Towards the Removal of HMTA Molecules in the Chemical Bath Deposition of ZnO Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition Techniques
2.2. Characterization Techniques
2.3. Thermodynamic Computations
3. Results & Discussion
3.1. Strategy for Substituting HMTA Molecules: State-of-the-Art
3.2. Effects of HMTA Molecules and Ammonia on the Physicochemical Processes in the Bath
3.3. Effects of HMTA Molecules and Ammonia on the Structural Morphology of ZnO
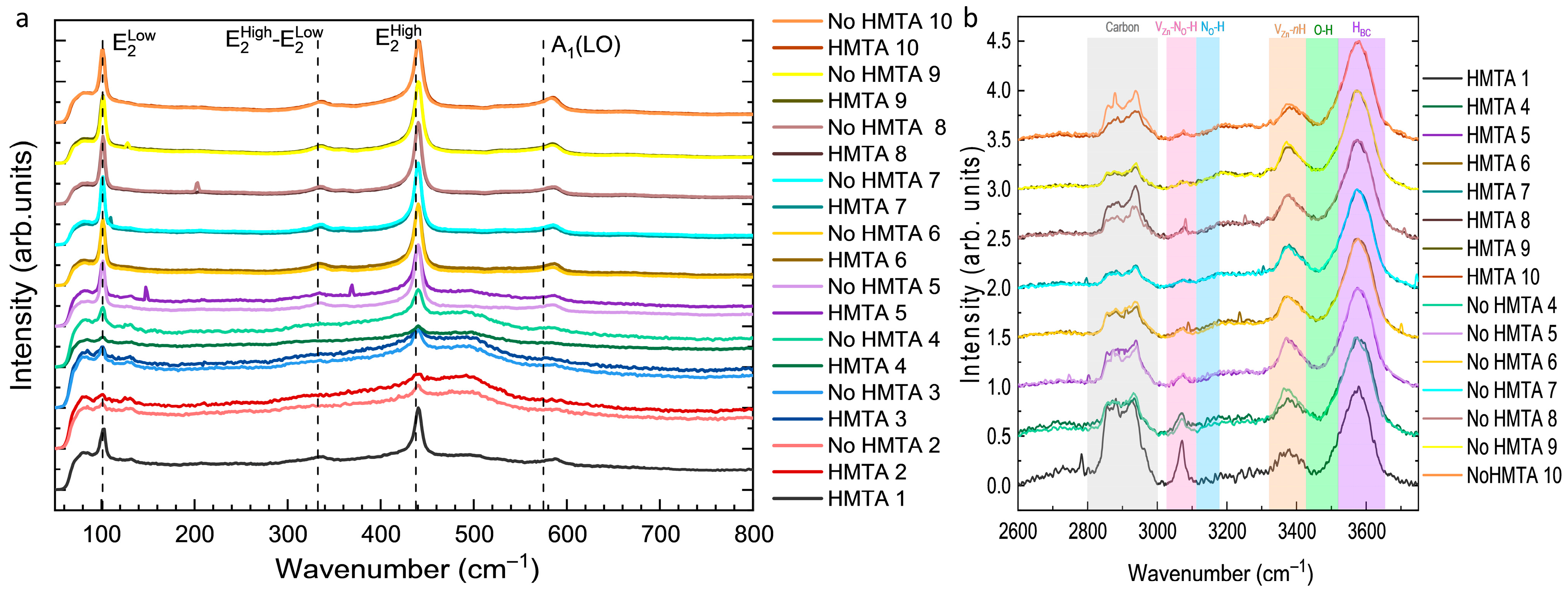
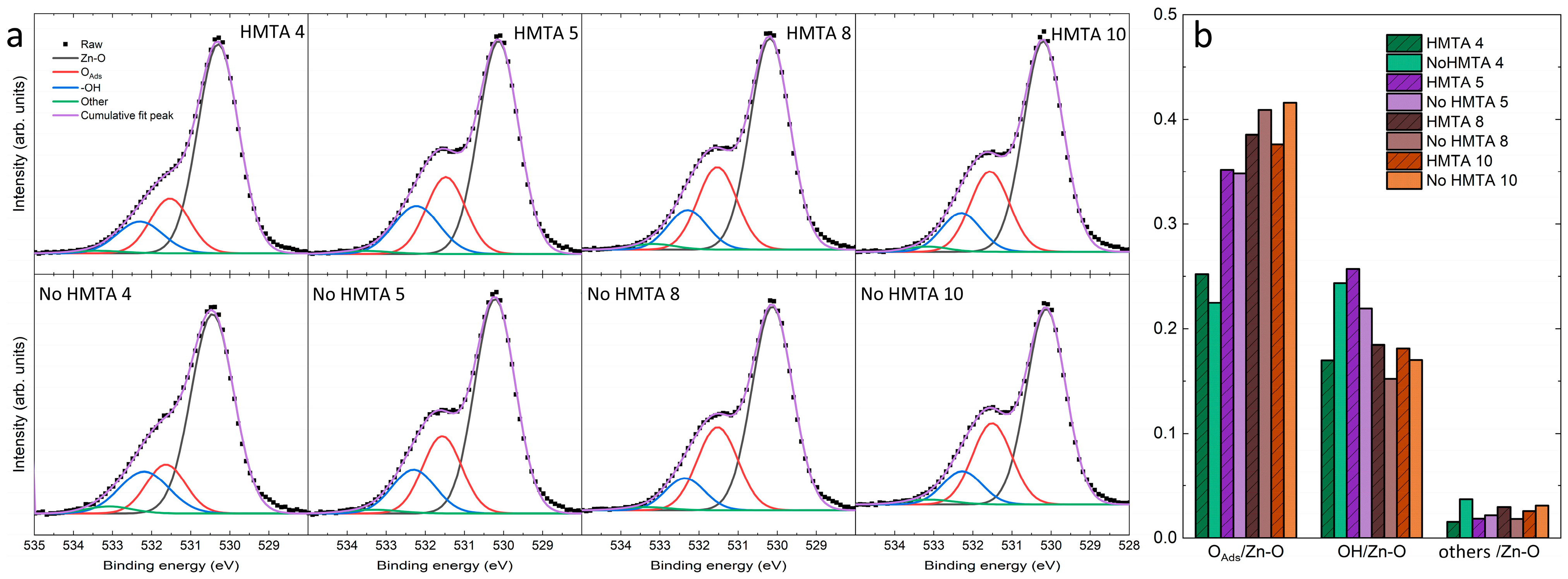
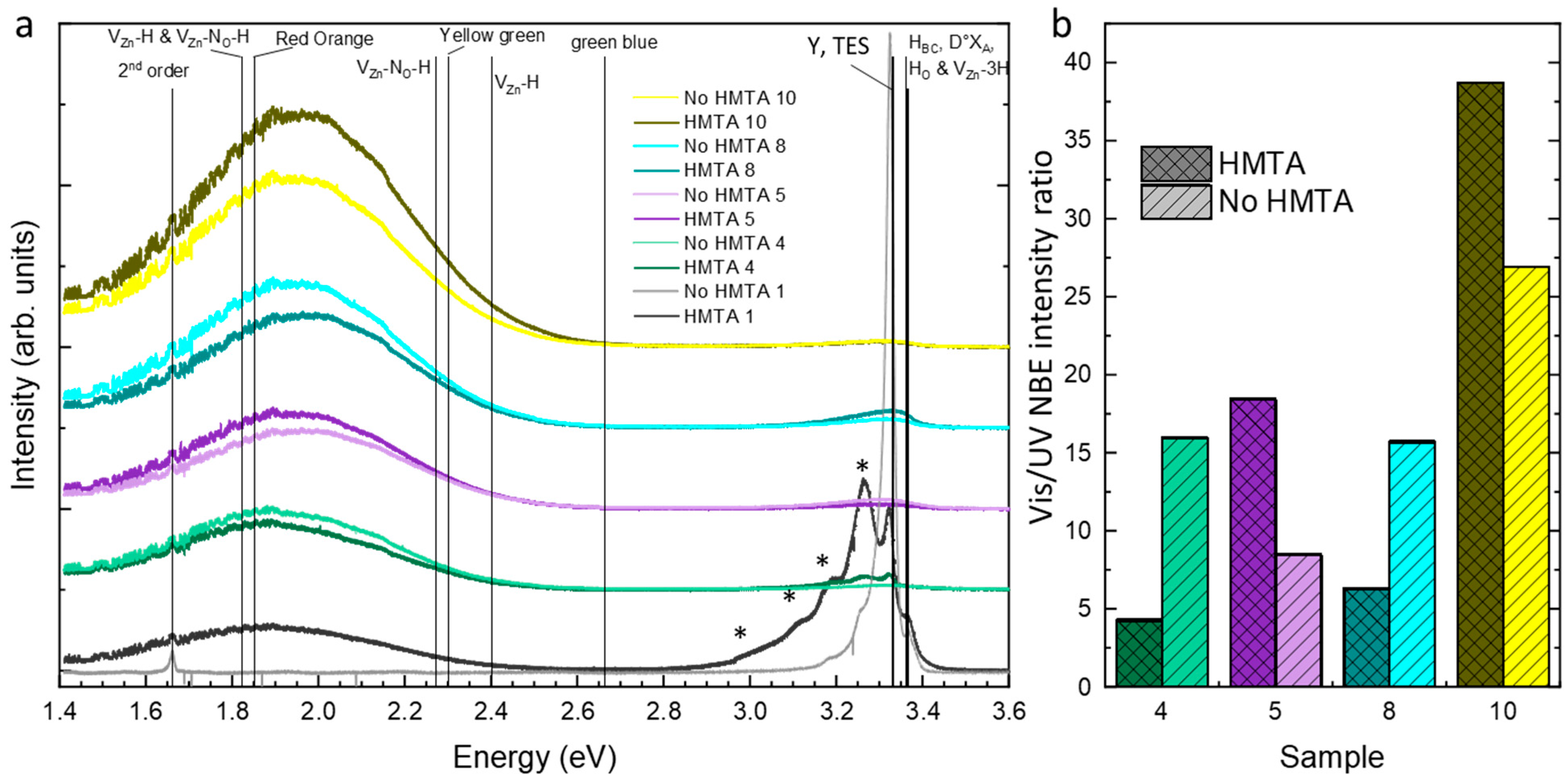
3.4. Effects of HMTA Molecules and Ammonia on the Crystalline Quality, Surface Chemical Composition, and Nature of Defects in ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lincot, D. Solution growth of functional zinc oxide films and nanostructures. MRS Bull. 2010, 35, 778–789. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Lindquist, S.E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Sugunan, A.; Warad, H.C.; Boman, M.; Dutta, J. Zinc oxide nanowires in chemical bath on seeded substrates: Role of hexamine. J. Sol-Gel Sci. Technol. 2006, 39, 49–56. [Google Scholar] [CrossRef]
- McPeak, K.M.; Le, T.P.; Britton, N.G.; Nickolov, Z.S.; Elabd, Y.A.; Baxter, J.B. Chemical Bath Deposition of ZnO Nanowires at Near-Neutral pH Conditions without Hexamethylenetetramine (HMTA): Understanding the Role of HMTA in ZnO Nanowire Growth. Langmuir 2011, 27, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- Strano, V.; Urso, R.G.; Scuderi, M.; Iwu, K.O.; Simone, F.; Ciliberto, E.; Spinella, C.; Mirabella, S. Double Role of HMTA in ZnO Nanorods Grown by Chemical Bath Deposition. J. Phys. Chem. C 2014, 118, 28189–28195. [Google Scholar] [CrossRef]
- Parize, R.; Garnier, J.; Chaix-Pluchery, O.; Verrier, C.; Appert, E.; Consonni, V. Effects of Hexamethylenetetramine on the Nucleation and Radial Growth of ZnO Nanowires by Chemical Bath Deposition. J. Phys. Chem. C 2016, 120, 5242–5250. [Google Scholar] [CrossRef]
- van Rijt, M.M.J.; Oosterlaken, B.M.; Joosten, R.R.M.; Wijkhuijs, L.E.A.; Bomans, P.H.H.; Friedrich, H.; de With, G. Counter-ion influence on the mechanism of HMTA-mediated ZnO formation. Crystengcomm 2020, 22, 5854–5861. [Google Scholar] [CrossRef]
- Lausecker, C.; Salem, B.; Baillin, X.; Consonni, V. Effects of Zinc Nitrate and HMTA on the Formation Mechanisms of ZnO Nanowires on Au Seed Layers. Cryst. Growth Des. 2023, 23, 2941–2950. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef]
- Salthammer, T. The formaldehyde dilemma. Int. J. Hyg. Environ. Health 2015, 218, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pontén, A.; Bruze, M. Formaldehyde. Dermatitis 2015, 26, 3–6. [Google Scholar] [CrossRef]
- Ahuja, I.S.; Yadava, C.L.; Singh, R. STructural information on manganese(II), cobalt(II), nickeL(II), zinC(II) and cadmium(II) sulphate complexes with hexamethylenetetramine (a potentially tetradentate ligand) from their magnetic moments, electronic and infrared spectra. J. Mol. Struct. 1982, 81, 229–234. [Google Scholar] [CrossRef]
- Guillemin, S.; Appert, E.; Roussel, H.; Doisneau, B.; Parize, R.; Boudou, T.; Bremond, G.; Consonni, V. Controlling the Structural Properties of Single Step, Dip Coated ZnO Seed Layers for Growing Perfectly Aligned Nanowire Arrays. J. Phys. Chem. C 2015, 119, 21694–21703. [Google Scholar] [CrossRef]
- Govender, K.; Boyle, D.S.; Kenway, P.B.; O’Brien, P. Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J. Mater. Chem. 2004, 14, 2575–2591. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Doherty, R.P.; Ndifor-Angwafor, N.G.; Riley, D.J.; Sun, Y. The kinetics of the hydrothermal growth of ZnO nanostructures. Thin Solid Film. 2007, 515, 8679–8683. [Google Scholar] [CrossRef]
- McBride, R.A.; Kelly, J.M.; McCormack, D.E. Growth of well-defined ZnO microparticles by hydroxide ion hydrolysis of zinc salts. J. Mater. Chem. 2003, 13, 1196–1201. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. pH-dependent growth of zinc oxide nanorods. J. Cryst. Growth 2009, 311, 2549–2554. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 2011, 269692. [Google Scholar] [CrossRef]
- Tak, Y.; Yong, K. Controlled Growth of Well-Aligned ZnO Nanorod Array Using a Novel Solution Method. J. Phys. Chem. B 2005, 109, 19263–19269. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Ji, X.; Li, Z.; He, X.; Sun, F. Vertically Aligned 1D ZnO Nanostructures on Bulk Alloy Substrates: Direct Solution Synthesis, Photoluminescence, and Field Emission. J. Phys. Chem. C 2007, 111, 4990–4997. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Zhou, J.; Nomenyo, K.; Ionescu, R.E.; Gokarna, A.; Lerondel, G. Facile, wafer-scale compatible growth of ZnO nanowires via chemical bath deposition: Assessment of zinc ion contribution and other limiting factors. Nanoscale Adv. 2020, 2, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, J.; Sarigiannidou, E.; Donatini, F.; Kioseoglou, J.; Chaix-Pluchery, O.; Pernot, J.; Consonni, V. Modulating the growth of chemically deposited ZnO nanowires and the formation of nitrogen- and hydrogen-related defects using pH adjustment. Nanoscale Adv. 2022, 4, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.; Kar, A.K. Counterion-Induced Tailoring of Energy Transfer in Hydrothermally Grown Nanostructured ZnO for Photocatalysis. Cryst. Growth Des. 2021, 21, 3656–3667. [Google Scholar] [CrossRef]
- Baillard, A.; Appert, E.; Weber, M.; Jacob, V.; Roussel, H.; Rapenne, L.; Chaix-Pluchery, O.; Consonni, V. Single and Co-Doping of ZnO Nanowires with Al and Cl Using One Precursor by Chemical Bath Deposition. J. Phys. Chem. C 2023, 127, 8306–8319. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Z.-Y.; Du, G.-H.; Ren, T.-Z.; Bouvy, C.; Halasa, M.; Su, B.-L. Simple approach to highly oriented ZnO nanowire arrays: Large-scale growth, photoluminescence and photocatalytic properties. Nanotechnology 2006, 17, 588. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Zhang, Y.; Hahn, S.H.; Kim, E.J. From Zn(OH)2 to ZnO: A study on the mechanism of phase transformation. CrystEngComm 2011, 13, 6024–6026. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. The mechanism for hydrothermal growth of zinc oxide. CrystEngComm 2012, 14, 1232–1240. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]
- McPeak, K.M.; Becker, M.A.; Britton, N.G.; Majidi, H.; Bunker, B.A.; Baxter, J.B. In Situ X-ray Absorption Near-Edge Structure Spectroscopy of ZnO Nanowire Growth During Chemical Bath Deposition. Chem. Mater. 2010, 22, 6162–6170. [Google Scholar] [CrossRef]
- Cheng, J.J.; Nicaise, S.M.; Berggren, K.K.; Gradečak, S. Dimensional Tailoring of Hydrothermally Grown Zinc Oxide Nanowire Arrays. Nano Lett. 2016, 16, 753–759. [Google Scholar] [CrossRef]
- Lausecker, C.; Salem, B.; Baillin, X.; Consonni, V. Modeling the Elongation of Nanowires Grown by Chemical Bath Deposition Using a Predictive Approach. J. Phys. Chem. C 2019, 123, 29476–29483. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Room Temperature Solution Synthesis of Monodispersed Single-Crystalline ZnO Nanorods and Derived Hierarchical Nanostructures. Langmuir 2004, 20, 4196–4204. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Jung, S.-H.; Lee, B.R.; Oh, E.; Lee, K.-H. Precursor Effects of Citric Acid and Citrates on ZnO Crystal Formation. Langmuir 2009, 25, 3825–3831. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Pramanik, A. Morphology control of ZnO with citrate: A time and concentration dependent mechanistic insight. CrystEngComm 2013, 15, 6349–6358. [Google Scholar] [CrossRef]
- Taubert, A.; Palms, D.; Weiss, Ö.; Piccini, M.-T.; Batchelder, D.N. Polymer-Assisted Control of Particle Morphology and Particle Size of Zinc Oxide Precipitated from Aqueous Solution. Chem. Mater. 2002, 14, 2594–2601. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, A.-W.; Deng, B.; Antonietti, M.; Cölfen, H. Polymer-Controlled Crystallization of Zinc Oxide Hexagonal Nanorings and Disks. J. Phys. Chem. B 2006, 110, 2988–2993. [Google Scholar] [CrossRef]
- Parize, R.; Garnier, J.D.; Appert, E.; Chaix-Pluchery, O.; Consonni, V. Effects of Polyethylenimine and Its Molecular Weight on the Chemical Bath Deposition of ZnO Nanowires. ACS Omega 2018, 3, 12457–12464. [Google Scholar] [CrossRef]
- Cho, S.; Jeong, H.; Park, D.-H.; Jung, S.-H.; Kim, H.-J.; Lee, K.-H. The effects of vitamin C on ZnO crystal formation. CrystEngComm 2010, 12, 968–976. [Google Scholar] [CrossRef]
- Cantelli, V.; Guillemin, S.; Sarigiannidou, E.; Carlá, F.; Bérini, B.; Chauveau, J.-M.; Fong, D.D.; Renevier, H.; Consonni, V. In situ analysis of the nucleation of O- and Zn-polar ZnO nanowires using synchrotron-based X-ray diffraction. Nanoscale 2022, 14, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Lausecker, C.; Salem, B.; Baillin, X.; Consonni, V. Implementing the Reactor Geometry in the Modeling of Chemical Bath Deposition of ZnO Nanowires. Nanomaterials 2022, 12, 1069. [Google Scholar] [CrossRef]
- Lausecker, C.; Salem, B.; Baillin, X.; Chaix-Pluchery, O.; Roussel, H.; Labau, S.; Pelissier, B.; Appert, E.; Consonni, V. Chemical Bath Deposition of ZnO Nanowires Using Copper Nitrate as an Additive for Compensating Doping. Inorg. Chem. 2021, 60, 1612–1623. [Google Scholar] [CrossRef]
- Sakai, D.; Nagashima, K.; Yoshida, H.; Kanai, M.; He, Y.; Zhang, G.; Zhao, X.; Takahashi, T.; Yasui, T.; Hosomi, T.; et al. Substantial Narrowing on the Width of “Concentration Window” of Hydrothermal ZnO Nanowires via Ammonia Addition. Sci. Rep. 2019, 9, 14160. [Google Scholar] [CrossRef] [PubMed]
- Cusco, R.; Alarcon-Llado, E.; Ibanez, J.; Artus, L.; Jimenez, J.; Wang, B.G.; Callahan, M.J. Temperature dependence of raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Weber, J.; Borrnert, F.; Van de Walle, C.G.; Helbig, R. Hydrogen-related defects in ZnO studied by infrared absorption spectroscopy. Phys. Rev. B 2002, 66, 165205. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Borrnert, F.; Weber, J. Dominant hydrogen-oxygen complex in hydrothermally grown ZnO. Phys. Rev. B 2005, 71, 035205. [Google Scholar] [CrossRef]
- Herklotz, F.; Hupfer, A.; Johansen, K.M.; Svensson, B.G.; Koch, S.G.; Lavrov, E.V. Infrared absorption on a complex comprising three equivalent hydrogen atoms in ZnO. Phys. Rev. B 2015, 92, 155203. [Google Scholar] [CrossRef]
- Nickel, N.H.; Fleischer, K. Hydrogen local vibrational modes in zinc oxide. Phys. Rev. Lett. 2003, 90, 197402. [Google Scholar] [CrossRef]
- Villafuerte, J.; Donatini, F.; Kioseoglou, J.; Sarigiannidou, E.; Chaix-Pluchery, O.; Pernot, J.; Consonni, V. Zinc Vacancy–Hydrogen Complexes as Major Defects in ZnO Nanowires Grown by Chemical Bath Deposition. J. Phys. Chem. C 2020, 124, 16652–16662. [Google Scholar] [CrossRef]
- Reynolds, J.G.; Reynolds, C.L.; Mohanta, A.; Muth, J.F.; Rowe, J.E.; Everitt, H.O.; Aspnes, D.E. Shallow acceptor complexes in p-type ZnO. Appl. Phys. Lett. 2013, 102, 152114. [Google Scholar] [CrossRef]
- Villafuerte, J.; Chaix-Pluchery, O.; Kioseoglou, J.; Donatini, F.; Sarigiannidou, E.; Pernot, J.; Consonni, V. Engineering nitrogen- and hydrogen-related defects in ZnO nanowires using thermal annealing. Phys. Rev. Mater. 2021, 5, 056001. [Google Scholar] [CrossRef]
- Li, X.N.; Keyes, B.; Asher, S.; Zhang, S.B.; Wei, S.H.; Coutts, T.J.; Limpijumnong, S.; Van de Walle, C.G. Hydrogen passivation effect in nitrogen-doped ZnO thin films. Appl. Phys. Lett. 2005, 86, 122107. [Google Scholar] [CrossRef]
- Jokela, S.J.; McCluskey, M.D. Unambiguous identification of nitrogen-hydrogen complexes in ZnO. Phys. Rev. B 2007, 76, 193201. [Google Scholar] [CrossRef]
- Gromyko, I.; Krunks, M.; Dedova, T.; Katerski, A.; Klauson, D.; Oja Acik, I. Surface properties of sprayed and electrodeposited ZnO rod layers. Appl. Surf. Sci. 2017, 405, 521–528. [Google Scholar] [CrossRef]
- Dedova, T.; Acik, I.O.; Chen, Z.; Katerski, A.; Balmassov, K.; Gromyko, I.; Nagyné-Kovács, T.; Szilágyi, I.M.; Krunks, M. Enhanced photocatalytic activity of ZnO nanorods by surface treatment with HAuCl4: Synergic effects through an electron scavenging, plasmon resonance and surface hydroxylation. Mater. Chem. Phys. 2020, 245, 122767. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Easton, C.D.; Morgan, D.J. Critical examination of the use of x-ray photoelectron spectroscopy (XPS) O 1s to characterize oxygen vacancies in catalytic materials and beyond. J. Vac. Sci. Technol. A 2025, 43, 53205. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Herklotz, F.; Weber, J. Identification of two hydrogen donors in ZnO. Phys. Rev. B 2009, 79, 65210. [Google Scholar] [CrossRef]
- Heinhold, R.; Neiman, A.; Kennedy, J.V.; Markwitz, A.; Reeves, R.J.; Allen, M.W. Hydrogen-related excitons and their excited-state transitions in ZnO. Phys. Rev. B 2017, 95, 054120. [Google Scholar] [CrossRef]
- Wagner, M.R.; Callsen, G.; Reparaz, J.S.; Schulze, J.H.; Kirste, R.; Cobet, M.; Ostapenko, I.A.; Rodt, S.; Nenstiel, C.; Kaiser, M.; et al. Bound excitons in ZnO: Structural defect complexes versus shallow impurity centers. Phys. Rev. B 2011, 84, 035313. [Google Scholar] [CrossRef]
- Guillemin, S.; Consonni, V.; Rapenne, L.; Sarigiannidou, E.; Donatini, F.; Bremond, G. Identification of extended defect and interface related luminescence lines in polycrystalline ZnO thin films grown by sol-gel process. RSC Adv. 2016, 6, 44987–44992. [Google Scholar] [CrossRef]
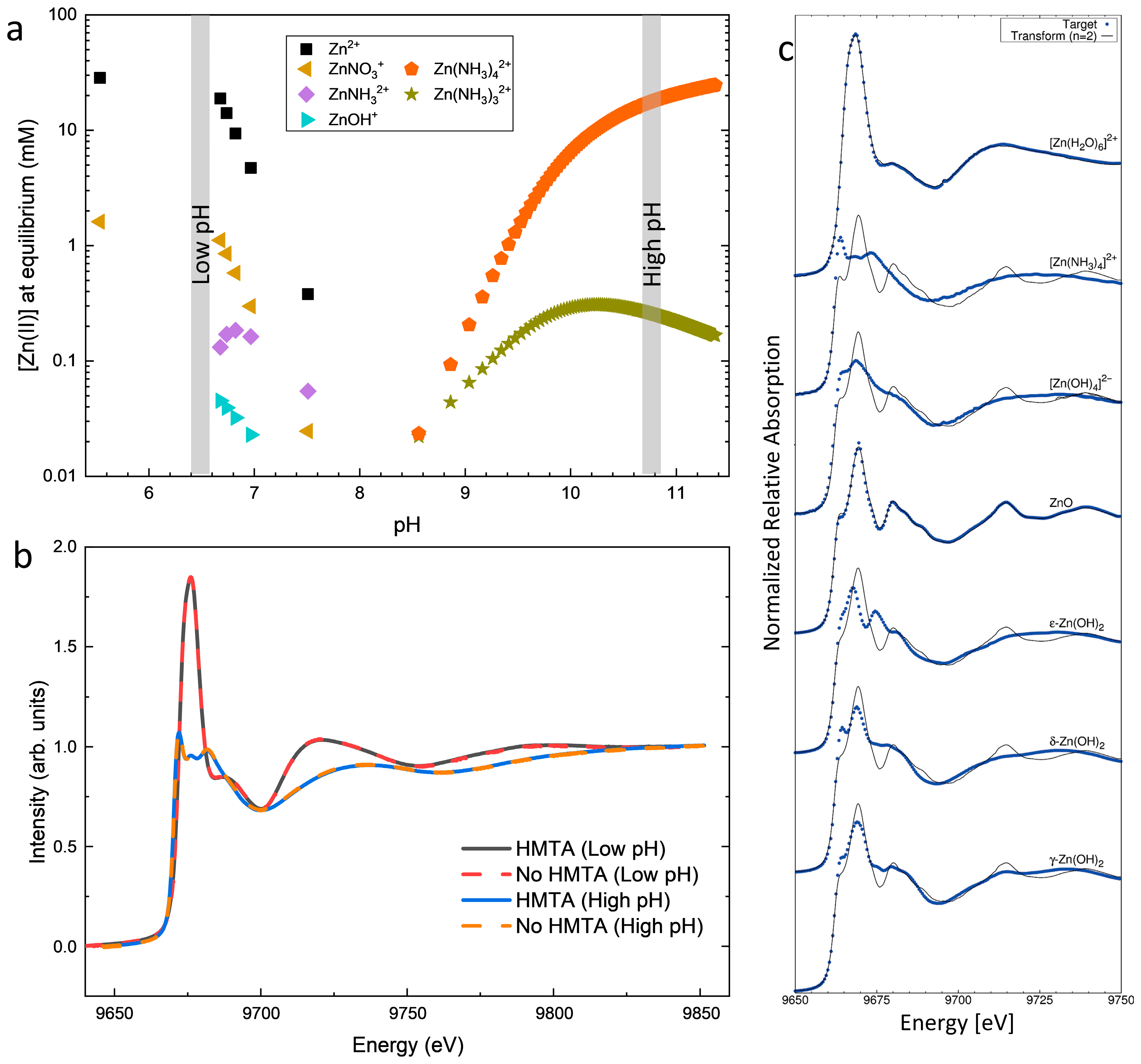
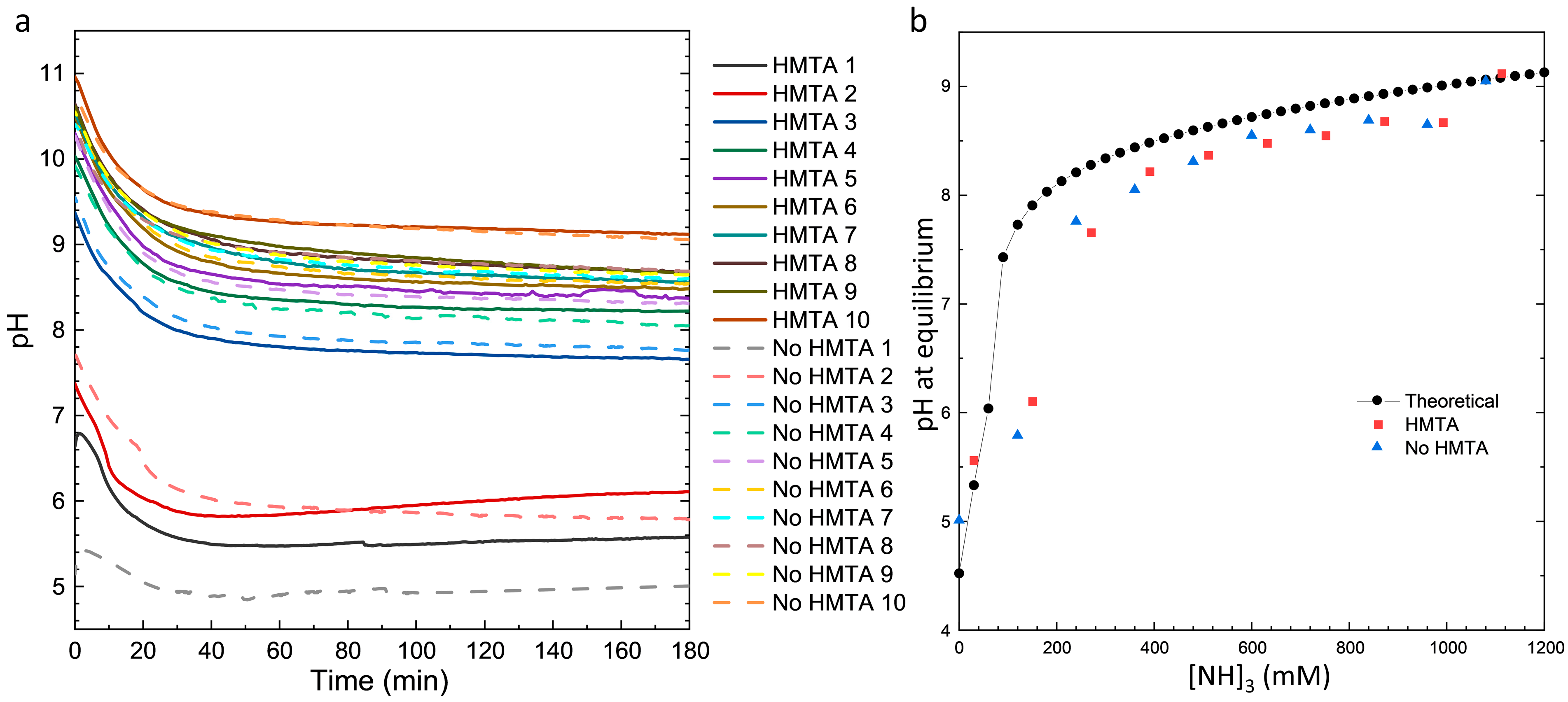
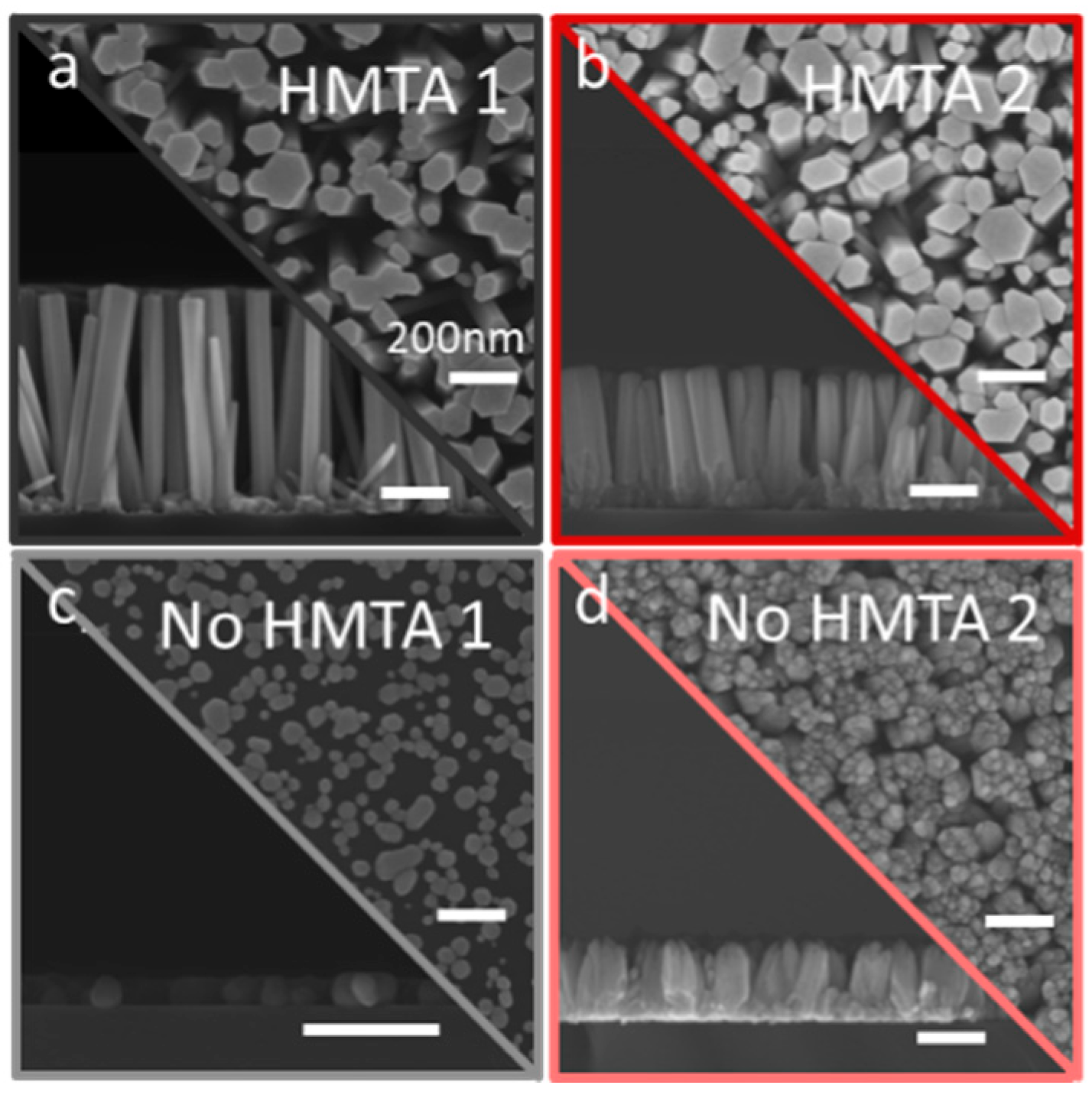


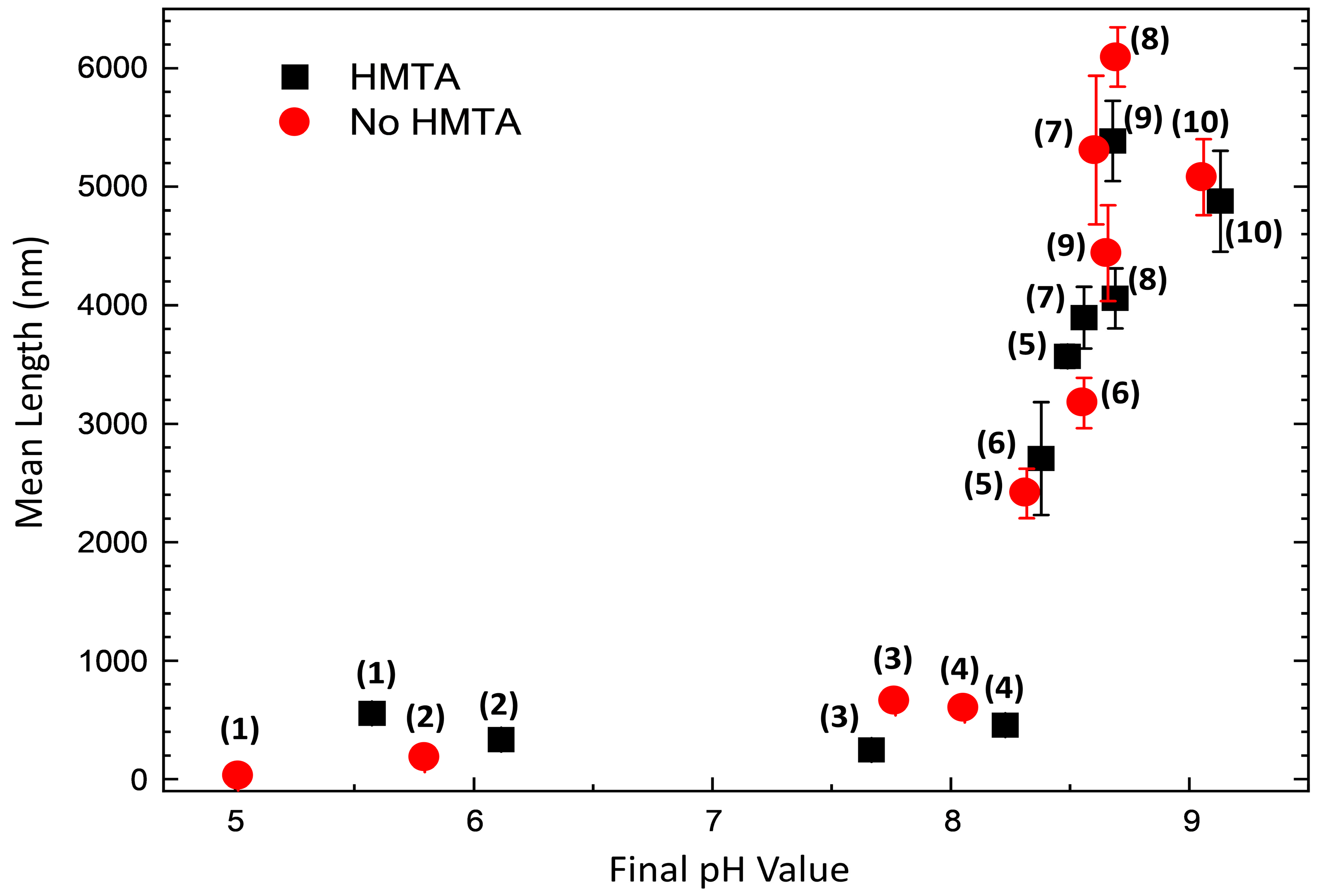
| Sample Number | Ammonia Concentration (mM) |
|---|---|
| 1 | 0 |
| 2 | 120 |
| 3 | 240 |
| 4 | 360 |
| 5 | 480 |
| 6 | 600 |
| 7 | 720 |
| 8 | 840 |
| 9 | 960 |
| 10 | 1080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baillard, A.; Appert, E.; Wilhelm, F.; Sarigiannidou, E.; Consonni, V. Towards the Removal of HMTA Molecules in the Chemical Bath Deposition of ZnO Nanowires. Nanomaterials 2025, 15, 1574. https://doi.org/10.3390/nano15201574
Baillard A, Appert E, Wilhelm F, Sarigiannidou E, Consonni V. Towards the Removal of HMTA Molecules in the Chemical Bath Deposition of ZnO Nanowires. Nanomaterials. 2025; 15(20):1574. https://doi.org/10.3390/nano15201574
Chicago/Turabian StyleBaillard, Adrien, Estelle Appert, Fabrice Wilhelm, Eirini Sarigiannidou, and Vincent Consonni. 2025. "Towards the Removal of HMTA Molecules in the Chemical Bath Deposition of ZnO Nanowires" Nanomaterials 15, no. 20: 1574. https://doi.org/10.3390/nano15201574
APA StyleBaillard, A., Appert, E., Wilhelm, F., Sarigiannidou, E., & Consonni, V. (2025). Towards the Removal of HMTA Molecules in the Chemical Bath Deposition of ZnO Nanowires. Nanomaterials, 15(20), 1574. https://doi.org/10.3390/nano15201574







