.; Gao, X.; et al. Oxygen Vacancy Mediation in SnO2 Electron Transport Layers Enables Efficient, Stable, and Scalable Perovskite Solar Cells. J. Am. Chem. Soc. 2024, 146, 19108–19117. [Google Scholar] [CrossRef]
- Hu, T.; Becker, T.; Pourdavoud, N.; Zhao, J.; Brinkmann, K.O.; Heiderhoff, R.; Gahlmann, T.; Huang, Z.; Olthof, S.; Meerholz, K.; et al. Indium-Free Perovskite Solar Cells Enabled by Impermeable Tin-Oxide Electron Extraction Layers. Adv. Mater. 2017, 29, 1606656. [Google Scholar] [CrossRef]
- Lee, S.-U.; Park, H.; Shin, H.; Park, N.-G. Atomic layer deposition of SnO2 using hydrogen peroxide improves the efficiency and stability of perovskite solar cells. Nanoscale 2023, 15, 5044–5052. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Z.; Li, X.; Guo, Y.; Li, P. Optimization of atomic layer deposited Pt-shell thickness of PtCu3@Pt/C catalyst for oxygen reduction reaction. Mater. Chem. Phys. 2024, 329, 130145. [Google Scholar] [CrossRef]
- Lausecker, C.; Muñoz-Rojas, D.; Weber, M. Atomic layer deposition (ALD) of palladium: From processes to applications. Crit. Rev. Solid State Mater. Sci. 2023, 49, 908–930. [Google Scholar] [CrossRef]
- Aqueel Ahmed, A.T.; Hafiyyan, A.f.; Nurhidayati, N.; Hidayah Rayanisaputri, F.R.; Alibrahim, K.A.; Khadtare, S.S.; Rahman, S.; Alodhayb, A.N.; Rochman, N.T.; Ansari, A.S. Thermal atomic layer deposition of aluminum oxide, nitride, and oxynitride: A mechanistic investigation. AIP Adv. 2024, 14, 035133. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.H.; Shong, B. Mechanistic analysis on low temperature thermal atomic layer deposition of nitrides utilizing H2S. J. Vac. Sci. Technol. A 2023, 41, 062405. [Google Scholar] [CrossRef]
- Liu, L.; Kong, M.; Xing, Y.; Wu, Z.; Chen, Y. Atomic Layer Deposition-Made MoS2–ReS2 Nanotubes with Cylindrical Wall Heterojunctions for Ultrasensitive MiRNA-155 Detection. ACS Appl. Mater. Interfaces 2022, 14, 10081–10091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Ren, Y.; Benamara, M.; Zhou, X.; Li, Y.; Chen, Z.; Zhou, H.; Xiao, X.; Liu, Y.; et al. High-performance LiNi0.8Mn0.1Co0.1O2 cathode by nanoscale lithium sulfide coating via atomic layer deposition. J. Energy Chem. 2022, 69, 531–540. [Google Scholar] [CrossRef]
- Lan, Z.; Huang, H.; Du, S.; Lu, Y.; Sun, C.; Yang, Y.; Zhang, Q.; Suo, Y.; Qu, S.; Wang, M.; et al. Cascade Reaction in Organic Hole Transport Layer Enables Efficient Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202402840. [Google Scholar] [CrossRef]
- Latypova, A.F.; Emelianov, N.A.; Balakirev, D.O.; Sukhorukova, P.K.; Kalinichenko, N.K.; Kuznetsov, P.M.; Luponosov, Y.N.; Aldoshin, S.M.; Ponomarenko, S.A.; Troshin, P.A.; et al. Design Principles for Organic Small Molecule Hole-Transport Materials for Perovskite Solar Cells: Film Morphology Matters. ACS Appl. Energy Mater. 2022, 5, 5395–5403. [Google Scholar] [CrossRef]
- Tan, W.; Hendricks, O.L.; Meng, A.C.; Braun, M.R.; McGehee, M.D.; Chidsey, C.E.D.; McIntyre, P.C. Atomic Layer Deposited TiO2–IrOx Alloy as a Hole Transport Material for Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800191. [Google Scholar] [CrossRef]
- Weiß, A.; Terletskaia, M.; Popov, G.; Mizohata, K.; Leskelä, M.; Ritala, M.; Kemell, M. Atomic Layer Deposition and Pulsed Chemical Vapor Deposition of SnI2 and CsSnI3. Chem. Mater. 2023, 35, 8722–8732. [Google Scholar] [CrossRef]
- Wu, Z.; Li, P.; Zhao, J.; Xiao, T.; Hu, H.; Sun, P.; Wu, Z.; Hao, J.; Sun, C.; Zhang, H.; et al. Low-Temperature-Deposited TiO2 Nanopillars for Efficient and Flexible Perovskite Solar Cells. Adv. Mater. Interfaces 2021, 8, 2001512. [Google Scholar] [CrossRef]
- Martin, B.; Yang, M.; Bramante, R.C.; Amerling, E.; Gupta, G.; van Hest, M.F.A.M.; Druffel, T. Fabrication of flexible perovskite solar cells via rapid thermal annealing. Mater. Lett. 2020, 276, 128215. [Google Scholar] [CrossRef]
- Jin, J.; Li, J.; Tai, Q.; Chen, Y.; Mishra, D.D.; Deng, W.; Xin, J.; Guo, S.; Xiao, B.; Wang, X. Efficient and stable flexible perovskite solar cells based on graphene-AgNWs substrate and carbon electrode without hole transport materials. J. Power Sources 2021, 482, 228953. [Google Scholar] [CrossRef]
- Tang, G.; Chen, L.; Cao, X.; Wang, Y.; Zhang, H.; Tai, Q. A Review on Recent Advances in Flexible Perovskite Solar Cells. Sol. RRL 2025, 9, 2400844. [Google Scholar] [CrossRef]
- Okawa, H.; Momose, Y.; Suyama, N.; Ishikawa, R.; Konagai, M. Flexible Perovskite Solar Cells with Current Collection Through-Hole Electrodes. Sol. RRL 2025, 9, 2500317. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Vergeer, K.; Dewi, H.A.; Wang, H.; Mhaisalkar, S.; Bruno, A.; Mathews, N. Interlayer Engineering for Flexible Large-Area Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 777–784. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, Y.; Xiao, Y.Y.; Sun, J.; Zhang, X.; Han, C.B.; Gao, H.; Zhang, Y.; Yan, H. Effect of temperature on the performance of perovskite solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 12784–12792. [Google Scholar] [CrossRef]
- Kim, N.-K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.-W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645. [Google Scholar] [CrossRef]
- Kumar, A.; Bansode, U.; Ogale, S.; Rahman, A. Understanding the thermal degradation mechanism of perovskite solar cells via dielectric and noise measurements. Nanotechnology 2020, 31, 365403. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, Q.; Sun, L.; Lan, Q.; Sheng, Y.; Wang, L.; Song, Q.; Yang, Z.; Ye, Q.; Wang, K. Perovskite solar cells prepared with multifunctional alcohol additives for enhanced thermal stability and optoelectronic performance. Phys. Scr. 2025, 100, 085983. [Google Scholar] [CrossRef]
- Kim, I.S.; Cao, D.H.; Buchholz, D.B.; Emery, J.D.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G.; Martinson, A.B.F. Liquid Water- and Heat-Resistant Hybrid Perovskite Photovoltaics via an Inverted ALD Oxide Electron Extraction Layer Design. Nano Lett. 2016, 16, 7786–7790. [Google Scholar] [CrossRef]
- Fan, W.; Shen, Y.; Deng, K.; Chen, Q.; Bai, Y.; Li, L. Synergistic bonding stabilized interface for perovskite solar cells with over 24% efficiency. Nano Energy 2022, 100, 107518. [Google Scholar] [CrossRef]
- Halvani Anaraki, E.; Kermanpur, A.; Mayer, M.T.; Steier, L.; Ahmed, T.; Turren-Cruz, S.-H.; Seo, J.; Luo, J.; Zakeeruddin, S.M.; Tress, W.R.; et al. Low-Temperature Nb-Doped SnO2 Electron-Selective Contact Yields over 20% Efficiency in Planar Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 773–778. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, Z.-C.; Jiang, B.-Q.; Kuang, X.-F.; Rao, Z.-Y.; Wang, Z.-J.; Lin, Y.-S.; Xu, Z. Enhanced perovskite solar cell performance via low-temperature ALD-Al2O3 interface modification. Rare Met. 2025, 44, 3060–3068. [Google Scholar] [CrossRef]
- Liang, J.; Hu, X.; Wang, C.; Liang, C.; Chen, C.; Xiao, M.; Li, J.; Tao, C.; Xing, G.; Yu, R.; et al. Origins and influences of metallic lead in perovskite solar cells. Joule 2022, 6, 816–833. [Google Scholar] [CrossRef]
- Hoffmann, L.; Brinkmann, K.O.; Malerczyk, J.; Rogalla, D.; Becker, T.; Theirich, D.; Shutsko, I.; Görrn, P.; Riedl, T. Spatial Atmospheric Pressure Atomic Layer Deposition of Tin Oxide as an Impermeable Electron Extraction Layer for Perovskite Solar Cells with Enhanced Thermal Stability. ACS Appl. Mater. Interfaces 2018, 10, 6006–6013. [Google Scholar] [CrossRef]
- Zhou, Y.; Herz, L.M.; Jen, A.K.Y.; Saliba, M. Advances and challenges in understanding the microscopic structure–property–performance relationship in perovskite solar cells. Nat. Energy 2022, 7, 794–807. [Google Scholar] [CrossRef]
- Koushik, D.; Hazendonk, L.; Zardetto, V.; Vandalon, V.; Verheijen, M.A.; Kessels, W.M.M.; Creatore, M. Chemical Analysis of the Interface between Hybrid Organic–Inorganic Perovskite and Atomic Layer Deposited Al2O3. ACS Appl. Mater. Interfaces 2019, 11, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, A.; Friedenberger, C.; Gahlmann, T.; Trost, S.; Becker, T.; Zilberberg, K.; Polywka, A.; Görrn, P.; Riedl, T. Highly Robust Transparent and Conductive Gas Diffusion Barriers Based on Tin Oxide. Adv. Mater. 2015, 27, 5961–5967. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Huang, S.-W.; Li, C.-F.; Huang, S.-H.; Tsai, F.-Y.; Su, W.-F. A comprehensive optimization of highly efficient MA-Free wide-bandgap perovskites for 4-T Perovskite/Silicon tandem solar cells. Chem. Eng. J. 2024, 503, 158272. [Google Scholar] [CrossRef]
- Luo, X.; Hu, Y.; Lin, Z.; Guo, X.; Zhang, S.; Shou, C.; Hu, Z.; Zhao, X.; Hao, Y.; Chang, J. Theoretical Analysis of All-Inorganic Wide Bandgap Perovskite/Sn-Based Narrow Bandgap Perovskite Tandem Solar Cells. Sol. RRL 2023, 7, 2300081. [Google Scholar] [CrossRef]
- Battaglia, C.; Cuevas, A.; De Wolf, S. High-efficiency crystalline silicon solar cells: Status and perspectives. Energy Environ. Sci. 2016, 9, 1552–1576. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, S.; Mao, K.; Meng, H.; Cai, F.; Li, T.; Feng, X.; Guo, H.; Tang, L.; Xu, J. Low-Temperature Atomic Layer Deposition of Hole Transport Layers for Enhanced Performance and Scalability in Textured Perovskite/Silicon Tandem Solar Cells. Adv. Energy Mater. 2024, 14, 2402365. [Google Scholar] [CrossRef]
- Palmstrom, A.F.; Eperon, G.E.; Leijtens, T.; Prasanna, R.; Habisreutinger, S.N.; Nemeth, W.; Gaulding, E.A.; Dunfield, S.P.; Reese, M.; Nanayakkara, S.; et al. Enabling Flexible All-Perovskite Tandem Solar Cells. Joule 2019, 3, 2193–2204. [Google Scholar] [CrossRef]
- Yun, H.J.; Kim, H.; Choi, B.J. Nucleation and growth behavior of aluminum nitride film using thermal atomic layer deposition. Ceram. Int. 2020, 46, 13372–13376. [Google Scholar] [CrossRef]
- Gong, J.; Adnani, M.; Jones, B.T.; Xin, Y.; Wang, S.; Patel, S.V.; Lochner, E.; Mattoussi, H.; Hu, Y.-Y.; Gao, H. Nanoscale Encapsulation of Hybrid Perovskites Using Hybrid Atomic Layer Deposition. J. Phys. Chem. Lett. 2022, 13, 4082–4089. [Google Scholar] [CrossRef]
- Raiford, J.A.; Chosy, C.; Reeves, B.A.; Bent, S.F. Tailoring the Surface of Metal Halide Perovskites to Enable the Atomic Layer Deposition of Metal Oxide Contacts. ACS Appl. Energy Mater. 2021, 4, 9871–9880. [Google Scholar] [CrossRef]
- David, M.-R.; Viet Huong, N.; César Masse de la, H.; Sara, A.; Carmen, J.; Daniel, B. Spatial Atomic Layer Deposition (SALD), an emerging tool for energy materials. Application to new-generation photovoltaic devices and transparent conductive materials. Comptes Rendus. Phys. 2017, 18, 391–400. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Z.; Gao, Y.; Li, Z.; Jiang, X.; Wen, Y.; Shan, B.; Yang, F.; Chen, R. Quantitative modeling of substrate velocity effects on deposition efficiency and precursor consumption in spatial ALD. Chem. Eng. J. 2025, 517, 164236. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Sekkat, A.; Jiménez, C.; Muñoz, D.; Bellet, D.; Muñoz-Rojas, D. Impact of precursor exposure on process efficiency and film properties in spatial atomic layer deposition. Chem. Eng. J. 2021, 403, 126234. [Google Scholar] [CrossRef]
- Masse de la Huerta, C.; Nguyen, V.H.; Dedulle, J.-M.; Bellet, D.; Jiménez, C.; Muñoz-Rojas, D. Influence of the Geometric Parameters on the Deposition Mode in Spatial Atomic Layer Deposition: A Novel Approach to Area-Selective Deposition. Coatings 2019, 9, 5. [Google Scholar]
- Pan, D. Numerical study on the effectiveness of precursor isolation using N2 as gas barrier in spatial atomic layer deposition. Int. J. Heat Mass Transf. 2019, 144, 118642. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen Thi Kieu, D.; Bui, H.V.; Le Thi Ngoc, L.; Nguyen, V.H. Enhancing control in spatial atomic layer deposition: Insights into precursor diffusion, geometric parameters, and CVD mitigation strategies. Nanotechnology 2024, 35, 205601. [Google Scholar] [CrossRef]
- Li, Z.; Cao, K.; Li, X.; Chen, R. Computational fluid dynamics modeling of spatial atomic layer deposition on microgroove substrates. Int. J. Heat Mass Transf. 2021, 181, 121854. [Google Scholar] [CrossRef]
- Seo, J.; Hwang, H.-S.; Park, S.; Lee, S.; Kim, D.S.; Lim, S.-T.; Seok, J. Deriving optimal atomic layer deposition process conditions using machine learning. J. Ind. Inf. Integr. 2025, 47, 100879. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Christofides, P.D. Multiscale computational fluid dynamics modeling of thermal atomic layer deposition with application to chamber design. Chem. Eng. Res. Des. 2019, 147, 529–544. [Google Scholar] [CrossRef]


| Device Layers | Temperature Range (°C) | Influencing Factors | Refs. |
|---|---|---|---|
| PET | ≤150 | The glass transition temperature of PET is circa 70 °C. Excessive temperature can cause the substrate to soften or shrink. | [176,177,178] |
| PI | ≤400 | PI has high thermal stability but high cost. | [179,180] |
| PEN | ≤150 | The glass transition temperature of PET is circa 120 °C. | [176,181] |
| Perovskite layers | ≤80–250 | The perovskite layer is highly sensitive to heat and is prone to decomposition or ion migration upon heating. The thermal decomposition temperature of perovskites can vary greatly depending on the preparation method, as well as the composition and ratio of their organic or inorganic components. Moreover, the duration of exposure at the given temperature also has a pronounced effect on their stability. | [72,92,182,183,184,185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Jiang, Y.; Wang, L.; Li, J.; Hou, Z.; Choy, K.L.; Li, Z. Atomic Layer Deposition for Perovskite Solar Cells: Interface Engineering, Stability Enhancement, and Future Prospects. Nanomaterials 2025, 15, 1674. https://doi.org/10.3390/nano15211674
Liao X, Jiang Y, Wang L, Li J, Hou Z, Choy KL, Li Z. Atomic Layer Deposition for Perovskite Solar Cells: Interface Engineering, Stability Enhancement, and Future Prospects. Nanomaterials. 2025; 15(21):1674. https://doi.org/10.3390/nano15211674
Chicago/Turabian StyleLiao, Xuanya, Youquan Jiang, Lirong Wang, Jiulong Li, Zhuoran Hou, Kwang Leong Choy, and Zhaodong Li. 2025. "Atomic Layer Deposition for Perovskite Solar Cells: Interface Engineering, Stability Enhancement, and Future Prospects" Nanomaterials 15, no. 21: 1674. https://doi.org/10.3390/nano15211674
APA StyleLiao, X., Jiang, Y., Wang, L., Li, J., Hou, Z., Choy, K. L., & Li, Z. (2025). Atomic Layer Deposition for Perovskite Solar Cells: Interface Engineering, Stability Enhancement, and Future Prospects. Nanomaterials, 15(21), 1674. https://doi.org/10.3390/nano15211674






